Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gerardo Rosati | -- | 2952 | 2022-05-12 07:00:10 | | | |
| 2 | Amina Yu | -13 word(s) | 2939 | 2022-05-13 03:57:20 | | | | |
| 3 | Amina Yu | -2 word(s) | 2937 | 2022-05-13 04:01:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rosati, G.; Aprile, G.; , .; De Stefano, A.; Avallone, A. Precision Medicine of Colorectal Cancer Heterogeneity. Encyclopedia. Available online: https://encyclopedia.pub/entry/22850 (accessed on 07 February 2026).
Rosati G, Aprile G, , De Stefano A, Avallone A. Precision Medicine of Colorectal Cancer Heterogeneity. Encyclopedia. Available at: https://encyclopedia.pub/entry/22850. Accessed February 07, 2026.
Rosati, Gerardo, Giuseppe Aprile, , Alfonso De Stefano, Antonio Avallone. "Precision Medicine of Colorectal Cancer Heterogeneity" Encyclopedia, https://encyclopedia.pub/entry/22850 (accessed February 07, 2026).
Rosati, G., Aprile, G., , ., De Stefano, A., & Avallone, A. (2022, May 12). Precision Medicine of Colorectal Cancer Heterogeneity. In Encyclopedia. https://encyclopedia.pub/entry/22850
Rosati, Gerardo, et al. "Precision Medicine of Colorectal Cancer Heterogeneity." Encyclopedia. Web. 12 May, 2022.
Copy Citation
Colorectal cancer (CRC) is a major global health issue, being the third most commonly diagnosed malignancy with an estimated number of more than 1.9 million new cases and about 935,000 deaths worldwide in 2020. For twenty percent, the disease occurs at an advanced stage at diagnosis, while up to 50% of patients with early-stage disease relapse, despite curative surgery, adjuvant chemotherapy, and/or radiation therapy. Advances in multidisciplinary treatment and care have led to significant improvements in survival, but a cure is not possible for most of these patients.
metastatic colorectal cancer
precision medicine
and inhibitors
immunotherapy
1. Introduction
Colorectal cancer (CRC) is a major global health issue, being the third most commonly diagnosed malignancy with an estimated number of more than 1.9 million new cases and about 935,000 deaths worldwide in 2020 [1][2]. In twenty percent of cases, the disease occurs at an advanced stage at diagnosis, while up to 50% of patients with early-stage disease relapse, despite curative surgery, adjuvant chemotherapy, and/or radiation therapy. Advances in multidisciplinary treatment and care have led to significant improvements in survival, but a cure is not possible for most of these patients [3].
Targeted therapies work on cancer cells by directly inhibiting cell proliferation, differentiation, and migration. The tumor microenvironment, including local blood vessels and immune cells, could also be altered by targeted drugs, so as to inhibit tumor growth. Various pathways that mediate CRC initiation, progression, and migration, as well as those that can activate signaling cascades, are ideal sites for these drugs [4].
Recently, clinical outcomes in metastatic colorectal patients (mCRC) have thus improved significantly. International guidelines have now first of all mandated, as a standard of care, the identification of approximately 40% of patients without rat sarcoma virus (RAS) and B-rapidly accelerated fibrosarcoma (BRAF) oncogene mutations [5]. The anti-epidermal growth factor receptor (EGFR) monoclonal antibodies cetuximab and panitumumab, combined with chemotherapy, are standard treatments. The most important limitation of these drugs is in inducing resistance sooner or later [6][7], although evidence suggests that some patients may benefit from a rechallenge strategy in the course of their disease [8]. On the contrary, patients with mutated tumors benefit from anti-vascular endothelial growth factor (VEGF) agents (bevacizumab and aflibercept) when combined with chemotherapy [9][10].
The refinement of the knowledge of molecular biology and new agents capable of more specifically targeting previously unknown mutated genes change the therapeutic perspectives of many patients. Since 2013, the National Cancer Institute (NCI) has launched, with renewed interest, programs, and initiatives to deepen the function of RAS and learn about its biology to identify innovative drugs [11]. Although BRAF inhibitors as single agents have shown only modest activity in BRAF-mutated mCRC, several clinical trials have demonstrated that combination therapies with EGFR and mitogen-activated protein kinase (MEK) inhibitors overcome resistance mechanisms [12]. Tumors with deficiency in mismatch repair genes (dMMR) are highly responsive to immune checkpoint inhibitors both in first- and second-line settings [13]. Human Epidermal Growth Factor Receptor (HER-2) amplification has emerged as a promising therapeutic target for mCRC patients displaying this molecular abnormality. Patients with RAS wild-type (wt) and HER-2 overexpression have been successfully treated with anti-HER-2 antibodies [14]. Other emerging actionable molecular alterations include rare gene fusions of neurotrophic tyrosine kinase receptor, type 1 (NTRK1) that can be targeted by specific inhibitors [15].
Although new techniques such as next-generation sequencing (NGS) and the availability of tumor panels allow the identification of many predictive markers (Figure 1), their application in clinical practice is often difficult. It is quite evident that recommendations are needed to guide the physician in these cases and support therapeutic decision making for patients with mCRC.
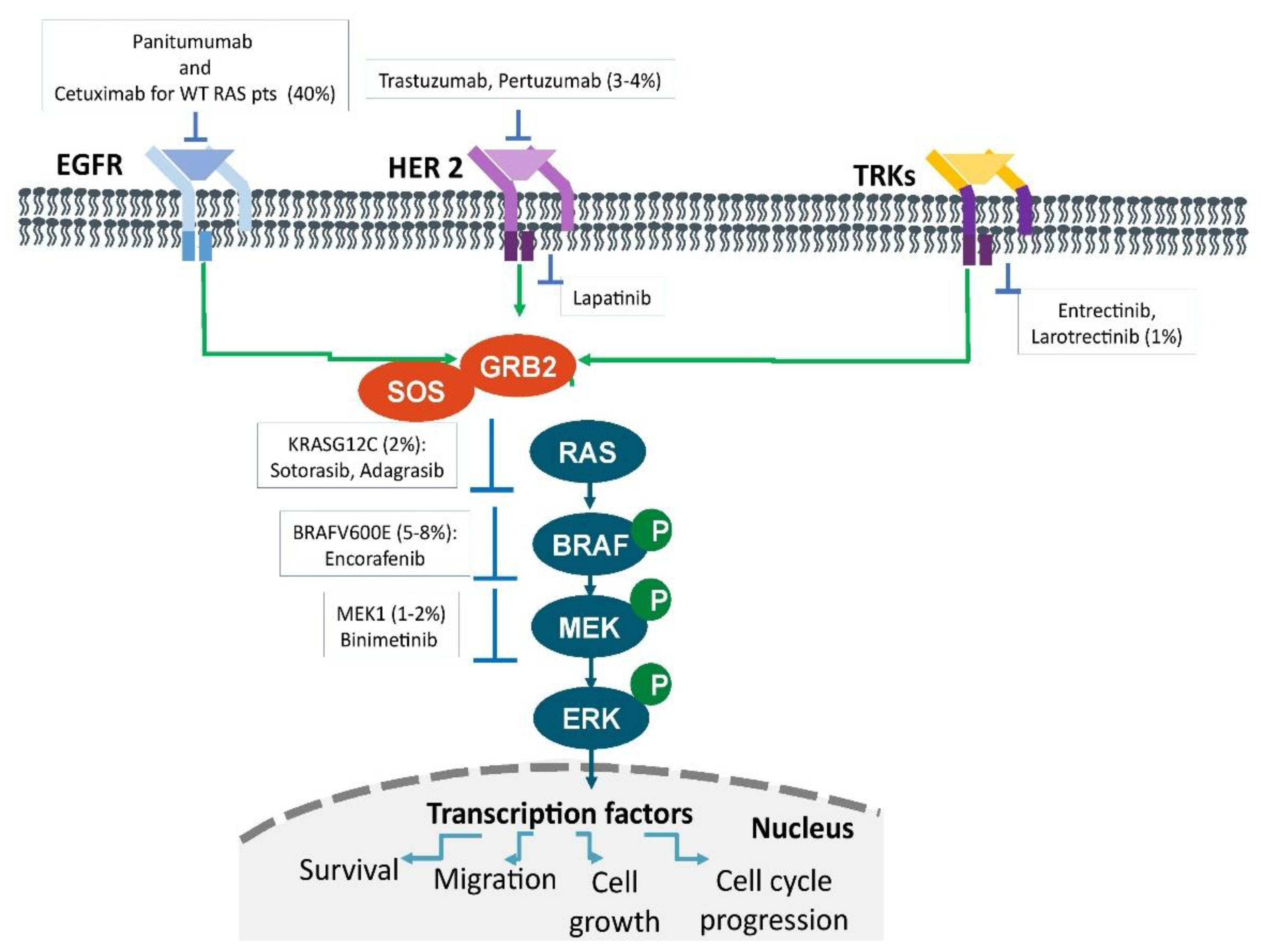
Figure 1. Therapeutic targets in metastatic colorectal cancer. The main oncogenic drivers. Signaling pathways and their prevalence in patients with metastatic colorectal cancer.
2. Mutational Status of RAS
RAS is a family of proteins expressed on all cells and responsible for transmitting signals through which proliferation, adhesion, migration, and cell differentiation as well as the apoptosis process are stimulated and controlled. When these proteins mutate, the cells acquire properties of invasion and metastatization. The main mutations concern Kirsten rat sarcoma virus (KRAS) and neuroblastoma ras viral oncogene homolog (NRAS) and consist in the change of a single nucleotide or its deletion or insertion into a deoxyribonucleic acid (DNA) sequence [16].
KRAS mutations are found in approximately 40% of patients with mCRC, mainly in exon 2, codons 12 (70–80%) and 13 (15–20%), less frequently in exons 3 and 4. The most common KRAS mutations of exon 2 (codons 12 and 13) include G12D (32.4%), G13D (14.1%), G12V (11.3%), G12S (9.9%), G12C (8.5%), and G12A (2.8%) [17]. Regardless of type and location, KRAS mutations play a prognostic role and, when grouped together, patients with KRAS mutated metastatic disease have a higher mortality rate (18.5% vs. 34%) and a shorter survival (14 months vs. 23.5 months) than wt ones [18]. Moreover, the various mutations of KRAS are not equal to each other and a pooled analysis of five randomized trials showed that they were associated with heterogeneous outcomes [19]. While patients harboring the KRAS G12C-variant correlated with inferior overall survival (OS) compared with unmutated tumors and a similar trend for OS was seen in the KRAS G13D-variant, more frequent KRAS exon 2 variants like G12D and G12V did not have a significant impact on OS. Although the reasons are not clear, these biological differences could be explained by a separate activation process for each individual variant of the KRAS-depending pathways.
NRAS mutations in codons 2, 3, and 4 are rare and found in 3–5% of metastatic patients, more frequently in left-side colon and mainly in women. An Italian one has shown that NRAS and KRAS mutated tumors did not show significant differences in terms of clinical and pathological characteristics, except for a lower prevalence of mucinous histology and lung metastases among NRAS mutated tumors. In uni- and multivariate analysis, NRAS mutations were associated with shorter OS than in all wt patients (median OS 25.6 vs. 42.7 months) [20]. NRAS mutations recorded at exon 3 identify patients with markedly lower OS not only compared to wt ones (HR 2.85; p < 0.01), but also to those with mutations in exon 2 (HR 2.0; p = 0.039) [21].
EGFR is a membrane receptor tyrosine kinase and is a key target for monoclonal antibodies which bind on the extracellular domain of the receptor. Several phase II and III trials indicate that an increased gene copy number of EGFR or mutations of KRAS and NRAS, responsible for downstream signalling, are important determinants of response to cetuximab and panitumumab [22][23][24][25]. While an improvement of treatment efficacy is proven only in wt patients, RAS mutated patients either had no benefit from the addition of anti-EGFRs or even showed a worse outcome than their comparators [7][26]. Thus, since 2013 extended RAS analysis is recommended at the time of diagnosis in metastatic disease for all patients [5][27].
The best treatment for RAS mutated patients is not sufficiently standardized due to the lack of clinical trials specifically designed for these patients. Although the major international guidelines that chemotherapy plus bevacizumab should be the preferred first-line therapy for patients with RAS mutation, the evidence is debatable [28][29]. First of all, there are no prospective randomized trials for this specific setting of patients. Secondly, the benefit of the addition of bevacizumab to first-line treatment significantly prolonged progression-free survival (PFS), while in RAS mutated patients it led to a relatively modest reduction in risk of death of 12%. Thirdly, data from randomized phase III is confusing as they include patients with RAS wt and RAS mutated disease. Only recently, a Chinese randomized one specifically enrolled patients with RAS mutation and with metastases limited exclusively to the liver showing that bevacizumab plus chemotherapy versus chemotherapy alone results in a higher conversion rate in liver surgery, an increase in response rate (RR), and an extension of PFS and OS [30]. The strategy of employing triplet chemotherapy [leucovorin, oxaliplatin, irinotecan, and fluorouracil (FOLFOXIRI)] plus bevacizumab also appears to confirm that mutated RAS patients have no improvement in OS, although this is partially confirmed by the TRIBE2 one including many patients with these characteristics [31][32].
3. Mutational Status of BRAF
The routine molecular characterization of mCRC patients includes, beyond RAS, the tumor BRAF mutational testing, according to the recommendations provided by the international clinical guidelines [33][34][35]. The BRAF gene encodes a serine-threonine protein kinase that is part of the MAPK pathway. BRAF mutations occur in about 10% of patients with mCRC and are usually mutually exclusive with RAS mutations. They are most frequently caused (>90%) by the replacement of valine with glutamic acid inside the 600 codon (BRAFV600E), leading to an overactive MAPK pathway [12][36].
The presence of somatic BRAFV600E alteration mostly characterizes a subgroup of mCRC patients associated with the female sex, right-sided colonic cancer, mucinous histology, microsatellite instability (MSI)/dMMR profile and metastatic spread mainly to lymph nodes and peritoneum [12][36][37]. BRAFV600E mutant mCRC patients show a shorter OS and achieve a very modest benefit from standard chemotherapy, highlighting their poor prognosis [12]. Moreover, the benefit of anti-EGFRs remains unclear and two meta-analyses have not been able to provide more clarity to the issue [38][39]. With the advent of next-generation sequencing, non-BRAFV600E mutations have been increasingly identified in clinical practice, more often observed in younger patients, males and showing fewer peritoneal metastases compared to BRAFV600E mutants [40]. The expected survival of this subgroup of patients is not negatively influenced, as happens for BRAFV600. Of note, most of the non-BRAFV600E mutations, in particular those belonging to class 3, retain a sensitivity to anti-EGFRs based treatments [41].
The optimal treatment of BRAFV600E mutant mCRC patients has been matter of active clinical research and controversial debate. In recent years, FOLFOXIRI plus bevacizumab was introduced as a standard of care for initial treatment of this subgroup of mCRC patients [33][34][42]. The use of this intensive combination was mainly supported by a subgroup analysis of 28 BRAFV600E mCRC patients enrolled in the TRIBE trial, which showed a median OS of 19.0 months in patients treated with FOLFOXIRI/bevacizumab, whereas patients treated with leucovorin, irinotecan and fluorouracil (FOLFIRI)/bevacizumab had a shorter median OS of 10.7 months [43]. However, the evidence of benefit from the intensified approach was not confirmed in the TRIBE 2 trial, in which patients were randomized to FOLFOXIRI plus bevacizumab or to leucovorin, oxaliplatin and fluorouracil (FOLFOX) plus bevacizumab [31]. In addition, a recent meta-analysis of five randomized trials comparing FOLFOXIRI plus bevacizumab to a doublet combination plus bevacizumab confirmed the absence of any advantage of FOLFOXIRI plus bevacizumab in BRAF mutated ones [44]. Based on this evidence, an intensification of treatment does not offer a clear benefit in the frontline treatment of BRAFV600E mutated mCRC patients. However, patients with BRAFV600E mutation tumors appear to benefit from anti-VEGF therapy, unlike that with anti-EGFRs, similarly to patients with BRAF wt tumors [12].
A major efficacy of an antiangiogenic agent in combination with chemotherapy has also been reported in the second line treatment of BRAFV600E mutant mCRC patients. Ramucirumab-a highly specific antiangiogenic agent directed against the extracellular domain of the VEGF receptor-2-may block the activating phosphorylation of the proangiogenic receptor. In the VELOUR trial, a subgroup it was showed that 11 patients treated with FOLFIRI plus aflibercept had a median PFS and OS compared with 19 patients receiving only chemotherapy for 5.5 and 10.3 months vs. 2.2 and 5.5 months, respectively [45]. Similar were also observed with FOLFIRI plus ramucirumab in the subgroup analysis of the RAISE one [46]. However, the value of these post-hoc analyses should be carefully considered given the small number of patients included.
4. Microsatellite Instability and Immunotherapy
Immunotherapy in cancer treatment arises from the concept that a condition of immunoevasion exists caused by neoplastic cells in the tumor microenvironment. Tumor cells, through the production of cytokines, stimulate suppressor myeloid cells and regulatory T cells (Treg) to inhibit the CD4+ and to increase CD8+ lymphocytes, braking immune responses. Furthermore, a loss of restricted major histocompatibility complex molecules has also been observed, resulting in an inability of the host to recognize non-self-antigens [47].
Microsatellites are repetitive sequences of coding, and non-coding DNA [48]. MSI results from the inability of the MMR gene to repeat DNA errors that occurred during the replication process. Gene insertions and deletions lead to somatic mutations in these repetitive DNA sequences resulting in genomic instability and production of immunogenic antigens and neoantigens, conditioning a response to checkpoint inhibitors [16]. Inactivation of MMR genes is the result of hypermethylation of the MLH1 promoter or of germline mutations of MLH1, MSH2, MSH6, and PMS2 [49].
Furthermore, MSI germline abnormalities also represent the molecular basis of Lynch syndrome [50]. It represents the most common hereditary form of this cancer. Latham et al. reported that dMMR is common in these patients, so those with MSI or dMMR tumors could predict Lynch syndrome through MSI related tests [51].
MSI is found in approximately 5% of patients with mCRC; only 3% are associated with Lynch syndrome and the other 12% are caused by sporadic hypermethylation of the MLH1 gene. CRC with MSI are most frequently localized on the right and in women over 70 years, are poorly differentiated, and have mucinous histology [52]. MSI tumor status could be a prognostic marker for a more favorable outcome. A large one reported that the percentage of mCRC patients with this characteristic was only 3.5% suggesting that these tumors have a lower probability of metastasizing [53].
The incidence of MSI in stage II and III is about 16%. Some have shown that dMMR or MSI tumor status are predictors of reduced benefit from adjuvant chemotherapy and that fluoropyrimidines given alone may even have a detrimental effect in patients with stage II disease [54][55][56]. Conversely, regarding patients with MSI and stage III, ACCENT, a pooled analysis of 12 adjuvant ones, has demonstrated that adding oxaliplatin to fluoropyrimidines improves DFS and OS of patients compared to those of stable microsatellite tumors (MSS) [57]. In particular, it was found a close relationship between the number of positive lymph nodes on histological examination and OS, documenting better outcomes in the N1 group, while data were similar in the N2 group.
5. HER2 Inhibition
The HER family plays a crucial role in the development and progression of several gastrointestinal tumors, including colorectal, gastric, and biliary adenocarcinomas; its aberrant activation-mainly due to overexpression via HER-2 gene amplification or to alternative genetic mechanisms-has been reported consistently in 5–20% of cancer patients [58][59]. The possibility of inhibiting HER-2 to tackle the progression of the disease is certainly not new, and pivotal randomized trials have shown that the use of trastuzumab either alone or combined with another HER-2 blockade agent has significantly extended survival in molecularly selected ones [60][61][62]. In addition, it stimulated the need for specific classifications and scoring systems to establish HER-2 positivity [63], which is usually scored with immunohistochemistry (IHC), and then confirmed with in situ hybridization or innovative, more sensitive techniques [64]. The IHC scoring system for HER-2 positivity in CRC was established by experienced pathologists involved in the HERACLES project. In more detail, IHC staining judged as intense (3+) in more than 10% of cancer cells with circumferential, basolateral, or lateral pattern was defined as positive; the expert panel recommended to confirm the positivity if the percentage of positive cells was inferior to 50% [63]. As an outstanding example for gastrointestinal oncology, in the open-label, multicenter, international, phase III ToGA trial the combination of standard chemotherapy and trastuzumab was compared to chemotherapy alone [60]. In patients with IHC 3+ HER-2-positive advanced gastric cancers treated with trastuzumab the reported median OS was about 4 months longer that that reported for patients treated with standard therapy (16.0 versus 11.8 months, HR 0.65), and the drug gained accelerated Food and Drug administrative approval. In mCRC, HER-2 has been shown to represent a notable therapeutic target, regardless of its primary or acquired resistance to EGFR inhibition [65][66], although prognostic impact of HER-2 overexpression/amplification has not yet been fully elucitated [67]. HERACLES, a proof-of-concept phase II academic trial, enrolled 35 HER-2-positive, RAS wt, mCRC patients refractory to standard therapies (including cetuximab or panitumumab), with 32 patients evaluable for response. Enrolled patients received intravenous trastuzumab at 4 mg/kg loading dose followed by 2 mg/kg once per week, and oral lapatinib at 1000 mg per day until evidence of disease progression. A RR of 28% was reported with one case of complete response lasting over 7 years, a DCR of 69%, a median PFS of 4.7 months (95% CI 3.7–6.1), and a median OS of 10 months (95% CI 7.9–15.8) [14][64]. Interestingly, progression in the central nervous system occurred in 6 (19%) out of 32 patients, suggesting that the evaluation of HER-2 expression in brain metastases from CRC is important [68].
6. Targeting NTRK, ALK, and ROS1 Fusions
Among novel actionable targets in mCRC, gene fusions such as NTRK rearrangements or fusions of anaplastic lymphoma kinase (ALK) or Proto-Oncogene 1 Receptor Tyrosine Kinase (ROS1) are of growing importance [69]. While several pathogenic alterations have been reported for such genes, including point mutations, amplifications, and splice variants, fusions are the most common genetic aberrations linked to cancer and cause constitutive gene activations and hyper-activation of the kinase domain. In mCRC, these fusions/rearrangements are rare (0.5–2%) and most frequently occur in elderly patients with right-sided, lymph-node positive, RAS wt, MSI cancers. They may suggest resistance to EGFR-inhibitors, have a negative prognostic survival impact and may be targeted with specific agents [70]. Larotrectinib and entrectinib are oral tropomyosin receptor kinases (TRK). Upon administration, these agents bind to TRK, preventing neurotrophin-TRK interaction and TRK activation, which results in both cellular apoptosis and the inhibition of cell growth in tumors that overexpress TRK. Based on the impressive results of agnostically testing larotrectinib and entrectinib in cancer patients with NTRK rearrangements, with a very high ORR in molecularly selected situations [71][72][73] and improvements in cancer-specific quality of life [70], both agents gained the Food and Drug Administration and the European Medicine Agency approval. In the phase II NAVIGATE one, larotrectinib produced an ORR of 50%, with a median duration of response of 15.5 months, and median OS of almost 30 months [74]. The possibility to use third-generation ALK inhibitors in mCRC has been suggested [75], but the rarity of this gene alteration makes it difficult to conduct large comparative trials. Novel NTRK/ROS1 inhibitors, including selitrectinib, repotrectinib, and belizatinib, are under investigation in early clinical trials [76].
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480.
- Koehler, A.; Bataille, F.; Schmid, C.; Ruemmele, P.; Waldeck, A.; Blaszyk, H.; Hartmann, A.; Hofstaedter, F.; Dietmaier, W. Gene expression profiling of colorectal cancer and metastases divides tumours according to their clinicopathological stage. J. Pathol. 2004, 204, 65–74.
- Sepulveda, A.R.; Hamilton, S.R.; Allegra, C.J.; Grody, W.; Cushman-Vokoun, A.M.; Funkhouser, W.K.; Kopetz, S.E.; Lieu, C.; Lindor, N.M.; Minsky, B.D.; et al. Molecular biomarkers for the evaluation of colorectal cancer: Guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 2017, 35, 1453–1486.
- Van Cutsem, E.; Lenz, H.J.; Kohne, C.H.; Heinemann, V.; Tejpar, S.; Melezinek, I.; Beier, F.; Stroh, C.; Rougier, P.; van Krieken, J.H.; et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J. Clin. Oncol. 2015, 33, 692–700.
- Douillard, J.Y.; Oliner, K.S.; Siena, S.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med. 2013, 369, 1023–1034.
- Parseghian, C.M.; Napolitano, S.; Loree, J.M.; Kopetz, S. Mechanisms of Innate and Acquired Resistance to Anti-EGFR Therapy: A Review of Current Knowledge with a Focus on Rechallenge Therapies. Clin. Cancer Res. 2019, 25, 6899–6908.
- Bang, Y.H.; Hong, Y.S.; Lee, J.S.; Lee, K.W.; Han, H.S.; Kim, S.Y.; Kim, J.W.; Kim, H.K.; Kim, J.W.; Eun, C.K.; et al. Effectiveness of Combining Bevacizumab With First-Line Chemotherapy Regimens for Metastatic Colorectal Cancer in Real-World Practice. Clin. Colorectal Cancer 2021, 20, 101–112.
- Saoudi Gonzalez, N.; Salva, F.; Ros, J.; Baraibar, I.; Marmolejo, D.; Valdivia, A.; Cuadra-Urteaga, J.L.; Mulet, N.; Tabernero, J.; Elez, E. Up-to-date role of aflibercept in the treatment of colorectal cancer. Expert Opin. Biol. Ther. 2021, 21, 1315–1324.
- Ryan, M.B.; Corcoran, R.B. Therapeutic strategies to target RAS-mutant cancers. Nat. Rev. Clin. Oncol. 2018, 15, 709–720.
- Grothey, A.; Fakih, M.; Tabernero, J. Management of BRAF-mutant metastatic colorectal cancer: A review of treatment options and evidence-based guidelines. Ann. Oncol. 2021, 32, 959–967.
- Andre, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218.
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): A proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 738–746.
- Kummar, S.; Lassen, U.N. TRK Inhibition: A New Tumor-Agnostic Treatment Strategy. Target Oncol. 2018, 13, 545–556.
- Afrăsânie, V.-A.; Marinca, M.V.; Alexa-Stratulat, T.; Gafton, B.; Păduraru, M.; Adavidoaiei, A.M.; Miron, L.; Rusu, C. KRAS, NRAS, BRAF, HER2 and microsatellite instability in metastatic colorectal cancer—Practical implications for the clinician. Radiol. Oncol. 2019, 53, 265–274.
- Gong, J.; Cho, M.; Sy, M.; Salgia, R.; Fakih, M. Molecular profiling of metastatic colorectal tumors using next-generation sequencing: A single-institution experience. Oncotarget 2017, 8, 42198–42213.
- Heinemann, V.; Stintzing, S.; Kirchner, T.; Boeck, S.; Jung, A. Clinical relevance of EGFR- and KRAS-status in colorectal cancer patients treated with monoclonal antibodies directed against the EGFR. Cancer Treat. Rev. 2009, 35, 262–271.
- Modest, D.P.; Ricard, I.; Heinemann, V.; Hegewisch-Becker, S.; Schmiegel, W.; Porschen, R.; Stintzing, S.; Graeven, U.; Arnold, D.; von Weikersthal, L.F.; et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: Pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann. Oncol. 2016, 27, 1746–1753.
- Schirripa, M.; Cremolini, C.; Loupakis, F.; Morvillo, M.; Bergamo, F.; Zoratto, F.; Salvatore, L.; Antoniotti, C.; Marmorino, F.; Sensi, E.; et al. Role of NRAS mutations as prognostic and predictive markers in metastatic colorectal cancer. Int. J. Cancer 2015, 136, 83–90.
- Matallanas, D.; Birtwistle, M.; Romano, D.; Zebisch, A.; Rauch, J.; von Kriegsheim, A.; Kolch, W. Raf family kinases: Old dogs have learned new tricks. Genes Cancer 2011, 2, 232–260.
- Van Cutsem, E.; Kohne, C.H.; Hitre, E.; Zaluski, J.; Chang Chien, C.R.; Makhson, A.; D'Haens, G.; Pinter, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 2009, 360, 1408–1417.
- Bokemeyer, C.; Bondarenko, I.; Hartmann, J.T.; de Braud, F.; Schuch, G.; Zubel, A.; Celik, I.; Schlichting, M.; Koralewski, P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: The OPUS study. Ann. Oncol. 2011, 22, 1535–1546.
- Au, H.J.; Karapetis, C.S.; O’Callaghan, C.J.; Tu, D.; Moore, M.J.; Zalcberg, J.R.; Kennecke, H.; Shapiro, J.D.; Koski, S.; Pavlakis, N.; et al. Health-related quality of life in patients with advanced colorectal cancer treated with cetuximab: Overall and KRAS-specific results of the NCIC CTG and AGITG CO.17 Trial. J. Clin. Oncol. 2009, 27, 1822–1828.
- Peeters, M.; Douillard, J.Y.; Van Cutsem, E.; Siena, S.; Zhang, K.; Williams, R.; Wiezorek, J. Mutant KRAS codon 12 and 13 alleles in patients with metastatic colorectal cancer: Assessment as prognostic and predictive biomarkers of response to panitumumab. J. Clin. Oncol. 2013, 31, 759–765.
- Ciardiello, F.; Lenz, H.-J.; Kohne, C.-H.; Heinemann, V.; Tejpar, S.; Esser, R.; Beier, F.; Stroh, C.; Duecker, K.; Van Cutsem, E. Effect of KRAS and NRAS mutational status on first-line treatment with FOLFIRI plus cetuximab in patients with metastatic colorectal cancer (mCRC): New results from the CRYSTAL trial. J. Clin. Oncol. 2014, 32, LBA443.
- Allegra, C.J.; Rumble, R.B.; Hamilton, S.R.; Mangu, P.B.; Roach, N.; Hantel, A.; Schilsky, R.L. Extended RAS Gene Mutation Testing in Metastatic Colorectal Carcinoma to Predict Response to Anti-Epidermal Growth Factor Receptor Monoclonal Antibody Therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update 2015. J. Clin. Oncol. 2016, 34, 179–185.
- Lakatos, G.; Kohne, C.H.; Bodoky, G. Current therapy of advanced colorectal cancer according to RAS/RAF mutational status. Cancer Metastasis Rev. 2020, 39, 1143–1157.
- Stahler, A.; Heinemann, V.; Ricard, I.; von Einem, J.C.; Giessen-Jung, C.; Westphalen, C.B.; Michl, M.; Heinrich, K.; Miller-Phillips, L.; Jelas, I.; et al. Current treatment options in RAS mutant metastatic colorectal cancer patients: A meta-analysis of 14 randomized phase III trials. J. Cancer Res. Clin. Oncol. 2020, 146, 2077–2087.
- Xu, J.; Liu, T.; Tang, W.; Chang, W.; Feng, Q.; Wei, Y.; Ren, L.; Ye, Q.; Cui, Y.; He, G.; et al. Bevacizumab plus chemotherapy versus chemotherapy alone as first-line treatment for patients with RAS mutant unresectable colorectal liver-limited metastases: A single center randomized control trial. Ann. Oncol. 2019, 30, v867.
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lupi, C.; Sensi, E.; Lonardi, S.; Mezi, S.; Tomasello, G.; Ronzoni, M.; Zaniboni, A.; et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015, 16, 1306–1315.
- Cremolini, C.; Antoniotti, C.; Rossini, D.; Lonardi, S.; Loupakis, F.; Pietrantonio, F.; Bordonaro, R.; Latiano, T.P.; Tamburini, E.; Santini, D.; et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020, 21, 497–507.
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422.
- Yoshino, T.; Arnold, D.; Taniguchi, H.; Pentheroudakis, G.; Yamazaki, K.; Xu, R.H.; Kim, T.W.; Ismail, F.; Tan, I.B.; Yeh, K.H.; et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann. Oncol. 2018, 29, 44–70.
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 874–901.
- Lebrun, H.; Turpin, A.; Zerbib, P. Therapeutic implications of B-RAF mutations in colorectal cancer. J. Visc. Surg. 2021, 158, 487–496.
- Loupakis, F.; Moretto, R.; Aprile, G.; Muntoni, M.; Cremolini, C.; Iacono, D.; Casagrande, M.; Ferrari, L.; Salvatore, L.; Schirripa, M.; et al. Clinico-pathological nomogram for predicting BRAF mutational status of metastatic colorectal cancer. Br. J. Cancer 2016, 114, 30–36.
- Rowland, A.; Dias, M.M.; Wiese, M.D.; Kichenadasse, G.; McKinnon, R.A.; Karapetis, C.S.; Sorich, M.J. Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br. J. Cancer 2015, 112, 1888–1894.
- Pietrantonio, F.; Petrelli, F.; Coinu, A.; Di Bartolomeo, M.; Borgonovo, K.; Maggi, C.; Cabiddu, M.; Iacovelli, R.; Bossi, I.; Lonati, V.; et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: A meta-analysis. Eur. J. Cancer 2015, 51, 587–594.
- Jones, J.C.; Renfro, L.A.; Al-Shamsi, H.O.; Schrock, A.B.; Rankin, A.; Zhang, B.Y.; Kasi, P.M.; Voss, J.S.; Leal, A.D.; Sun, J.; et al. (Non-V600) BRAF Mutations Define a Clinically Distinct Molecular Subtype of Metastatic Colorectal Cancer. J. Clin. Oncol. 2017, 35, 2624–2630.
- Yaeger, R.; Kotani, D.; Mondaca, S.; Parikh, A.R.; Bando, H.; Van Seventer, E.E.; Taniguchi, H.; Zhao, H.; Thant, C.N.; de Stanchina, E.; et al. Response to Anti-EGFR Therapy in Patients with BRAF non-V600-Mutant Metastatic Colorectal Cancer. Clin. Cancer Res. 2019, 25, 7089–7097.
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 359–369.
- Loupakis, F.; Cremolini, C.; Masi, G.; Lonardi, S.; Zagonel, V.; Salvatore, L.; Cortesi, E.; Tomasello, G.; Ronzoni, M.; Spadi, R.; et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N. Engl. J. Med. 2014, 371, 1609–1618.
- Cremolini, C.; Antoniotti, C.; Stein, A.; Bendell, J.; Gruenberger, T.; Rossini, D.; Masi, G.; Ongaro, E.; Hurwitz, H.; Falcone, A.; et al. Individual Patient Data Meta-Analysis of FOLFOXIRI Plus Bevacizumab Versus Doublets Plus Bevacizumab as Initial Therapy of Unresectable Metastatic Colorectal Cancer. J. Clin. Oncol. 2020, 38, 3314–3324.
- Wirapati, P.; Pomella, V.; Vandenbosch, B.; Kerr, P.; Maiello, E.; Jeffery, G.M.; Curca, R.-O.D.; Karthaus, M.; Bridgewater, J.A.; Mihailov, A.C.; et al. Velour trial biomarkers update: Impact of RAS, BRAF, and sidedness on aflibercept activity. J. Clin. Oncol. 2017, 35, 3538.
- Yoshino, T.; Portnoy, D.C.; Obermannova, R.; Bodoky, G.; Prausova, J.; Garcia-Carbonero, R.; Ciuleanu, T.; Garcia-Alfonso, P.; Cohn, A.L.; Van Cutsem, E.; et al. Biomarker analysis beyond angiogenesis: RAS/RAF mutation status, tumour sidedness, and second-line ramucirumab efficacy in patients with metastatic colorectal carcinoma from RAISE-a global phase III study. Ann. Oncol. 2019, 30, 124–131.
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571.
- Zhang, L. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part II. The utility of microsatellite instability testing. J. Mol. Diagn. 2008, 10, 301–307.
- Chang, S.C.; Li, A.F.; Lin, P.C.; Lin, C.C.; Lin, H.H.; Huang, S.C.; Lin, C.H.; Liang, W.Y.; Chen, W.S.; Jiang, J.K.; et al. Clinicopathological and Molecular Profiles of Sporadic Microsatellite Unstable Colorectal Cancer with or without the CpG Island Methylator Phenotype (CIMP). Cancer 2020, 12, 3487.
- Tiwari, A.K.; Roy, H.K.; Lynch, H.T. Lynch syndrome in the 21st century: Clinical perspectives. QJM Int. J. Med. 2016, 109, 151–158.
- Latham, A.; Srinivasan, P.; Kemel, Y. Microsatellite instability is associated with the presence of lynch syndrome pan-cancer. J. Clin. Oncol. 2018, 37, 286–297.
- Ashktorab, H.; Ahuja, S.; Kannan, L.; Llor, X.; Ellis, N.A.; Xicola, R.M.; Laiyemo, A.O.; Carethers, J.M.; Brim, H.; Nouraie, M. A meta-analysis of MSI frequency and race in colorectal cancer. Oncotarget 2016, 7, 34546–34557.
- Koopman, M.; Kortman, G.A.; Mekenkamp, L.; Ligtenberg, M.J.; Hoogerbrugge, N.; Antonini, N.F.; Punt, C.J.; van Krieken, J.H. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br. J. Cancer 2009, 100, 266–273.
- Ribic, C.M.; Sargent, D.J.; Moore, M.J.; Thibodeau, S.N.; French, A.J.; Goldberg, R.M.; Hamilton, S.R.; Laurent-Puig, P.; Gryfe, R.; Shepherd, L.E.; et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N. Engl. J. Med. 2003, 349, 247–257.
- Sinicrope, F.A.; Mahoney, M.R.; Smyrk, T.C.; Thibodeau, S.N.; Warren, R.S.; Bertagnolli, M.M.; Nelson, G.D.; Goldberg, R.M.; Sargent, D.J.; Alberts, S.R. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J. Clin. Oncol. 2013, 31, 3664–3672.
- Hutchins, G.; Southward, K.; Handley, K.; Magill, L.; Beaumont, C.; Stahlschmidt, J.; Richman, S.; Chambers, P.; Seymour, M.; Kerr, D.; et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J. Clin. Oncol. 2011, 29, 1261–1270.
- Cohen, R.; Taieb, J.; Fiskum, J.; Yothers, G.; Goldberg, R.; Yoshino, T.; Alberts, S.; Allegra, C.; de Gramont, A.; Seitz, J.F.; et al. Microsatellite Instability in Patients With Stage III Colon Cancer Receiving Fluoropyrimidine With or Without Oxaliplatin: An ACCENT Pooled Analysis of 12 Adjuvant Trials. J. Clin. Oncol. 2021, 39, 642–651.
- Fanotto, V.; Ongaro, E.; Rihawi, K.; Avallone, A.; Silvestris, N.; Fornaro, L.; Vasile, E.; Antonuzzo, L.; Leone, F.; Rosati, G.; et al. HER-2 inhibition in gastric and colorectal cancers: Tangible achievements, novel acquisitions and future perspectives. Oncotarget 2016, 7, 69060–69074.
- Hyman, D.M.; Piha-Paul, S.A.; Won, H.; Rodon, J.; Saura, C.; Shapiro, G.I.; Juric, D.; Quinn, D.I.; Moreno, V.; Doger, B.; et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 2018, 554, 189–194.
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697, Erratum in: Lancet 2010, 376, 1302.
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300.
- Meric-Bernstam, F.; Hurwitz, H.; Raghav, K.P.S.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C.; et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): An updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019, 20, 518–530.
- Valtorta, E.; Martino, C.; Sartore-Bianchi, A.; Penaullt-Llorca, F.; Viale, G.; Risio, M.; Rugge, M.; Grigioni, W.; Bencardino, K.; Lonardi, S.; et al. Assessment of a HER2 scoring system for colorectal cancer: Results from a validation study. Mod. Pathol. 2015, 28, 1481–1491.
- Roviello, G.; Catalano, M.; Iannone, L.F.; Marano, L.; Brugia, M.; Rossi, G.; Aprile, G.; Antonuzzo, L. Current status and future perspectives in HER2 positive advanced gastric cancer. Clin. Transl. Oncol. 2022, in press.
- Bertotti, A.; Migliardi, G.; Galimi, F.; Sassi, F.; Torti, D.; Isella, C.; Corà, D.; Di Nicolantonio, F.; Buscarino, M.; Petti, C.; et al. A molecularly annotated platform of patient-derived xenografts ("xenopatients") identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011, 1, 508–523.
- Sartore-Bianchi, A.; Amatu, A.; Porcu, L.; Ghezzi, S.; Lonardi, S.; Leone, F.; Bergamo, F.; Fenocchio, E.; Martinelli, E.; Borelli, B.; et al. HER2 Positivity Predicts Unresponsiveness to EGFR-Targeted Treatment in Metastatic Colorectal Cancer. Oncologist 2019, 24, 1395–1402.
- Tosi, F.; Sartore-Bianchi, A.; Lonardi, S.; Amatu, A.; Leone, F.; Ghezzi, S.; Martino, C.; Bencardino, K.; Bonazzina, E.; Bergamo, F.; et al. Long-term Clinical Outcome of Trastuzumab and Lapatinib for HER2-positive Metastatic Colorectal Cancer. Clin. Colorectal Cancer 2020, 19, 256–262.e2.
- Aprile, G.; De Maglio, G.; Menis, J.; Casagrande, M.; Tuniz, F.; Pisa, E.F.; Fontanella, C.; Skrap, M.; Beltrami, A.C.; Fasola, G.; et al. HER-2 Expression in Brain Metastases from Colorectal Cancer and Corresponding Primary Tumors: A Case Cohort Series. Int. J. Mol. Sci. 2013, 14, 2370–2387.
- Armstrong, S.A.; Malley, R.; Weinberg, B.A. Molecular Profiling in Metastatic Colorectal Cancer. Oncology 2020, 34, 352–355.
- Pietrantonio, F.; Di Nicolantonio, F.; Schrock, A.B.; Lee, J.; Tejpar, S.; Sartore-Bianchi, A.; Hechtman, J.F.; Christiansen, J.; Novara, L.; Tebbutt, N.; et al. ALK, ROS1, and NTRK Rearrangements in Metastatic Colorectal Cancer. J. Natl. Cancer Inst. 2017, 109, djx089.
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739.
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282.
- Paz-Ares, L.; Barlesi, F.; Siena, S.; Ahn, M.J.; Drilon, A.; Conley, A.; Rolfo, C.; Wolf, J.; Seto, T.; Doebele, R.; et al. Patient-reported outcomes from STARTRK-2: A global phase II basket study of entrectinib for ROS1 fusion-positive non-small-cell lung cancer and NTRK fusion-positive solid tumours. ESMO Open 2021, 6, 100113.
- Chou, A.; Fraser, T.; Ahadi, M.; Fuchs, T.; Sioson, L.; Clarkson, A.; Sheen, A.; Singh, N.; Corless, C.L.; Gill, A.J. NTRK gene rearrangements are highly enriched in MLH1/PMS2 deficient, BRAF wild-type colorectal carcinomas-a study of 4569 cases. Mod. Pathol. 2020, 33, 924–932.
- He, X.; Jiao, X.D.; Liu, K.; Qin, B.D.; Wu, Y.; Ling, Y.; Liu, J.; Xu, A.Q.; Song, K.; Zang, Y.S. Clinical Responses to Crizotinib, Alectinib, and Lorlatinib in a Metastatic Colorectal Carcinoma Patient With ALK Gene Rearrangement: A Case Report. JCO Precis. Oncol. 2021, 5, PO.20.00534.
- Jiang, T.; Wang, G.; Liu, Y.; Feng, L.; Wang, M.; Liu, J.; Chen, Y.; Ouyang, L. Development of small-molecule tropomyosin receptor kinase (TRK) inhibitors for NTRK fusion cancers. Acta Pharm. Sin. B 2021, 11, 355–372.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
766
Revisions:
3 times
(View History)
Update Date:
13 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




