Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francisco Cavilha Neto | -- | 6281 | 2022-05-11 20:12:37 | | | |
| 2 | Francisco Cavilha Neto | Meta information modification | 6281 | 2022-05-12 17:31:32 | | | | |
| 3 | Camila Xu | -2217 word(s) | 4064 | 2022-05-13 04:09:41 | | | | |
| 4 | Camila Xu | Meta information modification | 4064 | 2022-05-13 04:21:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cavilha Neto, F.; Aguilar, C.; Souza, M.; Neves, G.; , . Critical MIM + SH Processing Parameters. Encyclopedia. Available online: https://encyclopedia.pub/entry/22840 (accessed on 07 February 2026).
Cavilha Neto F, Aguilar C, Souza M, Neves G, . Critical MIM + SH Processing Parameters. Encyclopedia. Available at: https://encyclopedia.pub/entry/22840. Accessed February 07, 2026.
Cavilha Neto, Francisco, Claudio Aguilar, Marcelo Souza, Guilherme Neves, . "Critical MIM + SH Processing Parameters" Encyclopedia, https://encyclopedia.pub/entry/22840 (accessed February 07, 2026).
Cavilha Neto, F., Aguilar, C., Souza, M., Neves, G., & , . (2022, May 11). Critical MIM + SH Processing Parameters. In Encyclopedia. https://encyclopedia.pub/entry/22840
Cavilha Neto, Francisco, et al. "Critical MIM + SH Processing Parameters." Encyclopedia. Web. 11 May, 2022.
Copy Citation
Metal injection molding (MIM) combined with the use of a space holder (SH) is a very attractive route for the fabrication of highly porous titanium and titanium alloy components for biomedical applications. This approach allows fine control of the morphology, architecture, and purity of very complex net-shaped components.
titanium foams
metal injection molding
space holder
1. Introduction
Bone fractures are a global public health issue, and they impair quality of life and result in health-care costs. In fact, fractures are the most common musculoskeletal disorder, mostly related to wear and aging, especially in people with arthritis and osteoporosis. In many cases, the missing or damaged bone must be replaced by an orthopedic implant [1].
Orthopedic procedures mean large expenses, with the costs of implantable devices accounting for a large proportion of those procedure costs. According to one study [2], the current market for orthopedic implants accounts for around USD 45 bi and is estimated to exceed USD 60 bi in 2028. The rapid and continuous increase in the geriatric population around the globe, who are more prone to orthopedic problems, has been driving the demand for biomaterials. Technological innovations concerning implantable medical devices, robot-assisted tools, and the continuous development of new materials and processing procedures have also supplemented the market growth.
Bone tissue is a polymer–ceramic composite formed by minerals such as hydroxyapatite (Ca10(PO4)6(OH)2). It is found in two forms or types, and both have an anisotropic structure; that is, they have properties that depend on the direction analysis. The first type is the trabecular or cancellous bone, composed of an interconnected porous network of plates and rods arranged in various configurations, forming an open foam with a density around 0.05 to 1.0 g/cm³ and a low elastic modulus (0.76 to 4 GPa) [3], which varies considerably depending on the direction of the stress. The second type is called cortical or compact bone, characterized by having a higher density (1.99 g/cm³), lower porosity, greater metabolic activity [4], and higher elastic modulus (17 to 30 GPa) [5]. Normally, the two forms of bone can be found together in different configurations, giving the bone a combination of characteristics, such as excellent energy absorption capacity of the cancellous tissue and better mechanical resistance acquired from the cortical bone. In some cases, such as severe damage or fractures, the bone needs to be replaced. Successfully replacing this hard tissue with a complex structure that mimics its anisotropic features and mechanical properties can be challenging without the use of biocompatible materials.
Metals 316L stainless steels (316LSS), Co-Cr alloys, and Co-Cr-Mo alloys were the first artificial biomaterials, but they exhibited two disadvantages: the presence of toxic elements, such as Co [6] and Cr [7], and a high elastic modulus. Pure Ti and Ti-based alloys are currently the most exploited by the biomedical industry due to several characteristics, such as non-toxicity, good biocompatibility, and low density. The first generation of Ti alloys had several disadvantages, including relatively lower wear resistance, lower hardness, and higher stiffness compared with human bones [8]. Stiffness mismatch between the implant materials and human bones causes bone resorption and eventual loosening of the implants [9]. Moreover, alloying elements, such as Al, V, Ni, and Co, present in the Ti alloys, produce toxic effects when they are placed into the human body. Some diseases produced by these elements are dermatitis, Alzheimer’s, neuropathy, and osteomalacia [10][11][12]. The second generation of Ti alloys was produced with non-toxic elements, such as Nb, Mo, Zr, or Ta [13]. Even so, these alloys present problems associated with their mechanical performance.
One of the main difficulties in achieving a very high degree of osteointegration is related to the difference between the elastic modulus of the implant material and the underlying bone structure, as aforementioned. In this sense, the manufacture of porous components through processes such as metal injection molding (MIM) combined with the space holder (SH) method [14], powder compaction [15], and additive manufacturing (AM) [16] has been studied over the years to overcome these limitations. The pores generated from these processing methods, in addition to reducing the elastic modulus of the implant, provide the adherent surface necessary for cell proliferation and adhesion [17], thus avoiding the stress shielding effect. Furthermore, bone growth into the implant is determined by the porosity, pore size, and structure [18], allowing adequate vascularization of cells and fluids [19]. However, good control over the addition of porosity must be performed to achieve a precise balance between the mechanical properties and the biological and adhesion performance of the implant.
MIM has been demonstrated to be an excellent technique to obtain structures with controlled porosity. In addition, MIM combines some desirable characteristics, such as reproducibility, design simplicity, and flexibility in the choice of fitting materials. The manufacturing process has high design freedom, with the possibility of large-scale production in the processing of near-net-shaped parts. Geometrically complex parts, such as human bones, can be obtained by MIM. Furthermore, the MIM process associated with the SH method has great potential for obtaining porous structures with particular properties by creating voids with temporary materials. MIM combined with SH makes it possible to obtain parts with the desired size, shape, and percentage of pores. Several researchers have applied the combination of MIM and SH to produce titanium foams with elastic modulus values close to those of bone [20][21][22][23] and pore percentages in the range of 30% to 72% [24][25][26][27][28].
2. Critical MIM + SH Processing Parameters
2.1. Ti Powders
With regard to the characteristics of the raw material, the best commercially pure Ti powders for the MIM process are those where the particles are spherical, smaller than 45 µm with a non-uniform distribution, and the purity is greater than 99.5% [29]. These powders are preferred due to their better flowability [30], faster densification [31], and desirable characteristics during injection and sintering, respectively.
Concerning the size of the Ti particles, it is important to consider that they should be several times smaller than the SH to improve the sinterability [32]. The use of a fine spherical powder improves the surface finish and increases the apatite formation due to a higher surface energy [24], but, as the size of the particles becomes much smaller, the content of impurities tends to increase [33], which can have harmful effects on the properties of the component. However, finer and purer powders are more difficult to obtain due to the considerably higher price of the raw material. On the other hand, coarser powders or those with a wider particle size distribution can be used to increase the density of the green part and have the advantage of being less susceptible to impurities, but the disadvantages include less flowability and higher susceptibility to distortions. Moreover, in order to avoid substantial linear shrinkage and warping, it is important to use a powder with high packing density since the pressure used in injection molding is relatively low in comparison with other methods, such as press and sinter. Furthermore, low-packing-density powder can result in more micro-pores [31]. Since powder particles constitute the walls of the pores in porous foams, it is very important to choose a powder that results in a low number of micro-pores as these micro-pores can deteriorate the mechanical properties of foams by concentrating stress. Thus, it is preferred to use a powder with a broad particle size range in order to fill those empty spaces among the particles by finer particles, consequently minimizing the possibility of micro-pore formation.
Particle shape also has a great impact on the properties of the foams produced. As mentioned above, spherical high-purity powders are the most desirable for use in MIM. However, their higher cost represents an important disadvantage. The shape of the powder is normally dependent on the production method. Common particle shapes of Ti powders are spherical and angular or irregular. Spherical Ti powders are usually produced by gas atomization. In comparison, angular Ti powders are typically produced by the hydride–dehydride process, and they are relatively cheaper. Recently, researchers have used non-spherical titanium hydrogenated-dehydrogenated (HDH) powders [34][35][36], produced by mechanical milling of sponge, scrap, or ingots [37], and titanium hydride (TiH2) powders [20][38], which have lower levels of purity and flowability but are acquired at a lower cost. Figure 1 shows the (a) gas-atomized and (b) HDH powder morphologies used by Tuncer et al. [26].
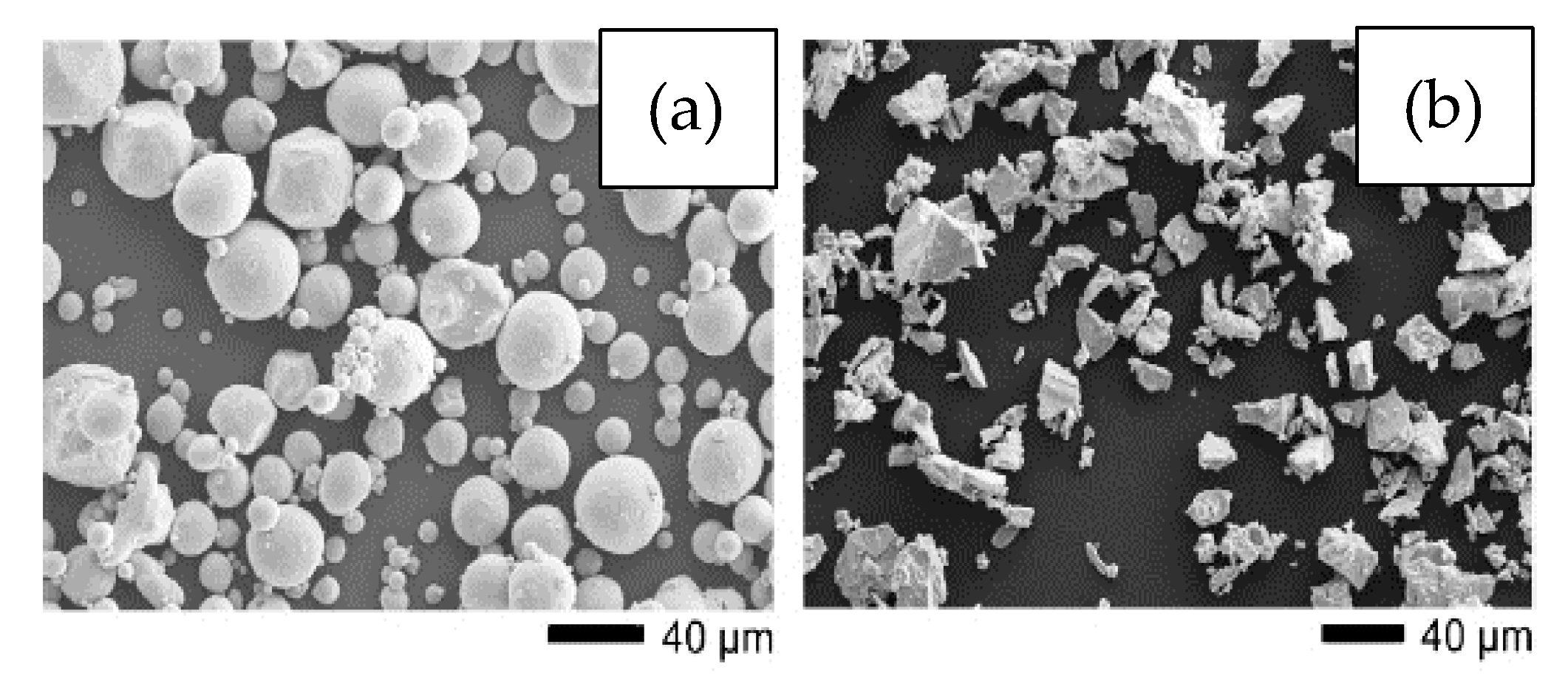 Figure 1. SEM images showing the morphology of (a) gas-atomized titanium powder, (b) hydride–dehydride (HDH) titanium powder. Adapted and reproduced with permission [26]. Copyright 2014, Elsevier.
Figure 1. SEM images showing the morphology of (a) gas-atomized titanium powder, (b) hydride–dehydride (HDH) titanium powder. Adapted and reproduced with permission [26]. Copyright 2014, Elsevier.These powders can be used to form the entire volume of the implant or be mixed with better quality powders [39] to allow a reduction in costs. Furthermore, the hydrogen released from TiH2 during sintering can prevent extra oxygen pickup by the part [40][41]. Among the disadvantages of using non-spherical powders such as HDH is the greater susceptibility to cracks due to the greater amount of stress concentrators [35]. Moreover, other studies have shown that irregular powders produced foams with less open porosity, a desirable characteristic aimed at bone growth and osteointegration; this was attributed to the low packing density of the irregular powder in comparison with the spherical ones [31][42]. Treatments such as induction plasma [43] can be performed to reduce the sharpness of irregular powders, improving volume density and packing capacity [34][44]. In this regard, Güden et al. [35] compared, after sintering, samples cold-compacted with both spherical and angular metal powders and found that the compressive strengths obtained were close to those of human bone due to the high degree of porosity (34–54%). The Ti structures compacted with different powders were very similar, showing close values for the porosity grades. However, the results showed that angular powders result in higher percentage of micro-porosity and poorer mechanical properties in comparison with spherical powders.
Contamination of the initial powder is one of the most important factors affecting the final oxygen content of the implant, a characteristic that can impair the mechanical properties of the part, reducing its toughness and increasing the elastic modulus [33] and the stress shielding effect. Thus, standards have been established regarding the chemical requirements for orthopedic applications [45][46][47]. During the entire processing route, if all the contamination precautions are followed, no step provides more oxygen as a contaminating element than the initial contamination level itself, as shown in the graph in Figure 2.
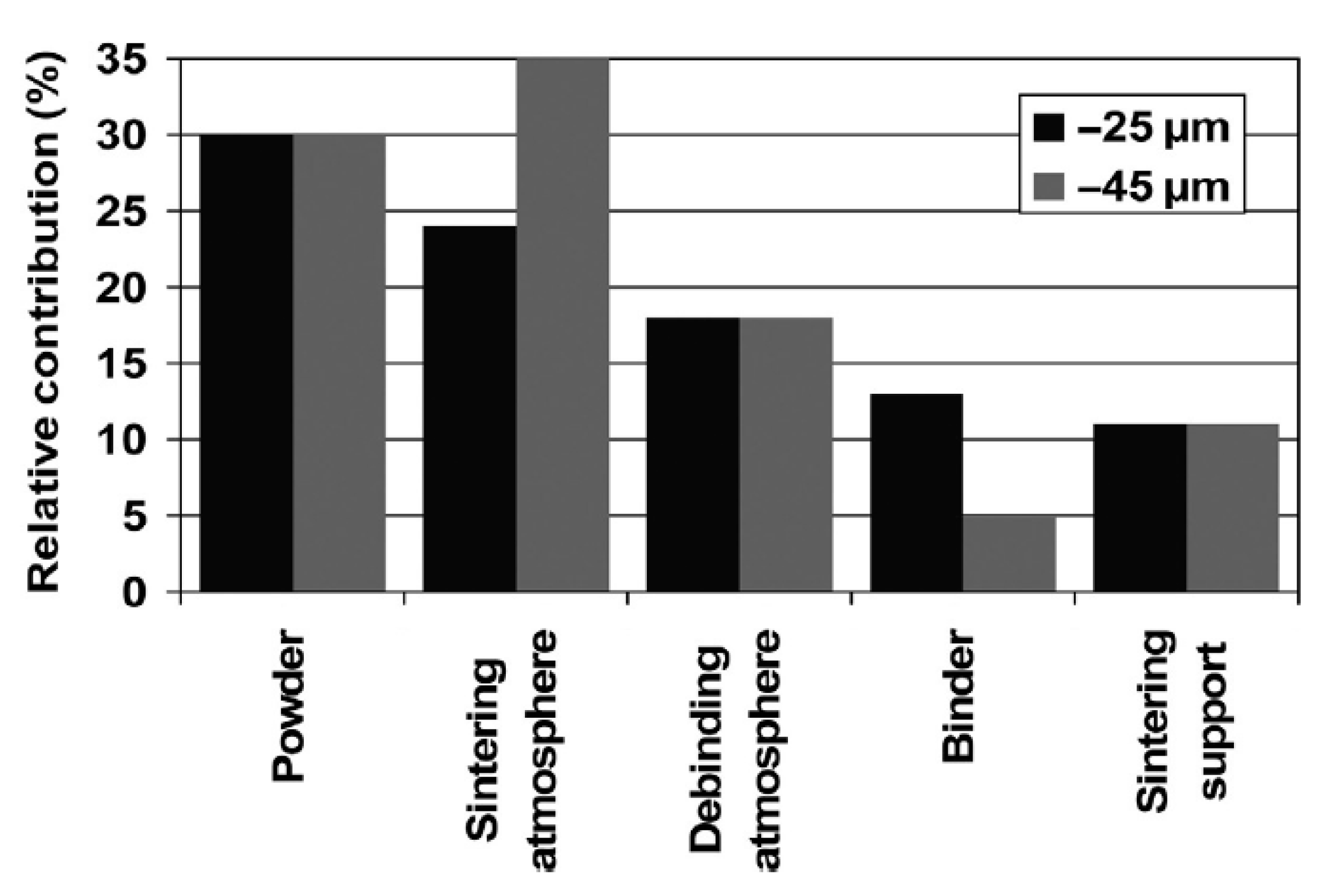 Figure 2. Contribution of each processing step to oxygen contamination in Ti powder subjected to MIM and sinter process. Reproduced with permission [33]. Copyright 2014, Taylor and Francis.
Figure 2. Contribution of each processing step to oxygen contamination in Ti powder subjected to MIM and sinter process. Reproduced with permission [33]. Copyright 2014, Taylor and Francis.Although very reactive, titanium powder with a particle size between 1 and 45 µm does not react strongly with air at temperatures below 200 °C, with most of its initial oxygen content coming from its manufacturing or atomization [33]. Therefore, its handling at room temperature does not require protective devices, such as gloveboxes or protective atmospheres. On the other hand, its reactivity increases exponentially at temperatures above 400 °C [33]. It is important to note that, the smaller the particle size, the more reactivity the powder will present due to a larger surface area, increasing the handling risks.
2.2. Space Holders (SH) Method for MIM
The porosity in titanium foams for biomedical implants, as previously mentioned, has the function of reducing the elastic modulus of the implant to values closer to the underlying bone. Moreover, it provides a better cell attachment and proliferation due to the natural porous aspect of the bone [48][49]. Pores can be introduced through several methods, including partial sintering [50], sintering with hollow spheres [51], trapping and gas expansion [52], slip casting [53], tape casting [54], gel casting [55], freeze casting [56], and polymeric impregnation [18]. However, few of these approaches compete with the SH method in controlling the amount and shape of pores, as well as the dimensional control of the parts produced at a low cost. In the SH procedure, the pore formers do not interact with the metal and are easily removed in later steps. Porosity is controlled by the amount and shape of the space support added. The SH is selected based on the following criteria: (a) available sizes and shapes, (b) cost, (c) no reactivity with Ti, (d) low presence of residue, (e) easy processability, (f) lack of toxicity, and (g) resistance to deformation. In general, the SH is removed through contact with solvents or heat. Table 1 includes the typical space holders used in combination with the MIM process found in the literature.
Table 1. Typical space holders used in the literature and their morphological and removal characteristics.
| Material | Particle Size | Removal | Observation | References |
|---|---|---|---|---|
| Sodium Chloride (NaCl) | 200–500 µm | Aqueous solution 50–60 °C for 40 to 72 h |
- Good water solubility and low cost - High melting point |
[20][26][57] |
| Potassium Chloride (KCl) | 250–500 µm | Aqueous solution 50–60 °C for 24 to 72 h or thermal removal at 750 °C for 2 h |
- High solubility in water, available in multiple shapes - Lower melting point |
[25][26][27] |
| Polymethylmethacrylate (PMMA) | D50 = 600 µm | Thermal 200–450 °C for 2 h |
- Control of the macropore morphology - Can be used as a binder - May contaminate the powder with C and O |
[20][58] |
| Magnesium | 300–1500 µm | Thermal during sintering |
- Can be leached with solvents | [59] |
| Ammonium bicarbonate NH4HCO3 | 500–800 µm | Thermal 175 °C |
- May contaminate the powder with interstitial elements - Easy and complete removable due to moderate decomposition temperature |
[24][60] |
| Tapioca Starch | 100–400 μm | Aqueous solution or in a furnace at 450 °C | - Low cost - Easy access - Easy removal |
[61] |
It can be noted that the salt-based SHs (NaCl and KCl) can be removed at lower temperatures and do not lead to contamination of the titanium. Moreover, they are inexpensive and easy to obtain. On the other hand, longer times (24 to 72 h) are required to remove these SHs in solvents. Tuncer et al. [26] tested NaCl with cubic morphology and KCl with rounded particles (see Figure 3a,b). The researchers observed that KCl offers several advantages over NaCl in terms of ease of removal and fluidity due to the shape of the particles. Morelli et al. [20] compared NaCl and polymethylmethacrylate (PMMA) to SHs. The researchers observed better shape retention of the green material in the case of PMMA. In addition, the foams produced with PMMA showed higher porosity when using the same volume of SH. Contamination was not measured.
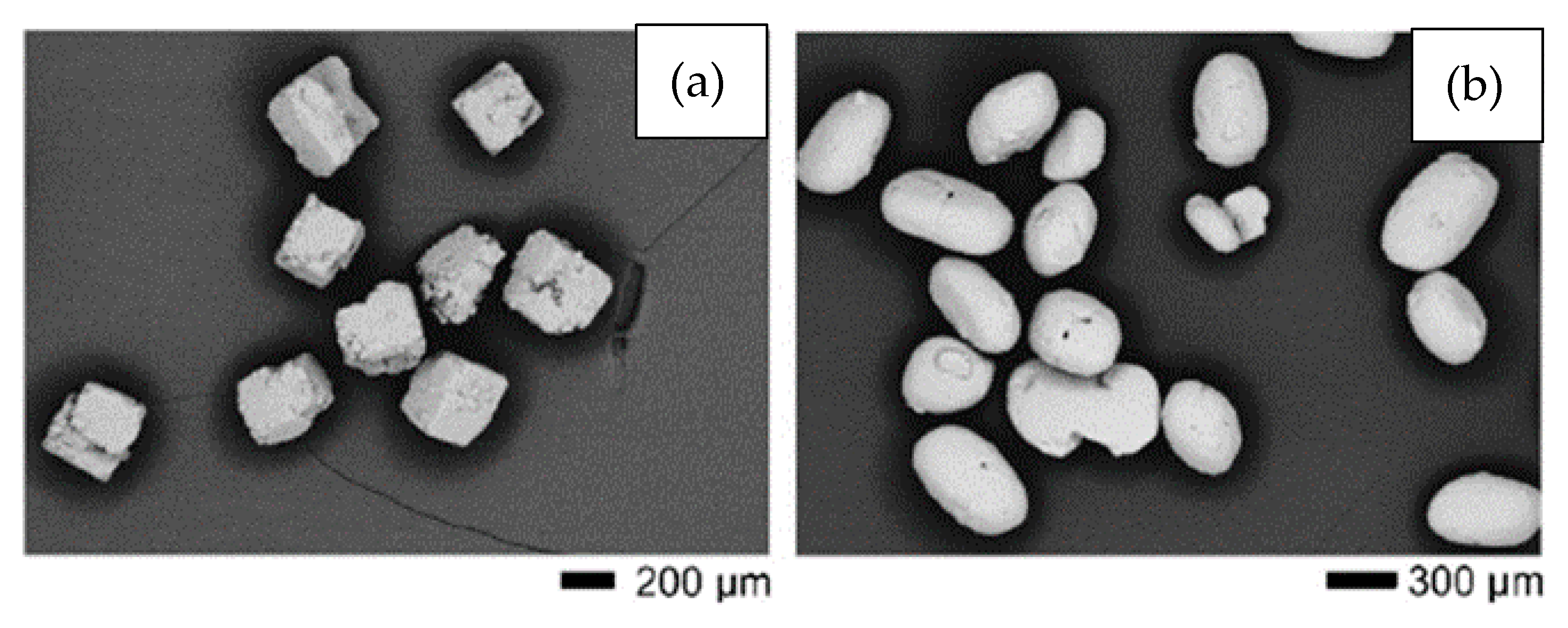 Figure 3. SEM images showing the morphology of (a) cubic NaCl and (b) rounded KCl powders used in experiments performed by Tuncer et al. Adapted and reproduced with permission [26]. Copyright 2014, Elsevier.
Figure 3. SEM images showing the morphology of (a) cubic NaCl and (b) rounded KCl powders used in experiments performed by Tuncer et al. Adapted and reproduced with permission [26]. Copyright 2014, Elsevier.The particle size and shape and the volume of the SH used are directly linked to the shape, size, and percentage of the pores [21]. Figure 4 shows a water-debinded sample illustrating the cubic-shaped pores, the structural binder, and the Ti particles. The images verify that the KCl space holder was dissolved in water and the pores retained the shape of the space holder particles, while the polyethylene glycol (PEG) and polymethyl-methacrylate (PMMA) networks of the binder system are still apparent after 23 h of dissolution at 20 °C. This research was carried out by Shbeh and Goodall [62], who successfully obtained Ti foams with 55% of porosity and low contamination.
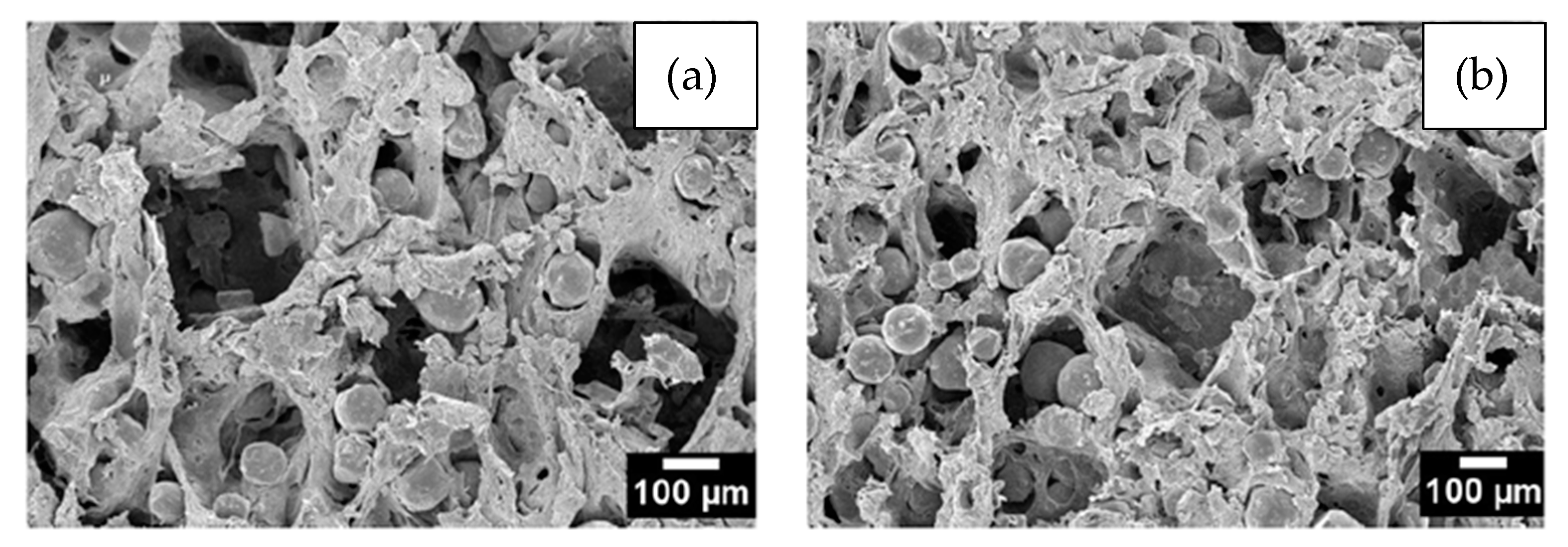 Figure 4. Secondary electron SEM images of a Ti sample debonded in water for 23 h showing in (a,b) the cubic voids formed due to the removal of the cubic-shaped space holder and the binder structure. Adapted and reproduced with permission [62]. Copyright 2015, Elsevier.
Figure 4. Secondary electron SEM images of a Ti sample debonded in water for 23 h showing in (a,b) the cubic voids formed due to the removal of the cubic-shaped space holder and the binder structure. Adapted and reproduced with permission [62]. Copyright 2015, Elsevier.Figure 5, taken from Shbeh et al. [28], shows the dependence of the amount of porosity, volume shrinkage, and mechanical strength on the percentage of SH feedstock. It can be seen in Figure 5a that, the higher the SH content, the greater the porosity. However, this relationship assumes a logarithmic behavior, with a large increase in porosity at lower SH contents. For contents higher than 50% SH, there is little porosity gain, and the curve becomes asymptote. The researchers attributed this behavior to the greater volume reduction in the samples (Figure 5b) with increasing content of SH since the Ti particles need to travel greater distances during sintering to initiate atomic bonds with each other and form cell walls. These movements result in greater volume shrinkage of the samples. Figure 5c shows the dependence of compressive strength on porosity. The stress–strain curves for foams made with a high porosity, especially those obtained with 52% and 60% of SH (by volume), showed a much longer plateau region before densification compared to those with a low porosity. The foams with a high porosity have a more uniform porous structure and undergo more uniform deformation until densification, while those with a lower porosity reach densification earlier. In the same study, Shbeh et al. [28] used different amounts, shapes, and sizes of the KCl as a space holder to evaluate the pore morphology in titanium foams. The impact of the shape of the space holder on the final porosity was not significant. On the other hand, as shown in Figure 5d, the shape of the SH particles has a great influence on the mechanical properties of the foams produced. The Ti foams produced with the cubic SH showed lower yield stress than those made with the spherical SH, probably due to the stress concentration at the corners of the cubic pores. The particle size and shape of the SH also influences the flowability of the material during MIM processing and the preparation of the powder mixture, which can lead to clogging or poor filling of the mold due to the low pressure and temperatures used in MIM.
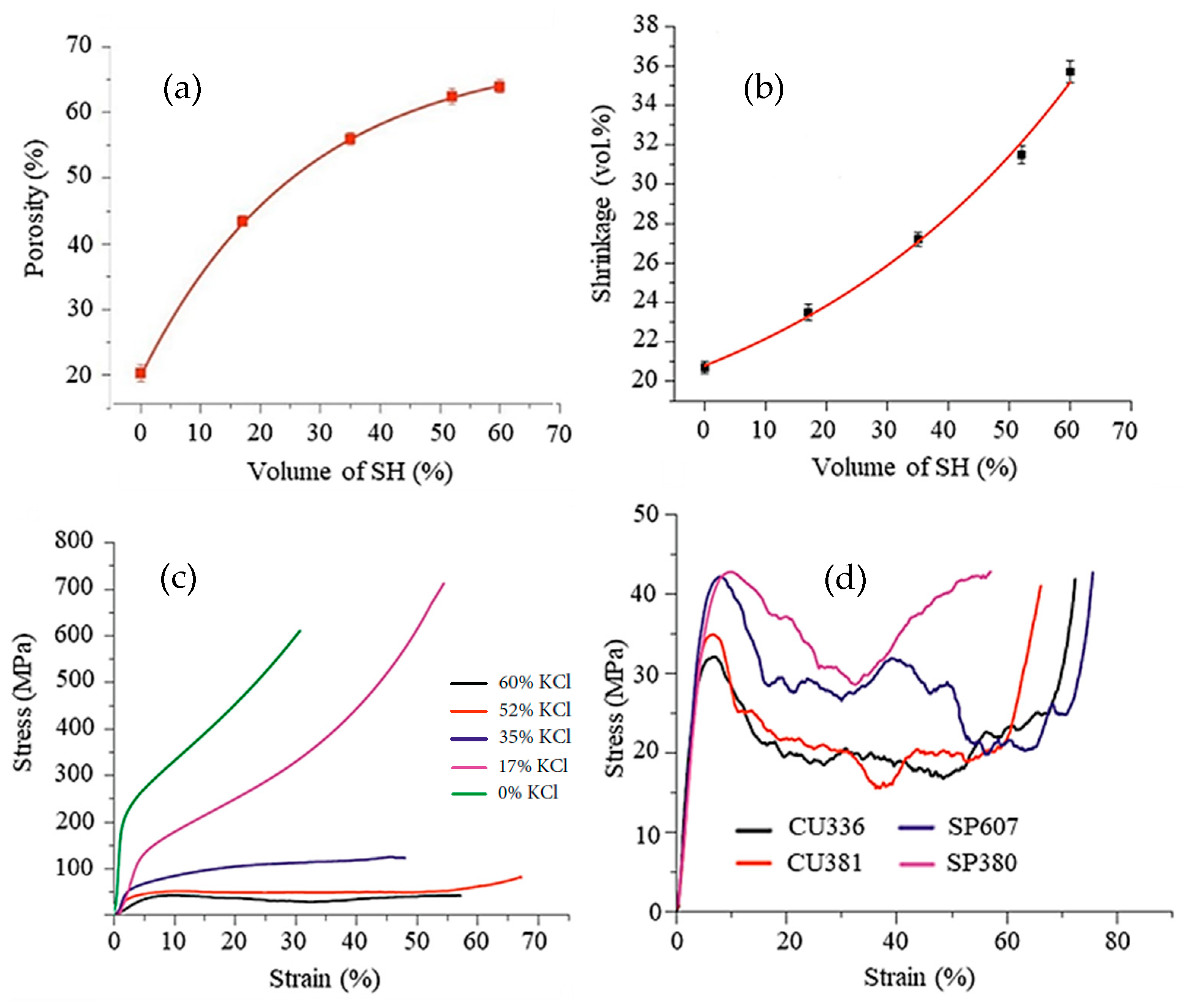 Figure 5. Properties of foams produced with commercially pure Ti powder (D50 = 75 μm) with a spherical shape, and spherical potassium chloride (D50 = 366 μm) as a space holder: (a) the relationship between the amount of space holder added and porosity in the sintered samples; (b) the correlation between the amount of space holder and volume shrinkage; (c) mechanical strength of samples with different amounts of porosity under compression; and (d) mechanical strength of foams under compression testing at a strain rate of 0.001 s−1 produced using cubic KCl with a mean particle size of 336 μm (CU336), cubic KCl with a mean particle size of 381 μm (CU381), spherical KCl with a mean particle size of 607 μm (SP607), and spherical KCl with a mean particle size of 380 μm (SP380). The samples were sintered at 1320 °C for 2 h. Adapted from Ref. [28]. Open Access Copyright.
Figure 5. Properties of foams produced with commercially pure Ti powder (D50 = 75 μm) with a spherical shape, and spherical potassium chloride (D50 = 366 μm) as a space holder: (a) the relationship between the amount of space holder added and porosity in the sintered samples; (b) the correlation between the amount of space holder and volume shrinkage; (c) mechanical strength of samples with different amounts of porosity under compression; and (d) mechanical strength of foams under compression testing at a strain rate of 0.001 s−1 produced using cubic KCl with a mean particle size of 336 μm (CU336), cubic KCl with a mean particle size of 381 μm (CU381), spherical KCl with a mean particle size of 607 μm (SP607), and spherical KCl with a mean particle size of 380 μm (SP380). The samples were sintered at 1320 °C for 2 h. Adapted from Ref. [28]. Open Access Copyright.Therefore, the proportion of titanium powder, SH, and binder must be adjusted so that the part develops the desired porosity as well as maintains its dimensions throughout the process, minimizing shrinkage and loss of shape. Daudt et al. [25] used spherical Ti powder (d90 = 32.8 µm) and KCl particles to produce Ti foams by MIM. The researchers observed that, when adding 70% of the SH, the powder charge had to be increased by 80% and the binder reduced by 20% to maintain the structure dimensions during sintering. Contents higher than 80% of SH can considerably reduce the fluidity and clog the nozzle of the MIM machine. Many biomedical implants require different levels of porosity or porosity gradients. This can be achieved by processing with the addition of space holders and binders in the appropriate proportions or applying 2-component-metal injection molding (2-C-MIM) [63]. This technique is derived from the plastics industry [64] and has been adapted to metal powders. Barbosa et al. [65] produced titanium foams with a gradient in porosity using this technology, starting with feedstocks with and without space holders. Implant shape stability was only possible using less than 50 vol.% of SH in a powder load. The final porosity was not reported, but it is probably under 50%.
2.3. Binders and Debinding
The binders used during MIM of titanium powders must be those that enable the least interaction of the powder with elements, such as oxygen, hydrogen, carbon, and nitrogen, which can considerably change the mechanical properties of the implant [66]. Some binders commonly used in MIM, such as paraffin-based ones, which are characterized by elements that are toxic to organisms, are being replaced by highly soluble water-based binders, such as polyethylene glycol (PEG) [37]. The binders can also be used as space holders and should give the powder mixture good flowability, wettability, and homogeneity, prompting researchers to seek the best materials to fill these roles. A good binder system must have several desirable characteristics, such as dimensional stability during its removal, low reactivity but good adhesion to titanium, a melting point at a temperature compatible with the MIM process, and it must leave no residue.
One of the difficulties in the production of titanium foams via MIM is the maintenance of the part dimensions during sintering, mainly due to poor properties of the green material, phase change, a sudden variation in temperature during thermal debinding or sintering, and contamination by the binder. Moreover, in the case of parts with proportions of space holders above 55%, it is difficult to maintain their shape after injection, hindering the manufacture of implants with porosity greater than 50%, an appreciable percentage for foams. Several studies have focused on better understanding the dimensional stability of parts produced via MIM. Daudt et al. [25], for example, applied a plasma treatment to MIM samples after the chemical removal of binders and before thermal removal. The microwave plasma treatment cleaned the surface of the component, keeping the properties of the part intact and facilitating the thermal removal of binders. Figure 6 shows the samples obtained by the researchers. The researchers noted that the plasma helped to maintain the shape of the part by stimulating a pre-sintering phenomenon (Figure 6c), creating small connections between the powder particles even for porosity above 50% and at a much lower temperature than that used in sintering in addition to maintaining open porosity.
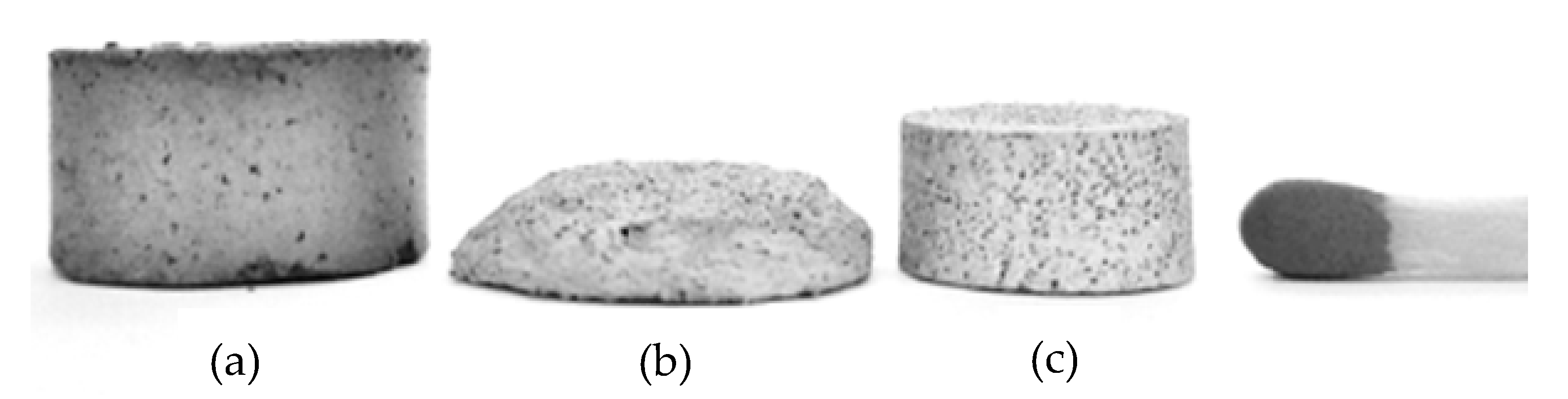 Figure 6. MIM samples: (a) unsintered; (b) sintered at 1200 °C, 3 h without plasma treatment; (c) plasma-treated (15 min, 294 W) and sintered at 1200 °C, 3 h. Adapted and reproduced with permission [25]. Copyright 2017, Elsevier.
Figure 6. MIM samples: (a) unsintered; (b) sintered at 1200 °C, 3 h without plasma treatment; (c) plasma-treated (15 min, 294 W) and sintered at 1200 °C, 3 h. Adapted and reproduced with permission [25]. Copyright 2017, Elsevier.The binder system used to produce porous titanium implants via MIM is generally composed of three materials: one with low viscosity, one with high viscosity, and a lubricant. Materials with low viscosity give the mixture a good flowability, which facilitates injection at low pressures. The most commonly used are PEG and paraffin, polymers that have a low melting point. The former can be removed with water solutions without any hazards [67]. The high-viscosity materials consist, in general, of polymers with a high melting point, such as PMMA and polyethylene (PE), providing mechanical stability, mainly after the removal of the SH. The lubricant gives the mixture greater fluidity, and stearic acid (SA) is commonly used. The proportion of these materials can vary, but, in general, the low viscosity polymer accounts for more than half of the binder mixture, the high-viscosity material makes up one-third, and the SA represents around 6% [26][32][58].
Binders are usually removed using chemical solutions and/or the application of heat. The biggest difference between paraffin and PEG is that the agents used to remove the latter are less toxic to the human body and the environment [68][69]. The mixture of powder and binder is designed to give it characteristics that guarantee good injection. The viscosity must be below 1000 Pa.s at the injection temperature [70]. Pseudoplastic polymeric characteristics are also desirable since the reduction in viscosity with deformation helps the injection of parts with more complex dimensions [71].
There are currently two main methods for debinding, namely: thermal and solvent debinding. The former is usually performed in the same furnace and cycle as the sintering step. The parts are heated and maintained at a certain temperature equal to or close to the degradation temperature of the polymer (obtained by termogravimetry) in which the binder can be completely removed. On the other hand, solvent debinding consists of plunging the parts inside a (usually) heated chemical solution (hexane or water), which reacts with part of the binder system, removing the non-structural component. In the literature on MIM + SH foams, many researchers report very long debinding and dissolution times, in some cases over two days [26]. In this regard, Shbeh and Goodall [62] assessed the effect on the processing speed of different water debinding and dissolution techniques for PEG and KCl as the binder and SH, respectively. The results showed that ultrasonic water solutions at the peak melting point of PEG were the fastest way (4 h) to remove PEG and KCl completely, which can be a promising technique for the KCl removal in other feedstock compositions. The best temperature was found to be equal to 70 °C, above which the rate of dissolution was reduced due to a significant dilatation in the sample, slowing the SH removal process.
In a similar study, Thian et al. [72] assessed the thermal debinding parameters of wax binders. They observed that very fast heating rates increased the formation of internal and external cracks, deteriorating the mechanical properties of the part. The researchers also noted that higher argon fluxes reduced the percentage of contamination due to the faster purging of gases from the binder, preventing them from condensing into carbon. The research showed that modulating the extraction of binders in stages improved the dimensional stability of the components due to the creation of bonds between the particles in intermediate stages.
References
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425.
- Louis, G.P. Rapid Growth in the Elderly Population of the World. In Brain and Spine Surgery in the Elderly; Berhouma, M., Krolak-Salmon, P., Eds.; Springer: Cham, Switzerland, 2017.
- Gibson, L.J. The mechanical behaviour of cancellous bone. J. Biomech. 1985, 18, 317–328.
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. CJASN 2008, 3 (Suppl. 3), 131–139.
- Bayraktar, H.H.; Morgan, E.F.; Niebur, G.L.; Morris, G.E.; Wong, E.K.; Keaveny, T.M. Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J. Biomech. 2004, 37, 27–35.
- Garcia, M.D.; Hur, M.; Chen, J.J.; Bhatti, M.T. Cobalt toxic optic neuropathy and retinopathy: Case report and review of the literature. Am. J. Ophthalmol. Case Rep. 2020, 17, 100606.
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloys Compd. 2017, 701, 698–715.
- Liu, X.; Chu, P.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121.
- Xiong, J.; Li, Y.; Wang, X.; Hodgson, P.; Wen, C. Mechanical properties and bioactive surface modification via alkali-heat treatment of a porous Ti–18Nb–4Sn alloy for biomedical applications. Acta Biomater. 2008, 4, 1963–1968.
- Wapner, K.L. Implications of metallic corrosion in total knee arthroplasty. Clin. Orthop. Related Res. 1991, 271, 12–20.
- Nag, S.; Banerjee, R.; Fraser, H.L. Microstructural evolution and strengthening mechanisms in Ti–Nb–Zr–Ta, Ti–Mo–Zr–Fe and Ti–15Mo biocompatible alloys. Mater. Sci. Eng. C 2005, 25, 357–362.
- Eisenbarth, E.; Velten, D.; Müller, M.; Thull, R.; Breme, J. Biocompatibility of β-stabilizing elements of titanium alloys. Biomaterials 2004, 25, 5705–5713.
- Taddei, E.B.; Henriques, V.A.R.; Silva, C.R.M.; Cairo, C.A.A. Production of new titanium alloy for orthopedic implants. Mater. Sci. Eng. C 2004, 24, 683–687.
- Dehghan-manshadi, A.; St. John, D.H.; Dargusch, M.S.; Chen, Y.; Sun, J.F.; Qian, M. Metal injection moulding of non-spherical titanium powders: Processing, microstructure and mechanical properties. J. Manuf. Proces. 2018, 31, 416–423.
- Sharma, M.; Gupta, G.K.; Dasgupta, R.; Kumar, M.; Kumar, P. Titanium Foams Processed Through Powder Metallurgy Route Using Lubricant Acrawax as Space Holder Material. Trans. Indian Inst. Met. 2018, 71, 1933–1940.
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C 2016, 59, 690–701.
- Li, J.P.; Habibovic, P.; Yuan, H.; van den Doel, M.; Wilson, C.E.; de Wijn, J.R.; van Blitterswijk, C.A.; de Groot, K. Biological performance in goats of a porous titanium alloy-biphasic calcium phosphate composite. Biomaterials 2007, 28, 4209–4218.
- Wang, C.; Chen, H.; Zhu, X.; Xiao, Z.; Zhang, K.; Zhang, X. An improved polymeric sponge replication method for biomedical porous titanium scaffolds. Mater. Sci. Eng. C 2017, 70, 1192–1199.
- Hollister, S.J. Scaffold design and manufacturing: From concept to clinic. Adv. Mater. 2009, 21, 3330–3342.
- Carrenõ-Morelli, E.; Rodríguez-Arbaizar, M.; Amherd, A.; Bidaux, J.E. Porous titanium processed by powder injection moulding of titanium hydride and space holders. Powder Metall. 2014, 57, 93–96.
- Zheng, J.P.; Chen, L.J.; Chen, D.Y.; Shao, C.S.; Yi, M.F.; Zhang, B. Effects of pore size and porosity of surface-modified porous titanium implants on bone tissue ingrowth. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2019, 29, 2534–2545.
- Lascano, S.; Arévalo, C.; Montealegre-Melendez, I.; Muñoz, S.; Rodriguez-Ortiz, J.A.; Trueba, P.; Torres, Y. Porous titanium for biomedical applications: Evaluation of the conventional powder metallurgy frontier and space-holder technique. Appl. Sci. 2019, 9, 982.
- Cabezas-Villa, J.L.; Olmos, L.; Bouvard, D.; Lemus-Ruiz, J.; Jiménez, O. Processing and properties of highly porous Ti6Al4V mimicking human bones. J. Mater. Res. 2018, 33, 650–661.
- Chen, X.B.; Li, Y.C.; Hodgson, P.D.; Wen, C. The importance of particle size in porous titanium and nonporous counterparts for surface energy and its impact on apatite formation. Acta Biomater. 2009, 5, 2290–2302.
- de Daudt, N.; Bram, M.; Barbosa, A.P.C.; Laptev, A.M.; Alves, C. Manufacturing of highly porous titanium by metal injection molding in combination with plasma treatment. J. Mater. Process. Technol. 2017, 239, 202–209.
- Tuncer, N.; Bram, M.; Laptev, A.; Beck, T.; Moser, A.; Buchkremer, H.P. Study of metal injection molding of highly porous titanium by physical modeling and direct experiments. J. Mater. Process. Technol. 2014, 214, 1352–1360.
- Laptev, A.M.; Daudt, N.F.; Guillon, O.; Bram, M. Increased Shape Stability and Porosity of Highly Porous Injection-Molded Titanium Parts. Adv. Eng. Mater. 2015, 17, 1579–1587.
- Shbeh, M.; Oner, E.; Al-Rubaye, A.; Goodall, R. Production and Digital Image Correlation Analysis of Titanium Foams with Different Pore Morphologies as a Bone-Substitute Material. Adv. Mater. Sci. Eng. 2019, 2019, 1670837.
- Dehghan-Manshadi, A.; Yu, P.; Dargusch, M.; StJohn, D.; Qian, M. Metal injection moulding of surgical tools, biomaterials and medical devices: A review. Powder Technol. 2020, 364, 189–204.
- German, R.M. Progress in titanium metal powder injection molding. Materials 2013, 6, 3641–3662.
- Gülsoy, H.Ö.; Gülsoy, N.; Calişici, R. Particle morphology influence on mechanical and biocompatibility properties of injection molded Ti alloy powder. Bio-Med. Mater. Eng. 2014, 24, 1861–1873.
- Torres, Y.; Pavón, J.J.; Rodríguez, J.A. Processing and characterization of porous titanium for implants by using NaCl as space holder. J. Mater. Processing Technol. 2012, 212, 1061–1069.
- Baril, E.; Lefebvre, L.P.; Thomas, Y. Interstitial elements in titanium powder metallurgy: Sources and control. Powder Metall. 2011, 54, 183–187.
- Yu, C.; Cao, P.; Jones, M.I. Titanium powder sintering in a graphite furnace and mechanical properties of sintered parts. Metals 2017, 7, 67.
- Güden, M.; Çelik, E.; Hizal, A.; Altindiş, M.; Çetiner, S. Effects of compaction pressure and particle shape on the porosity and compression mechanical properties of sintered Ti6Al4V powder compacts for hard tissue implantation. J. Biomed. Mater. Res.-Part B Appl. BioMater. 2008, 85, 547–555.
- Thavanayagam, G.; Pickering, K.L.; Swan, J.E.; Cao, P. Analysis of rheological behaviour of titanium feedstocks formulated with a water-soluble binder system for powder injection moulding. Powder Technol. 2015, 269, 227–232.
- McCracken, C.G.; Barbis, D.P.; Deeter, R.C. Key characteristics of hydride-Dehydride titanium powder. Powder Metall. 2011, 54, 180–183.
- Hu, K.; Zou, L.; Shi, Q.; Hu, K.; Liu, X.; Duan, B. Effect of titanium hydride powder addition on microstructure and properties of titanium powder injection molding. Powder Technol. 2020, 367, 225–232.
- Peng, Q.; Yang, B.; Friedrich, B. Porous Titanium Parts Fabricated by Sintering of TiH2 and Ti Powder Mixtures. J. Mater. Eng. Perform. 2018, 27, 228–242.
- Ivasishin, O.M.; Savvakin, D.G.; Gumenyak, M.M.; Bondarchuk, O.B. Role of surface contamination in titanium PM. Key Eng. Mater. 2012, 520, 121–132.
- Ebel, T. Metal injection molding (MIM) of titanium and titanium alloys. In Handbook of Metal Injection Molding, 2nd ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 415–445.
- Park, S.J.; Wu, Y.; Heaney, D.F.; Zou, X.; Gai, G.; German, R.M. Rheological and thermal debinding behaviors in titanium powder injection molding. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2009, 40, 215–222.
- Vert, R.; Pontone, R.; Dolbec, R.; Dionne, L.; Boulos, M.I. Induction plasma technology applied to powder manufacturing: Example of Titanium-based materials. Key Eng. Mater. 2016, 704, 282–286.
- Liang, Y.; Wu, Y. Methods to Prepare Spherical Titanium Powders and Investigation on Spheroidization of HDH Titanium Powders. In Proceedings of the 13th World Conference on Titanium, San Diego, CA, USA, 16–20 August 2015; pp. 139–143.
- ASTM. ASTM Standard F67–00; Standard Specification for Unalloyed Titanium, for Surgical Implant Applications (UNS R50250, UNS R50400, UNS R50550, UNS R50700); ASTM International: West Conshohocken, PA, USA, 2000.
- ASTM. ASTM standard E 1409–05; Standard Test Method for Determination of Oxygen and Nitrogen in Titanium and Titanium Alloys by the Inert Gas Fusion Technique; ASTM International: West Conshohocken, PA, USA, 2005.
- ASTM. ASTM standard 817–08; Standard Specification for Powder Metallurgy (PM) Titanium Alloy Structural Components; ASTM International: West Conshohocken, PA, USA, 2008.
- Torres-Sanchez, C.; al Mushref, F.R.A.; Norrito, M.; Yendall, K.; Liu, Y.; Conway, P.P. The effect of pore size and porosity on mechanical properties and biological response of porous titanium scaffolds. Mater. Sci. Eng. C 2017, 77, 219–228.
- Xu, W.; Liu, Z.; Lu, X.; Tian, J.; Chen, G.; Liu, B.; Li, Z.; Qu, X.; Wen, C. Porous Ti-10Mo alloy fabricated by powder metallurgy for promoting bone regeneration. Sci. China Mater. 2019, 62, 1053–1064.
- Thieme, M.; Wieters, K.P.; Bergner, F.; Scharnweber, D.; Worch, H.; Ndop, J.; Kim, T.J.; Grill, W. Titanium powder sintering for preparation of a porous functionally graded material destined for orthopaedic implants. J. Mater. Sci. Mater. Med. 2001, 12, 225–231.
- Yu, P.; Stephani, G.; Luo, S.D.; Goehler, H.; Qian, M. Microwave-assisted fabrication of titanium hollow spheres with tailored shell structures for various potential applications. Mater. Lett. 2012, 86, 84–87.
- Oppenheimer, S.; Dunand, D.C. Solid-state foaming of Ti-6A1-4V by creep or superplastic expansion of argon-filled pores. Acta Mater. 2010, 58, 4387–4397.
- Neirinck, B.; Mattheys, T.; Braem, A.; Fransaer, J.; van der Biest, O.; Vleugels, J. Preparation of titanium foams by slip casting of particle stabilized emulsions. Adv. Eng. Mater. 2009, 11, 633–636.
- Rak, Z.S.; Walter, J. Porous titanium foil by tape casting technique. J. Mater. Processing Technol. 2006, 175, 358–363.
- Erk, K.A.; Dunand, D.C.; Shull, K.R. Titanium with controllable pore fractions by thermoreversible gelcasting of TiH2. Acta Mater. 2008, 56, 5147–5157.
- Chino, Y.; Dunand, D.C. Directionally freeze-cast titanium foam with aligned, elongated pores. Acta Mater. 2008, 56, 105–113.
- Özbilen, S.; Liebert, D.; Beck, T.; Bram, M. Fatigue behavior of highly porous titanium produced by powder metallurgy with temporary space holders. Mater. Sci. Eng. C 2016, 60, 446–457.
- Engin, G.; Aydemir, B.; Gülsoy, H.Ö. Injection molding of micro-porous titanium alloy with space holder technique. Rare Met. 2011, 30, 565–571.
- Esen, Z.; Bor, Ş. Characterization of Ti–6Al–4V alloy foams synthesized by space holder technique. Mater. Sci. Eng. A 2011, 528, 3200–3209.
- Wen, C.; Mabuchi, M.; Yamada, Y.; Shimojima, K.; Chino, Y.; Asahina, T. Processing of biocompatible porous Ti and Mg. Scr. Mater. 2001, 45, 1147–1153.
- Mansourighasri, A.; Muhamad, N.; Sulong, A.B. Processing titanium foams using tapioca starch as a space holder. J. Mater. Processing Technol. 2012, 212, 83–89.
- Shbeh, M.M.; Goodall, R. Design of water debinding and dissolution stages of metal injection moulded porous Ti foam production. Mater. Des. 2015, 87, 295–302.
- Thomsen, O.T.; Bozhevolnaya, E.; Lyckegaard, A. (Eds.) Sandwich Structures 7: Advancing with Sandwich Structures and Materials; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005.
- Malloy, R.A. Plastic Part Design for Injection Molding 2010; Carl Hanser Verlag GmbH & Company KG: Munich, Germany, 2005; Volume I–XIV.
- Barbosa, A.P.C.; Bram, M.; Stöver, D.; Buchkremer, H.P. Realization of a titanium spinal implant with a gradient in porosity by 2-component-metal injection moulding. Adv. Eng. Mater. 2013, 15, 510–521.
- Bootchai, S.; Taweejun, N.; Manonukul, A.; Kanchanomai, C. Metal Injection Molded Titanium: Mechanical Properties of Debinded Powder and Sintered Metal. J. Mater. Eng. Perform. 2020, 29, 4559–4568.
- Sidambe, A.T.; Derguti, F.; Todd, I. Metal Injection Moulding of Low Interstitial Titanium. Key Eng. Mater. 2020, 520, 145–152.
- Deing, A.; Luthringer, B.; Laipple, D.; Ebel, T.; Willumeit, R. A porous TiAl6V4 implant material for medical application. Int. J. BioMater. 2014, 2014, 904230.
- Ismail, M.H.; Goodall, R.; Davies, H.A.; Todd, I. Porous NiTi alloy by metal injection moulding/sintering of elemental powders: Effect of sintering temperature. Mater. Lett. 2012, 70, 142–145.
- Nor, N.H.M.; Muhamad, N.; Jamaludin, K.R.; Ahmad, S.; Ibrahim, M.H.I. Characterisation of titanium alloy feedstock for metal injection moulding using palm stearin binder system. Adv. Mater. Res. 2011, 264–265, 586–591.
- Jamaludin, K.R.; Muhamad, N.; Abolhasani, H.; Murtadhahadi; Rahman, M.N.A. An influence of a binder system to the rheological behavior of the SS316l Metal Injection Molding (MIM) feedstock. Adv. Mater. Res. 2011, 264–265, 554–558.
- Thian, E.S.; Loh, N.H.; Khor, K.A.; Tor, S.B. Effects of debinding parameters on powder injection molded Ti-6Al-4V/HA composite parts. Adv. Powder Technol. 2001, 12, 361–370.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
4 times
(View History)
Update Date:
13 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




