Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Argyris Costas Hadjimichael | -- | 2975 | 2022-05-12 12:40:45 | | | |
| 2 | Camila Xu | Meta information modification | 2975 | 2022-05-13 04:04:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hadjimichael, A.; Theocharis, S.; Pergaris, A.; Kaspiris, A.; , .; Kokkali, S.; Tsourouflis, G. EPH/Ephrin Signaling Pathways in Bone Sarcomas. Encyclopedia. Available online: https://encyclopedia.pub/entry/22870 (accessed on 07 February 2026).
Hadjimichael A, Theocharis S, Pergaris A, Kaspiris A, , Kokkali S, et al. EPH/Ephrin Signaling Pathways in Bone Sarcomas. Encyclopedia. Available at: https://encyclopedia.pub/entry/22870. Accessed February 07, 2026.
Hadjimichael, Argyris, Stamatios Theocharis, Alexandros Pergaris, Angelos Kaspiris, , Stefania Kokkali, Gerasimos Tsourouflis. "EPH/Ephrin Signaling Pathways in Bone Sarcomas" Encyclopedia, https://encyclopedia.pub/entry/22870 (accessed February 07, 2026).
Hadjimichael, A., Theocharis, S., Pergaris, A., Kaspiris, A., , ., Kokkali, S., & Tsourouflis, G. (2022, May 12). EPH/Ephrin Signaling Pathways in Bone Sarcomas. In Encyclopedia. https://encyclopedia.pub/entry/22870
Hadjimichael, Argyris, et al. "EPH/Ephrin Signaling Pathways in Bone Sarcomas." Encyclopedia. Web. 12 May, 2022.
Copy Citation
Erythropoietin-producing human hepatocellular receptors (EPHs) is a large family of membrane-bound tyrosine kinases receptors (RTKs) which bind the Eph family receptor interacting proteins (ephrins) located on the surfaces of neighboring cells. Bone and soft tissue sarcomas represent a family of rare connective tissue malignancies with mesenchymal origin and very aggressive behavior.
EPH
ephrin
bone sarcoma
1. Introduction
Bone and soft tissue sarcomas represent a family of rare connective tissue malignancies with mesenchymal origin and very aggressive behavior [1][2][3]. Based on epidemiological reports from EUROCARE (European Cancer Registry based study on survival and care of cancer patients), primary bone sarcomas account for less than 0.2% of malignant neoplasms [4]. Meanwhile, the incidence of soft tissue sarcomas in Europe is approximately 3.6–4.7 cases per 100,000 people annually, representing less than 1% of all cancer types [5]. The most common histological types of bone sarcomas are osteosarcoma, Ewing’s sarcoma and chondrosarcoma [6], whereas rhabdomyosarcoma, synovial sarcoma, undifferentiated pleomorphic sarcoma and leiomyosarcoma are the most usually diagnosed histological types for soft tissue sarcomas [6].
Regarding prognosis of bone sarcomas, the age-standardized relative survival reduces to 78% at one year, 60% at three years and 53% at five years after diagnosis [7]. Similarly, the age-standardized relative survival for all ages is deteriorated to 82% at one-year, 66% at three years and 60% at five years after a soft tissue sarcoma is confirmed. The American Joint Committee on Cancer (AJCC) correlates the poorer prognosis with predictors such as the local expansion of tumor and the presence of metastatic disease in lymph nodes and distant sites (e.g., lungs, liver, bones) [8]. Oftentimes, localized sarcomas can be successfully treated with surgery and radiation, but metastatic widespread disease can be prevented only by chemotherapy [9]. The necessity for the development of novel anti-metastatic drugs has emerged in recent years, prompting the investigation of metastatic pathways and patterns [10].
Erythropoietin-producing human hepatocellular receptors (EPHs) is a large family of membrane-bound tyrosine kinases receptors (RTKs) which bind the Eph family receptor interacting proteins (ephrins) located on the surfaces of neighboring cells [11]. EPHs are further divided in two subfamilies. The first family consists of type-A EPHs (EPHA1–EPHA8 and EPHA10), which interact with type-A ephrins (ephrin-A1–ephrin-A5) [12]. The second family consists of type-B, EPHs (EPHB1–EPHB4 and EPHB6), which interact with type-B ephrins (ephrin-B1–ephrin-B3). However, cross-interaction between members of the two categories has been described [12]. For instance, EPHB2 can activate ephrin-A5, and EPHA4 can activate ephrin-B ligands. Likewise, the interaction and activation between EPHs and multiple ephrins can be seen within categories (e.g., EPHB2 activates ephrin-B1, B2 and B3) [12]. The EPH/ephrin signaling and cell–cell interactions regulate many physiologic and homeostatic events (Figure 1). For example, one of the roles of the EPH/ephrin system is to regulate neurogenesis and neuronal migration during embryogenesis and to guide axonal growth during early brain development [13]. Furthermore, it has been suggested that EPHA/ephrin-A signaling enhances osteoclastogenesis and suppresses osteoblastogenesis in vitro [13]. Additionally, it has been found that ephrin-B reverse signaling inhibits osteoclast differentiation and deteriorates bone resorption, whereas EPHB promotes the differentiation of osteoblasts, leading to increased bone formation [13]. Therefore, the EPH/ephrin system may function as an important regulator in normal bone homeostasis. Moreover, there is strong evidence that the interaction between EPHB4 and ephrin-B2 contributes to the formation of new blood vessels (angiogenesis) and lymphatic vasculature (lymphangiogenesis) [13]. This is one of the most significant mechanisms that malignant tumors use to achieve rapid growth and distant metastatic dissemination. An effective strategy to inhibit the progression of primary tumors and the expansion of lethal metastatic sites is to block the process of neo-angiogenesis that supports tumor cells with oxygen and adequate blood supply. Clearly, targeted anti-EPH/ephrin agents could influence the aggressive potential of bone and soft tissue sarcomas and open up the possibility to generate novel chemotherapeutic drugs.
 Figure 1. Structure of EPHs and ephrins and the presentation of some physiologic processes mediated through the EPH/ephrin signaling pathway. Created with BioRender.com, accessed on 2 May 2022.
Figure 1. Structure of EPHs and ephrins and the presentation of some physiologic processes mediated through the EPH/ephrin signaling pathway. Created with BioRender.com, accessed on 2 May 2022.In addition, the EPH/ephrin system is involved in malignant processes such as metastatic development [13]. Subsequently, several in vitro and in vivo studies have elucidated the molecular mechanisms through which the EPH/ephrin signaling pathway has impact on tumorigenesis and cancer metastasis [14]. For example, the EPHB4/ephrin-B2 interaction enhances angiogenesis via the VEGFR2 and Notch signaling pathways in glioblastoma [14]. In addition, the phosphorylation of EPHA3 activates the PI3K/Akt and MAPK pathways and induces resistance to trastuzumab in breast cancer [14]. On the contrary, EPHA3 activates the phosphorylation of the PI3K/BMX/STAT3 signaling pathway, leading to the apoptosis of lung carcinoma cells [14].
2. EPH/Ephrin Signaling Pathways in Bone Sarcomas
2.1. EPH/Ephrin Expression in Osteosarcoma and Interaction with Ras/MAPK Pathway
The experimental study by Fritsche-Guenther et al. revealed that EPHs/ephrins are either being upregulated or de novo expressed during the oncogenic signaling of osteosarcoma, stimulating its metastatic phenotype [15]. According to their findings, the expression of ephrin-A1, a ligand with high affinity with the EPHA tyrosine kinase receptors, was found 10-fold higher in osteosarcoma cells compared to normal osteoblasts. Furthermore, EPHA2 was present only in osteosarcoma samples but absent in non-malignant bone cells [15]. In addition, ephrin-B1 levels were found de novo increased in primary osteosarcoma tumors but were significantly downregulated in metastatic osteosarcoma of the lungs [15]. Likewise, the expression of ephrin-B3 and EPHA3 were found elevated in osteosarcoma without a significant difference being reported between primary and metastatic tissue samples [15]. The interaction between EPHA2 and ephrin-A1 enhanced the phosphorylation of EPHA2′s tyrosine and increased the mitogenic process via the Ras/MAPK pathway, which eventually enhanced the proliferation and migration of SaOS2 and MNNG/HOS human osteosarcoma cells [15].
2.2. EPHA2/Ephrin-A1–CAV1 Axis Activates AKT Signaling in EWS
The impact of EPH/ephrin signaling in neo-angiogenesis and the tumor neovascularization process was correlated with the highly aggressive behavior of Ewing’s sarcoma by Sáinz-Jaspeado et al. [16]. Their in vitro trial demonstrated that the silencing of the metastasis-associated CAV1 gene, which expresses the CAV1 integral protein, was related to reduced endothelial cell migration in Ewing’s sarcoma [16]. According to their findings, the interaction between CAV1 and EPHA2 in the presence of ephrin-A1 activates the AKT signaling pathway and promotes the expression of the pre-angiogenic basic fibroblast growth factor (bFGF). Subsequently, the secreted bFGF acts as a chemoattractant for endothelial cells and promoter of angiogenesis in Ewing’s sarcoma [16]. Likewise, their in vitro findings were in line with in vivo outcomes after the implantation of three Ewing’s sarcoma cells with the CAV1 gene silencing profile and low expression of CAV1 transmembrane protein. The primary tumor growth, along with the number of vessels supplying it, were found significantly reduced, leading to excessive tumor necrosis. The EPHA2/ephrin-A1–CAV1 axis activates AKT signaling to secrete bFGF and has been proposed as a potential target of chemotherapeutic agents [16].
2.3. Activation of Notch Signaling Pathway by EphrinB1 in Osteosarcoma
Another study by Yu et al. provided pre-clinical and clinical in vitro evidence that the interaction between Notch signaling and the EPH/ephrin axis contributes to osteosarcoma progression [17]. The Notch signaling pathway consists of four Notch receptors (Notch 1–4), which are single-pass type I transmembrane molecules, and their transmembrane ligands Delta-like and Jagged [18]. Upon ligand activation, the Notch intracellular domain (NICD) is translocated to the nucleus to activate the transcriptional cofactor CBF1, leading to the expression of HES and HEY genes [18]. In vitro, the mRNA expression levels of Hes1 from the Notch pathway and ephrin-B1 from the EPH/ephrin axis were found to be both elevated in 143B highly metastatic human osteosarcoma cells. Further in silico analysis revealed that −1438 to −1431, −2430 to −2423, and −2911 to −2904 in the ephrin-B1 promoter region are the three recognized binding sites for NICD1 [17]. The activation of the Notch pathway promotes the phosphorylation and overexpression of ephrin-B1 [17]. Based on the experimental observations by Yu, eprin-B1 enhances the Notch-driven osteosarcoma cells’ proliferation and chemoresistance, properties which were successfully reversed in ephrin-B1 knockdown in the aforementioned cells [17]. Eventually, specific anti-ephrin-B1 agents represent potential drug candidates that could eliminate the expansion and metastatic burden of osteosarcoma as well as counter the cells’ chemoresistance toward existing chemotherapeutic regiments.
2.4. The Key Role of EPHA2 in Osteosarcoma, Chondrosarcoma, Ewing’s Sarcoma
Among others, one of the synergistic antitumor activities that pazopanib and trametinib (receptor tyrosine kinase inhibitors) exhibit in osteosarcoma cells is carried out through the down-modulation of EPHA2 and Interleukin (IL)-7 Receptor (IL-7R), according to the in vitro findings by Chiabotto et al. [19]. Initially, a significant suppression of migration and proliferation properties was observed in four EPHA2-silenced osteosarcoma cell lines (HOS, KHOS/NP, MNNG/HOS and U2OS) compared to their controls. Importantly, for the first time, the combination of drugs such as pazopanib and trametinib has been proposed as an EPHA2 oncogene suppressor with potential therapeutic effect in the clinical setting for unresectable or metastatic osteosarcoma cases [19].
The potential role of EPHA2 as a monitoring molecule and pharmacological target in osteosarcoma, Ewing’s sarcoma and chondrosarcoma was interpreted by a study that was conducted by Giordano et al. in 2021 [20]. Using bioinformatic analysis, the EPHA2 expression levels were correlated to patients’ clinical outcomes according to TARGET-OS project data extracted from the NCI Genomic Data Commons (88 osteosarcoma patients) as well as from three public gene expression experiments deposited in the Gene Expression Omnibus (90 osteosarcoma patients) [20]. The researchers confirmed in silico that the expression of EPHA2 in samples retrieved from patients with Ewing’s sarcoma was higher compared to normal tissues [20]. In addition, higher measured levels of EPHA2 were indicative of advanced Huvos grade in osteoblastic osteosarcoma and poorer prognosis in patients with dedifferentiated chondrosarcoma. Likewise, the expression levels of EPHA2 were found to be significantly higher among male compared to female Ewing’s sarcoma and osteosarcoma patients, which was in accordance with the prognostic value of gender for those bone malignancies [20]. Another interesting data extracted from this research was that the phosphorylation of EPHA2 at its critical serine 897 contributes to the activation of the oncogenic non-canonical process in both osteosarcoma and chondrosarcoma patient-derived xenograft models. The generation of p-EPHA2Ser897 might be responsible for the evolution to a more aggressive phenotype and to metastatic disease. This finding was not confirmed in Ewing’s sarcoma patient-derived xenografts due to the variability of expression levels for p-EPHA2Ser897 in this type of sarcoma [20]. Finally, the potent EPHA2 inhibitor ALW-II-41-27 demonstrated remarkable impeding effects on cell growth and cell viability when applied in osteoblastic osteosarcoma, Ewing’s sarcoma and conventional chondrosarcoma [20]. Despite the association between EPHs/ephrins with tumorigenesis and cancer progression, a study by Kalinski et al. revealed that ephrin-A5 has a protective role in chondrosarcoma pathogenesis [21]. Results from their in vitro analysis showed a significant downregulation of ephrin-A5 at the transcriptional level in chondrosarcoma cells compared to normal ones. Therefore, ephrin-A5 is not implicated in cell–cell adhesion interactions that could remodel the microenvironment to promote the expansion of chondrosarcoma. Thus, ephrin-A5 could have been investigated as a potential tumor-suppressing ligand through the interaction with its tumor promoting receptors such as EPHA2, EPHA3, EPHA4, EPHA5, EPHA7, EPHA8, and EPHB2 in sarcomas [21].
2.5. The Role of EPHA7 in HCP5/miR-101/EPHA7 Axis in Osteosarcoma
A novel mechanism involving the expression of EPHA7 in the progression of osteosarcoma has been recently proposed by Tu et al. in 2021 [22]. EPHA7 has the ability to interact with HCP5 (long non-coding RNAs (lncRNA) of human histocompatibility leukocyte antigen (HLA) complex P5) and miR-101, a non-coding microRNA [22]. In the clinical setting, a high expression of HCP5 in tissue samples retrieved from osteosarcoma patients was significantly correlated to low survival and poor prognosis. On the contrary, the in vitro downregulation of HCP5 led to a notable inhibition of proliferation, migration, invasion and enhanced apoptosis in osteosarcoma cell lines [22]. The experimental data of this research revealed that HCP5 directly targets and regulates the expression levels of miR-101. Likewise, the miR-101 directly targets the EPHA7 (the binding site of miR-101 is EPHA7 3′UTR), regulating its expression. Therefore, the HCP5/miR-101/EPHA7 axis has been correlated to osteosarcoma malignant development, as HCP5 promotes the increased expression of EPHA7 via targeting miR-101 competitively [22]. Consequently, HCP5, miR-101 and EPHA7 could be further considered as potential prognostic biomarkers and therapeutic targets for osteosarcoma treatment.
2.6. Ephrin-Specific Expression Profile in Osteosarcoma
Varelias et al. revealed that a specific ephrin profile is present in human osteosarcoma specimens and human osteosarcoma cell lines, which is correlated with the progression of malignancy [23]. According to their findings, two mRNA profile patterns were recognized between normal bone tissues, osteosarcoma samples and osteosarcoma cells. The first mRNA profile included the expression of ephrin-A1, ephrin-A4 and ephrin-B2, which coordinate migration and cell–cell contact, oftentimes being involved in osteoblasts regulating bone homeostasis [23]. The second mRNA profile was composed of ephrin-A3, ephrin-A5, and ephrin-B1 in a subset of osteosarcoma patients with possibly worse prognosis [23]. The most significant observation was that increased levels of ephrin-B1 were detected in osteosarcoma cells and blood vessels and were associated with local recurrence, metastatic disease and poorer clinical prognosis [23]. Therefore, the interaction between B-subclass ephrins and EPHs may influence patients’ prognosis via excessive tumor neovascularization, which promotes metastatic spread through newly formed blood vessels and assists the construction of tumor vascular networks for nutritional and oxygen supplies at metastatic sites [23].
The EPHs/ephrins investigated in bone sarcomas’ pathogenesis as well as the results reported are summarized in Table 1 and Figure 2.
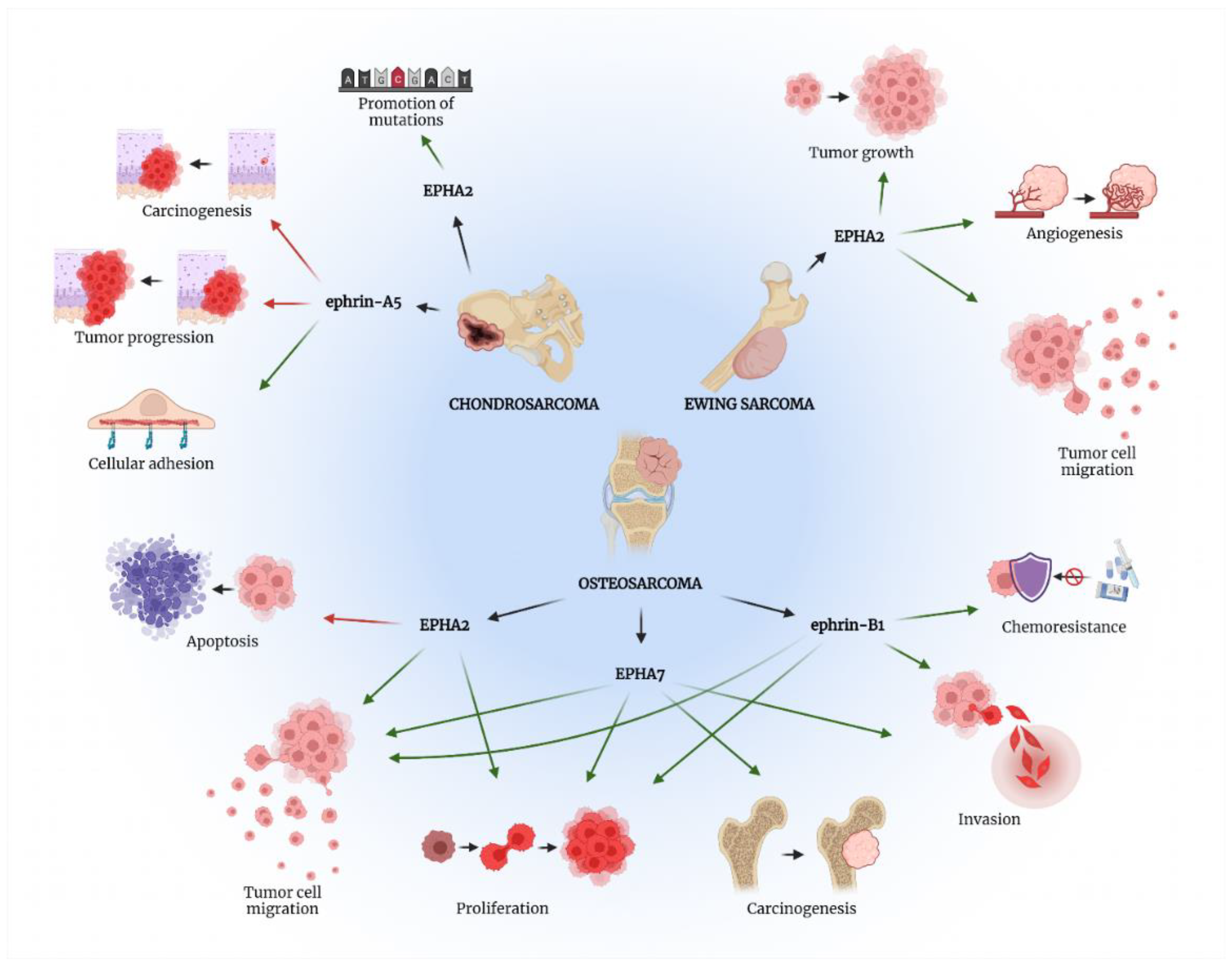 Figure 2. The role of EPH/ephrin axis in bone sarcomas’ pathogenesis. Green arrows present procedures promoted by the specific EPH/ephrin member, while red arrows show processes suppressed by the aforementioned molecules. Created with BioRender.com, accessed on 2 May 2022.
Figure 2. The role of EPH/ephrin axis in bone sarcomas’ pathogenesis. Green arrows present procedures promoted by the specific EPH/ephrin member, while red arrows show processes suppressed by the aforementioned molecules. Created with BioRender.com, accessed on 2 May 2022.Table 1. Molecular mechanisms involving the EPH/ephrin axis in bone sarcomas leading to specific laboratory and clinicopathological outcomes.
| EPH/Ephrin | Tumor Type | Cell Lines/Tissues | Mechanism | Result/Clinicopathological Correlations | References |
|---|---|---|---|---|---|
| EPHA2 | OS | 8 OS cell lines: SaOS2, HOS, MNNG⁄HOS, OST, SJSA, MG63, ZK58 19 OS tissue samples: 7/19 metastatic OS 12/19 conventional OS Control:
|
|
EPHA2/ephrin-A1 interaction induces:
|
[12] |
| EWS | In vitro: A673, TC252, RH1 and STAET1 cell lines In vivo: knocked down Caveolin-1 (CAV1) expression in RDES, TC71 and SKES1 cells of EWS xenograft mice Control: Non-transfected cells and cells transfected with an empty vector |
|
|
[13] | |
| OS | 7 OS cell lines: EPHA2-silenced osteosarcoma cell lines (HOS, KHOS/NP, MNNG/HOS and U2OS) vs. Controls OS xenograft mice MNNG/HOS or KHOS Application or not of RTK pathway’s inhibitors (pazopanib + trametinib) |
pazopanib + trametinib down-modulated the expression of EPHA2 and IL7R and upregulated MEK6 expression |
EPHA2-silenced OS and pazopanib + trametinib treated cells:
|
[16] | |
| OS EWS CHS |
EPHA2 expression retrieved using Bioinformatics Analyses Patients(n): 232 OS, 197 EWS, 102 CHS Cell lines: 10 OS, 12 EWS, 4 CHS Patient-Derived Xenograft Models (PDX models) Osteoblastic OS (metastatic) EWS (localized) CHS (conventional) |
↑ expression of EPHA2 in the following bone sarcoma cell lines: (Saos-2, U2OS, MG63) followed by EWS cell lines (SK-NEP-1, RD-ES, CADO-ES1) and CHS cell lines (CAL-78, Hs 819.T, SW 1353) Phosphorylation of serine 897 in EPHA2 (p-EPHA2Ser897) Activates oncogenesis in OS and CHS PDX models |
OS patients:
EPHA2 inhibitor ALW II-41-27 reduced cell viability and tumor growth in OS, EWS and CHS |
[17] | |
| EPHA7 | OS | Tissue samples from 40 OS patients | ↑ HCP5 expression OS tissues compared to control ↑ HCP5 expression in OS cell lines (MG-63, U2OS, 143B, and HOS) compared with normal cells (hFOB1.19) Knockdown of HCP5 suppressed cell proliferation, migration, invasion and enhanced cell apoptosis in MG-63 and U2OS cells HCP5 regulates the expression of miR-101 by targeting miR-101 in OS miR-101 directly targets the 3′UTR region of EPHA7 and mediates the EPHA7 expression in OS cell lines |
HCP5 expression is enhanced in OS cell lines and tissues The HCP5/miR-101/EPHA7 axis is involved in OS development HCP5 induces OS cell proliferation, migration, and invasion by up-regulation of EPHA7 (targeting miR-101 competitively) |
[19] |
| OS cell lines: MG-63, U2OS, 143B, HOS, human osteoblast cell line (hFOB) |
|||||
| ephrin-B1 | OS | Tissues from 12 OS patients vs. controls OS cell lines: U2OS, MG63,143B OS xenografts mice: OS cells transfected with the NICD1-OE, RBPJ-shRNA vs. control |
Activation of Notch signaling → phosphorylation of ephrin-B1 and increases the expression of ephrin-B1 Inhibition of Notch signaling is able to reduce tumor growth and metastasis in xenografts mice. Notch intracellular domain (NICD) is transferred to the nucleus and activates the transcriptional cofactor CBF1, leading to the overexpression of HES and HEY genes and ephrin-B1 in 143B OS cell lines |
Notch signaling promotes proliferation, migration, invasion, upregulation of stem-cell like abilities and chemoresistance by targeting ephrin-B1 |
[14][15] |
| ephrin-A5 | CHS | 19 patients: 15 conventional CHS 6 CHS grade I 9 CHS grade II 4 dedifferentiated CHS vs. 3 patients normal articular cartilage Cell lines: Human CHS cell lines C3842 and SW1353 |
Not identified mechanism of ephrin-A5 downregulation in CHS. No significant differences in the expression of ephrin-A5 in C3842 and SW1353 cells treated with or without hypoxia. Ephrin-A5 gene promoter hypermethylation is not the cause of ephrin-A5 gene downregulation in CHSs. |
Protective function in tumor progression ↓ ephrin-A5 leads to tumorigenesis, tumor progression and ↓ cellular adhesion |
[18] |
Abbreviations: OS: osteosarcoma; EWS: Ewing’s sarcoma; CHS: chondrosarcoma; CAV: caveolin; PDX: patient-derived xenografts; RTK: receptor tyrosine kinase; HCP5: histocompatibility leukocyte antigen (HLA) complex P5 (HCP5); NICD: Notch intracellular domain.
References
- Hui, J.Y.C. Epidemiology and Etiology of Sarcomas. Surg. Clin. N. Am. 2016, 96, 901–914.
- Goldblum, J.R.; Folpe, A.L.; Weiss, S.W. Enzinger and Weiss’s Soft Tissue Tumors; Elsevier: USA, 2019; p. 1270.
- Klijanienko, J.; Lagace, R. Soft Tissue Tumors: A Multidisciplinary, Decisional, Diagnostic Approach; Wiley-Blackwell: Hoboken, NJ, USA, 2010; p. 426.
- Casali, P.G.; Bielack, S.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brennan, B.; et al. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv79–iv95.
- Weiss, S.; Korthaus, A.; Baumann, N.; Yamamura, J.; Spiro, A.S.; Lübke, A.M.; Frosch, K.H.; Schlickewei, C.; Priemel, M. Musculoskeletal soft-tissue sarcoma: Quality assessment of initial MRI reports shows frequent deviation from ESSR guidelines. Diagnostics 2021, 11, 695.
- Beckingsale, T.B.; Shaw, C. Epidemiology of bone and soft-tissue sarcomas. Orthop. Trauma 2017, 31, 144–150.
- Stiller, C.A.; Botta, L.; Brewster, D.H.; Ho, V.K.; Frezza, A.M.; Whelan, J.; Casali, P.G.; Trama, A.; Gatta, G. EUROCARE-5 Working Group. Survival of adults with cancers of bone or soft tissue in Europe—Report from the EUROCARE-5 study. Cancer Epidemiol. 2018, 56, 146–153.
- Steffner, R.J.; Jang, E.S. Staging of Bone and Soft-Tissue Sarcomas. J. Am. Acad. Orthop. Surg. 2018, 26, E269–E278.
- Amankwah, E.K.; Conley, A.P.; Reed, D.R. Epidemiology and therapies for metastatic sarcoma. Clin. Epidemiol. 2013, 5, 147–162.
- Hadjimichael, A.C.; Pergaris, A.; Kaspiris, A.; Foukas, A.F.; Theocharis, S.E. Liquid Biopsy: A New Translational Diagnostic and Monitoring Tool for Musculoskeletal Tumors. Int. J. Mol. Sci. 2021, 22, 11526.
- Barquilla, A.; Pasquale, E.B. Eph receptors and ephrins: Therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 467–487.
- Taylor, H.; Campbell, J.; Nobes, C.D. Ephs and ephrins. Curr. Biol. 2017, 27, R90–R95.
- Kania, A.; Klein, R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat. Rev. Mol. Cell Biol. 2016, 17, 240–256.
- Pergaris, A.; Danas, E.; Goutas, D.; Sykaras, A.G.; Soranidis, A.; Theocharis, S. The clinical impact of the eph/ephrin system in cancer: Unwinding the thread. Int. J. Mol. Sci. 2021, 22, 8412.
- Fritsche-Guenther, R.; Noske, A.; Ungethüm, U.; Kuban, R.J.; Schlag, P.M.; Tunn, P.U.; Karle, J.; Krenn, V.; Dietel, M.; Sers, C. De novo expression of EphA2 in osteosarcoma modulates activation of the mitogenic signalling pathway. Histopathology 2010, 57, 836–850.
- Sainz-Jaspeado, M.; Huertas-Martinez, J.; Lagares-Tena, L.; Martin Liberal, J.; Mateo-Lozano, S.; de Alava, E.; de Torres, C.; Mora, J.; Muro, X.G.D.; Tirado, O.M. EphA2-Induced Angiogenesis in Ewing Sarcoma Cells Works through bFGF Production and Is Dependent on Caveolin-1. PLoS ONE 2013, 8, e71449.
- Yu, L.; Xia, K.; Gao, T.; Chen, J.; Zhang, Z.; Sun, X.; Simões, B.M.; Eyre, R.; Fan, Z.; Guo, W.; et al. The notch pathway promotes osteosarcoma progression through activation of ephrin reverse signaling. Mol. Cancer Res. 2019, 17, 2383–2394.
- Ntziachristos, P.; Lim, J.S.; Sage, J.; Aifantis, I. From fly wings to targeted cancer therapies: A centennial for notch signaling. Cancer Cell 2014, 25, 318–334.
- Chiabotto, G.; Grignani, G.; Todorovic, M.; Martin, V.; Centomo, M.L.; Prola, E.; Giordano, G.; Merlini, A.; Miglio, U.; Berrino, E.; et al. Pazopanib and trametinib as a synergistic strategy against osteosarcoma: Preclinical activity and molecular insights. Cancers 2020, 12, 1519.
- Giordano, G.; Merlini, A.; Ferrero, G.; Mesiano, G.; Fiorino, E.; Brusco, S.; Centomo, M.L.; Leuci, V.; D’Ambrosio, L.; Aglietta, M.; et al. Epha2 expression in bone sarcomas: Bioinformatic analyses and preclinical characterization in patient-derived models of osteosarcoma, ewing’s sarcoma and chondrosarcoma. Cells 2021, 10, 2893.
- Kalinski, T.; Röpke, A.; Sel, S.; Kouznetsova, I.; Röpke, M.; Roessner, A. Down-regulation of ephrin-A5, a gene product of normal cartilage, in chondrosarcoma. Hum. Pathol. 2009, 40, 1679–1685.
- Tu, Y.; Cai, Q.; Zhu, X.; Xu, M. Down-regulation of hcp5 inhibits cell proliferation, migration, and invasion through regulating epha7 by competitively binding mir-101 in osteosarcoma. Braz. J. Med. Biol. Res. 2021, 54, 1–9.
- Varelias, A.; Koblar, S.A.; Cowled, P.A.; Carter, C.D.; Clayer, M. Human osteosarcoma expresses specific ephrin profiles: Implications for tumorigenicity and prognosis. Cancer 2002, 95, 862–869.
More
Information
Subjects:
Orthopedics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
13 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




