Covalent organic frameworks (COFs) are a class of crystalline porous organic polymers with polygonal porosity and highly ordered structures. The most prominent feature of the COFs is their excellent crystallinity and highly ordered modifiable one-dimensional pores. Since the first report of them in 2005, COFs with various structures were successfully synthesized and their applications in a wide range of fields including gas storage, pollution removal, catalysis, and optoelectronics explored. In the meantime, COFs also exhibited good performance in chemical and biological sensing, because their highly ordered modifiable pores allowed the selective adsorption of the analytes, and the interaction between the analytes and the COFs’ skeletons may lead to a detectable change in the optical or electrical properties of the COFs.
1. Designing a COF or COF-Based Hybird Material for Selective Adsorption
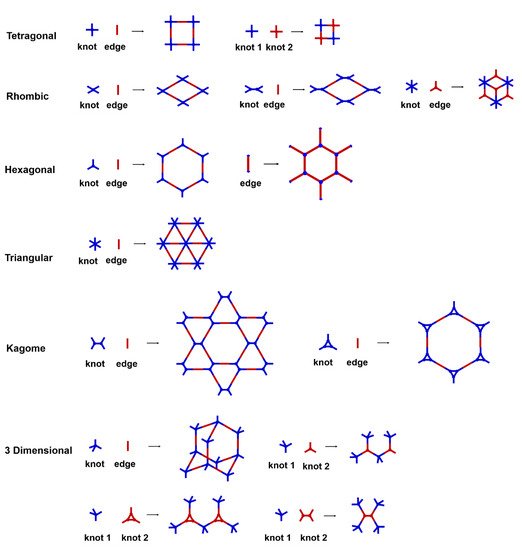
The porous structure of the COF results in its extraordinary adsorption capacity. However, compared with other types of porous materials, the predominant characteristic of COFs is the designability of the pores. Inspired by the recognition between the enzyme and its substrate, the key points to design a COF that can enrich the analyte are creating a suitable space allowing the load of the analyte and introducing binding sites to selectively interact with the analyte. Hence, the shape and size of the COF pores should be firstly considered. In general, the shape of the pores is mainly determined by the topology of the “knot” monomer (
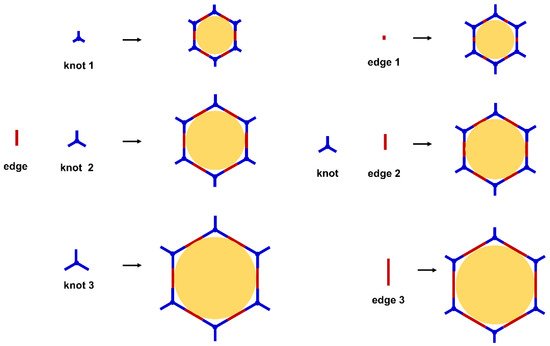
Figure 1). For example, to obtain a hexagonal pore, three-armed planar rigid “knot” monomers, such as the derivatives of triazine, triphenyl benzene, triphenylene derivatives, et al. were usually applied. In addition, four-armed planar rigid “knot” monomers were often used for fabricating a tetragonal pore, while the size of the COF pores depends on the length of both the “edge” monomer and the arms of the “knot” monomers. As shown in
Figure 2, it can be easily enlarged by choosing a longer “edge” monomer or a “knot” molecule with longer arms. Thus, it can be found that both the shape and size of the COFs pores can be pre-designed by choosing the “knot” and “edge” monomers
[1][2]. To effectively adsorb the analyte, the pore shape and size should be carefully designed to at least allow the analyte molecule to enter. Furthermore, for discriminating the interferents of a similar molecular shape and size, in general, a binding site is needed that can selectively interact with the analyte. The binding site can be the innate groups on the COF skeleton. For example, as shown in
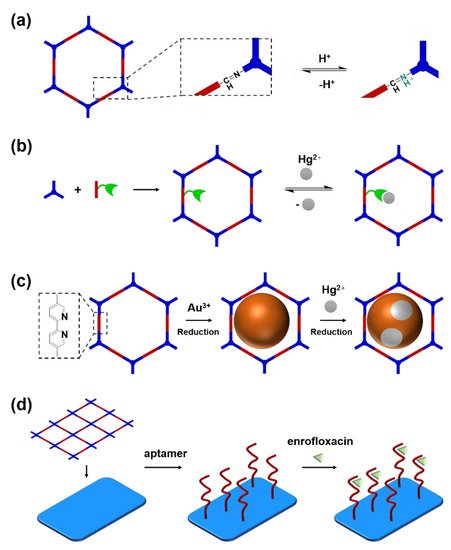
Figure 3a, the imine linkage is pH sensitive. It is a good binding site for capturing H
+ in the solution or the acid gases like HCl and Trifluoroacetic acid (TFA)
[3][4]. However, in other cases, as shown in
Figure 3b, the binding site is pre-linked onto one of the monomers before the synthesis of the COFs. For example, thiol or thioether groups are able to selectively adsorb the heavy metal ions like Hg
2+. Thus, thioether was covalently pre-linked onto the “edge” monomer, yielding a thioether-containing COF (COF−LZU8)
[5]. It was shown that COF−LZU8 performed well in selectively capturing and detecting Hg
2+. In addition, the binding site can be also introduced after the formation of COFs, which is also called “post-functionalization” of COFs. For instance, Au nanoparticles (AuNPs) can form an amalgam layer with Hg
2+ under the reduction of citric acid (
Figure 3c). Hence, to efficiently capture and detect the trace amount of Hg
2+, AuNPs were doped on the nanosheet of a bipyridine-containing COF by the in situ growth method to form a COF-based hybrid material. It was found that the huge specific surface area of the COF-AuNPs hybrid material can significantly enhance the sensitivity of the Hg
2+ detection
[6]. Moreover, except for in situ growth, the binding site can be also externally introduced onto the 2D sheet of the COFs. For example, as shown in
Figure 3d, to selectively detect enrofloxacin, a kind of antibiotic, an enrofloxacin-targeted aptamer was immobilized onto a 2D sheet of COF via π-π stacking and electrostatic interaction to yield an aptamer-functionalized COF hybrid
[7]. Then the hybrid was coated onto a gold electrode. It can selectively capture the enrofloxacin in the sample solution and result in a detectable change in the electrode conductivity.
Figure 1. The basic topology of the COFs.
Figure 2. Modulating the pore size of the COFs.
Figure 3. Introducing the binding site that can selectively capture the analyte. (a) capturing H+ by imine linkage; (b) Intruding thioether binding site for capturing Hg2+; (c) Introducing AuNPs by in situ growth for mercury capture; (d) Externally immobilizing enrofloxacin-targeted aptamer onto the 2D sheet of the COF for capturing enrofloxacin.
2. Signals Produced by the Adsorption of the Analyte
Apart from the selective uptake and enrichment of the analyte, the other key problem in constructing a COF-based sensor is to make the uptake of the analyte produce a detectable signal. By ingenious structural design, a large number of COFs of which the color, fluorescence, conductivity or capacitance can quantitatively change with the uptake of the analyte have been synthesized
[8]. Besides that, in some reports the adsorption of the analyte can also produce some detectable signals which do not originate from COFs
[6][9].
2.1. Fluorescence
Among the detectable signals caused by the uptake of the analyte, fluorescence change of COFs including fluorescence enhancement (“turn on”) and fluorescence quenching (“turn off”) is the most frequently used to respond to the concentration of analyte. Due to the rigid structure, the large π-conjugated fluorescent chromophores, such as phenyl, naphthalenyl, pyrenyl, perylenyl, triazine, and triphyenyl-benzene are usually applied for building COFs
[10][11]. Moreover, the conjugated linkages like imine and olefins can further enlarge the π-conjugated structure in the COFs. Therefore, it is not unusual to find a COF with fluorescent emission. However, it was found that the adsorption of some analytes may influence the fluorescent emission, including the change of the intensity and movement of the emission peak. The fluorescence change may be induced by various mechanisms, such as the charge and energy transfer between the COF and analyte, aggregation-induced emission, and exciplex formation, et al. The most common one among the above mechanisms is the charge transfer from an electron-rich COF to an electron-deficient analyte. It can lead to a significant fluorescent quenching which is relative to the concentration of the analyte. In the following demonstration, it can be found that many COF-based nitroaromatic explosive sensors, iodine sensors, and transition metal ions sensors are constructed by the charge transfer mechanism.
2.2. Chromism
Chromism is a signal that can be directly found by naked eyes. The advantage of the sensing approach based on chromism is easy-operate. It is very convenient to be further developed into rapid testing equipment like test paper, test kit, and wearable monitoring devices. Therefore, the COF-based sensors based on chromism are frequently reported. The color change of the COFs can be induced in different manners. Firstly, the non-covalent interaction between COFs and the analyte disturbs the electronic transition responsible for the coloration. For example, the adsorption of molecules with different polarities can influence the intramolecular charge transfer from the electron donor to the electron receptor on the COF skeleton, causing a red or blue shift of the UV-Vis absorbance
[12]. Secondly, in some COF-based chromism sensors, the interaction with the analyte can isomerize some groups on the COF skeleton, resulting in the color change
[13][14]. In addition, it should be noted that there is also a type of COF-based chromism sensor of which the color change is not caused by the COF itself. For example, in some sensors, COFs are used as a catalyst or a catalyst carrier, and the adsorption of the analyte can influence the catalytic capacity which can be detected by the formation of the colored product. Thus, the concentration of the analyte can be determined by the color change
[6][9].
2.3. Capacitance and Conductivity
The adsorption of the analyte can lead to a change in the capacitance and conductivity of the COFs
[15][16][17]. Hence the concentration of the analyte can be also determined by the change of such two electrical signals. In general, the quantitative measurement of electrical signals is much more convenient compared with photo signals. The measuring devices involved are much simpler. Therefore, COF-containing sensors based on the change of capacitance and conductivity are more likely to be realized on a chip-like device. Many presented works have demonstrated that a fingertip-size interdigital electrode (IDE) coated with a specific COF can be applied for real-time detection of humidity, corrosive gases, or toxic gases since their adsorption results in the change of the capacitance or conductivity of the COFs. It can be expected that such a type of device can be further developed into portable, wearable, or even implantable sensors for real-time sensing.
2.4. Electrochemical or Photoelectrochemical Signals
Electrochemical or photoelectrochemical signals are generated by the redox reaction of the active substance. Plenty of analytical techniques based on electrochemistry or photoelectrochemistry have been built. The signal produced by electrochemical or photoelectrochemical reactions can be influenced by many factors such as the concentration of the active substances, the conductivity of the electrode/electrolyte interface, the existence of catalysts, and so on. In recent years, a number of examples of electrochemical or photoelectrochemical analysis with the participation of COFs were reported. The COFs coated on the electrode in these examples can play various roles including: (1) enriching the analyte that can be directly detected by the electrochemical or photoelectrochemical method; (2) concentrating the analytes to inhibit or facilitate the transportation of charges or substances; (3) a carrier for immobilizing the catalyst which adsorption can be affected by the analytes. All of the above allow a significant influence of the analyte on the electrochemical or photoelectrochemical signal. Compared with the above type of COF-based sensors the advantage of the electrochemical or photoelectrochemical signal is the high sensitivity. It is not rare to realize trace amounts of the analyte at or lower than ppb or ng/mL level with this type of COF-based sensor.