| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Parbati Phuyal | + 1562 word(s) | 1562 | 2020-09-25 04:09:39 | | | |
| 2 | Parbati Phuyal | -75 word(s) | 1487 | 2020-09-30 12:56:09 | | | | |
| 3 | Vicky Zhou | -2 word(s) | 1485 | 2020-10-10 08:30:34 | | | | |
| 4 | Vicky Zhou | + 56 word(s) | 1543 | 2020-10-10 08:31:36 | | | | |
| 5 | Vicky Zhou | + 3 word(s) | 1546 | 2020-10-12 06:08:34 | | | | |
| 6 | Vicky Zhou | Meta information modification | 1546 | 2020-10-23 11:30:00 | | | | |
| 7 | Vicky Zhou | -58 word(s) | 1488 | 2020-10-27 08:35:10 | | | | |
| 8 | Parbati Phuyal | + 17 word(s) | 1505 | 2020-10-27 09:32:26 | | |
Video Upload Options
Dengue and chikungunya are the vector-borne diseases, that are transmitted to humans by the mosquitoes Aedes aegypti and Aedes albopictus. This study aimed to show the spatial and temporal distribution of those diseases in the Hindu Kush Himalayan region.
1. Introduction
Dengue (DEN) is one of the fastest spreading infectious human diseases of the twenty-first century, and chikungunya (CHIK) is an emerging public health threat worldwide [1]. DEN is caused by the dengue virus (DENV), which is distinguished in 4 serotypes, DENV-1 to -4 and CHIK, by the chikungunya virus (CHIKV) [2][3]. According to estimates of the World Health Organization (WHO), around 100 million DEN infections occur worldwide annually, and approximately 2.5 billion of the world’s population live in DEN-endemic areas [4]. Thus, it has a major socioeconomic and public health impact on the epidemic regions [5]. As CHIKV/DENV/malaria share almost the same geographic areas and show similar clinical signs and symptoms, including fever, headache, nausea, and, in a few cases, hemorrhage, it is difficult to distinguish between these vector-borne diseases by clinical symptoms alone [4][6][7]. Due to this similarity of symptoms, misdiagnosis and under-reporting of actual DEN/CHIK cases in malaria-endemic areas are very common [7].
The distribution of these VBDs is generally determined by a complex dynamic of environmental and social factors [8]. Rapid unplanned urbanization, massive increases of international travel and trade, different agricultural practices, and other environmental changes can favor the new establishment and spread of vectors and can place healthy populations of nonendemic regions at risk [9]. In addition, vector control programs (e.g., vector surveillance, source reduction, elimination of container habitats) and socioeconomic (income, education, gender, education), medical (drug resistance), and climatic (seasonal weather variation, climatic variability, climate change) factors are highly likely to influence the epidemiology of VBDs [10].
The Hindu-Kush Himalayan (HKH) region is among the most diverse regions of the world in terms of environmental, sociocultural, and economic aspects [11]. This region covers a wide range of lowlands to highlands, extending from Afghanistan in the west to Myanmar in the east and includes all of Nepal and Bhutan and the mountainous areas of Afghanistan, Bangladesh, China, India, Myanmar, and Pakistan [11][12]. In 2017, approximately 240 million people were living in the HKH region, and, in 2030, the population is predicted to increase to ~300 million (www.icimod.org). Anthropogenic climate change, along with rapid landscape and demographic changes, is altering the environment of the HKH region dramatically, causing the shifting of disease vectors and disease transmission from tropical into temperate regions and highlands [12][13][14]. The literature reports outbreaks of VBDs from new areas of the HKH region and also a higher number of VBD cases, for example, in Nepal, the outbreak of 2019 [15].
2. DEN and CHIK in the Hindu Kush Himalayan Region
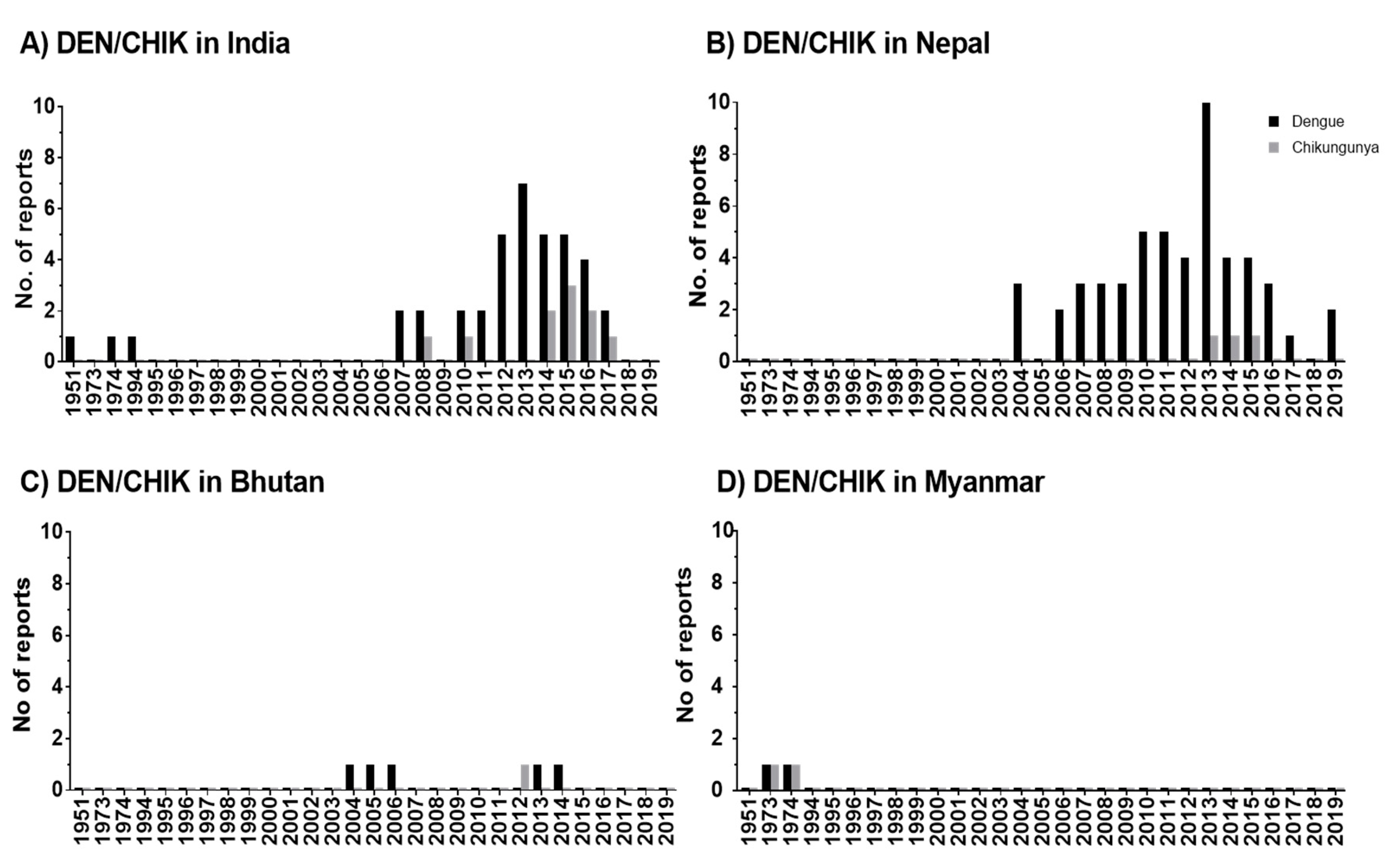
The studies reveal that DEN occurs in seven out of eight HKH countries and CHIK in four out of eight HKH countries. DEN fever emerged in the HKH region in 1951, thus, twenty years earlier than CHIK fever. An increase of reported DEN and CHIK cases in the HKH region was observed from 2004 onwards.
In the HKH region, DENV was first recognized in tea gardens of Northern Assam, India, in 1951 [16]. According to the literature, a large number of states (eight states and two provinces) in the Himalayan and sub-Himalayan region of India have been affected by DEN after 2005 onwards [17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][16] The virus circulating in India in the 1950s, causing mild diseases, was replaced or evolved into genotypes with bigger virulence and transmissibility [39]. The movement of the human population from DEN-endemic areas of India to nonendemic areas might have significantly contributed to the outbreak of DEN in new areas and more frequent DEN epidemics after 2005 [33].
The DEN cases in Pakistan were earlier reported among employees of a construction contractor at the power generation plant in Baluchistan in 1995 [40], but no further DEN cases were reported in this HKH country until 2007. Lack of proper surveillance systems or complexity in laboratory diagnosis due to infection with other diseases like malaria, typhoid, and hepatitis might cause an underreporting of cases [41]. In the meantime, frequently reported DEN incidence in this region from 2007 onwards [42] might be driven, in part, by increasing human mobility, particularly in areas with climatic suitability for the mosquito vector [43]. A mobile-phone-based study conducted in Pakistan shows the mobility of infected travelers from endemic regions to all other parts of the country during the outbreak in 2013 [44]. In Nepal as well, the improvement in social and infrastructure development such as the expansion of roads, rapid urbanization, and increase in trade and business opportunities after the end of decade-long armed conflict (1996–2006) has increased the mobility of people within and from neighboring countries [45]. This fact might be the cause for the rapid expansion of DENV in this HKH country, supported by entomological investigations that show the presence of Ae. albopictus in Nepal in 1956 already [46]. However, the primary vector for DEN Ae. aegypti was reported for the first time in 2006 from the lowlands of Nepal [47], later in 2009 from Kathmandu, which is a hilly region of Nepal [48], and reports of Ae. aegypti and Ae. albopictus from at least 2000 m above sea level [49]. In parallel, cases of DEN were reported from Terai (lowland) and hill and mountain regions (68 out of 77 districts of Nepal) in 2019 [15]. Although the recent study in Afghanistan [50] reported the first locally acquired DENV cases in 2019, our study shows that dengue antibodies were detected earlier, between 2010 and 2011, among Afghan National Army recruits in Afghanistan. However, DEN was distributed more frequently or rapidly in the HKH countries of India, Nepal, Pakistan, and Bhutan. China is the only HKH country with no reported cases of DEN in the HKH region.
The distribution of CHIK was observed in Myanmar [51], India [52][53][22][54][55][34], Nepal [56][57][58], and in Bhutan [59]. Although CHIK was observed quite early in Myanmar in 1973 and 1974, it was most frequently reported in India. However, no clear conclusions can be drawn for CHIK due to the low number of articles in our database and due to the unavailability of local governmental reports.
The period of outbreaks of DEN and CHIK varies only slightly within the HKH region. Most of studies from India [17][20][27][30][32] and Nepal [60][61][62][63][64][65][66][67] show almost consistent seasonality of DEN outbreaks, lasting from June to December. The majority of the studies from India and Nepal reported maximum DEN cases in September, October, and November in the postmonsoon period [17][60][18][61][20][21][22][23][62][26][27][64][28][65][31][66][35]. Our findings concerning a seasonal distribution of DEN in HKH countries are similar to the studies on Brazil [68] and Thailand [69]. These findings are further supported by entomological studies conducted in Nepal [49] and in Assam, India [70], that show a high abundance of vectors Ae. aegypti and Ae. albopictus at the end of the monsoon and postmonsoon season (September to November) compared to the winter season. The peak for DEN transmission during the postmonsoon season might be due to the most favorable weather conditions, including moderate rainfall, mild mean temperature, and optimum temperature range, which help vectors to breed, survive, and reproduce [49]. Temperature fluctuations also influence DEN infection in mosquitos [71]. Accordingly, adult Ae. aegypti live longer and, thus, were more likely to become infected under moderate temperature fluctuations. Moderate temperature fluctuations are typical during high DEN transmission season, whereas large temperature fluctuations favor a low dengue transmission season [71]. In Ae. albopictus, a temperature fluctuation from 28°-23°-18°C showed a probability of lower DEN transmission (in regard to virus titer in the salivary glands) than a constant 28 °C temperature [72]. Accordingly, vector activity and, thus, virus distribution are linked since temperatures influence, especially during the DEN season, virus transmission of the mosquitos.
The scientific reports of DEN and CHIK outbreaks in the HKH region have gradually increased from 2004/2005 until 2020. The reported incidence rates show an increase in disease burden over time (Figure 1). Accordingly, this increasing trend indicates the expansion of these disease vectors in the HKH region. The distributional shifts and increasing abundance of those disease vectors are also directly or indirectly influenced by weather variables such as temperature, humidity, and precipitation [33]. The Intergovernmental Panel on Climate Change (IPCC) also concludes that anthropogenic climate change, in particular, the changing temperature and precipitation patterns, has already altered the distribution of vector-borne diseases worldwide [73]. Nevertheless, the impacts of climate change on the distribution of DEN vectors should always be considered in the context of multiple social, behavioral, economic, environmental, and health system factors. For instance, human mobility from DEN endemic areas to non-DEN-endemic areas is vital for determining the changes in exposure and susceptibility to the DEN virus in the face of climate variability and change [74]. Furthermore, problems with water scarcity and, consequently, human behavior can even increase the breeding opportunities for DEN vectors [45]. The dry climate could force local communities to store water in containers, which ultimately increases the breeding sites for Ae. aegypti and Ae. albopictus [49][75].
Figure 1. Temporal distribution of DEN and CHIK reports in the HKH region of India (A), Nepal (B), Bhutan (C), and Myanmar (D). In publications, the study has been conducted within one or multiple years. Therefore, here, the numbers are given by considering the individual study year as individual reports. Zero reports of DEN/CHIK are indicated as 0.1 value.
References
- World Health Organization (WHO). Vector-Borne Diseases; An information Booklet; World Health Organization, Regional Office for South-East Asia: New Delhi, India, 2014.
- Seitz, R. Dengue fever virus (DENV). Transfus. Med. Hemotherapy 2011, 38, 318–330.
- Powers, A.M.; Logue, C.H. Changing patterns of chikunya virus: Re-emergence of a zoonotic arbovirus. J. Gen. Virol. 2007, 88, 2363–2377.
- World Health Organization. A global Brief on Vector-Borne Diseases; World Health Organization: Geneva, Switzerland, 2014.
- Gubler, D.J. The economic burden of dengue. Am. J. Trop. Med. Hyg. 2012, 86, 743–744.
- Leroy, E.M.; Nkoghe, D.; Ollomo, B.; Nze-Nkogue, C.; Becquart, P.; Grard, G.; Pourrut, X.; Charrel, R.; Moureau, G.; Ndjoyi-Mbiguino, A.; et al. Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg. Infect. Dis. 2009, 15, 591–593.
- Chahar, H.S.; Bharaj, P.; Dar, L.; Guleria, R.; Kabra, S.K.; Broor, S. Co-infections with chikungunya virus and dengue virus in Delhi, India. Emerg. Infect. Dis. 2009, 15, 1077–1080.
- WHO. Vector-Borne Diseases; World Health Organization: Geneva, Switzerland, 2009; pp. 1–3.
- WHO. New Vector Control Response Seen as Game-Changer. Available online: http://www.who.int/features/2017/new-vector-control/en/ (accessed on 5 February 2018).
- Githeko, A.; Lindsay, S.; Confalonieri, U.; Patz, J. Climate change and vector-borne diseases: A regional analysis. Bull. World Health Organ. 2000, 78, 1136–1147.
- Wester, P.; Mishra, A.; Mukherji, A.; Shrestha, A.B. The Hindu Kush Himalaya Assessment-Mountains, Climate Change, Sustainability and People; Wester, P., Mishra, A., Mukherji, A., Shrestha, A.B., Eds.; Springer Nature: Cham, Switzerland, 2019; ISBN 9783319922874.
- Singh, S.P.; Bassignana-Khadka, I.; Karky, B.S.; Sharma, E. Climate Change in the Hindu Kush-Himalayas: The State of Current Knowledge; International Centre for Integrated Mountain Development: Kathmandu, Nepal, 2011; ISBN1 978-92-9115-220-9. ISBN2 978-92-9115-221-6.
- Liu-Helmersson, J.; Stenlund, H.; Wilder-Smith, A.; Rocklöv, J. Vectorial capacity of Aedes aegypti: Effects of Temperature and Implications for Global Dengue Epidemic Potential. PLoS ONE 2014, 9, e89783.
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate warming. Science (80-) 2011, 333, 1024–1026.
- Pandey, B.D.; Costello, A. The Dengue Epidemic and Climate Change in Nepal. Lancet 2019, 394, 2150–2151.
- Birks, P.H. Dengue in Northern Assam tea gardens. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 195–200.
- Mondal, R.; Devi, N.P.; Jauhari, R.K. Dengue fever incidences and meteorological variables in Dehradun city ( Uttarakhand), India: Temporal Analysis. J. Exp. Zool. India 2018, 21, 985–989.
- Rahman, M.; Sharma, A.; Patgiri, S.; Hussain, E.; Borah, A.K.; Saikia, L. Serotyping of Degue Viruses Circulating During 2014-2015 in Assam, India. Indian J. Med. Microbiol. 2018, 36, 429–431.
- Borkakoty, B.; Das, M.; Sarma, K.; Jakharia, A.; Das, P.K.; Bhattacharya, C.; Apum, B.; Biswas, D. Molecular characterisation and phylogenetic anlysis of dengue outbreak in Pasighat, Arunachal Pradesh, Northeast India. Indian J. Med. Microbiol. 2018, 36, 37–42.
- Sargiary, P.; Das, A.; Rajkhowa, P.; Hussain, P.R.; Nath, R. First Outbreak of Dengue in Jorhat District of Assam. J. Clin. Dianostic Res. 2018, 12, 1–3.
- Singh, L.K.; Yengkokpam, C.; Singh, L.S. A Study of Seroprevalence and Changing Trend of Dengue in a Tertiary Care Hospital in Manipur. J. Evol. Med. Dent. Sci. 2018, 7, 3530–3535.
- Sudhan, S.S.; Sharma, M.; Sharma, P.; Gupta, R.K.; Sambyal, S.S.; Sharma, S. Serosurveillance of Dengue, Chikungunya and Zika in Jammu, a Sub-Himalayan Region of India. J. Clin. Diagnostic Res. 2017, 11, DC05–DC08.
- Singh, A.K.; Chawla, S.; Chawla, B.; Bhaglani, D.K.; Sharma, K.C. Role of a Surveillance System in the Management of an Outbreak of Dengue in the Mid Hills of Himachal Pradesh, India. J. Clin. Diagnostic Res. 2017, 11, LC01–LC05.
- Sapkota, S.; Bhandari, S.; Sapkota, S.; Hamal, R. Dengue and Scrub Typhus Coinfection in a Patient Presenting with Febrile Illness. Case Rep. Infect. Dis. 2017, 2017, 1–3.
- Ahmad, S.; Dhar, M.; Mittal, G.; Bhat, N.K.; Shirazi, N.; Kalra, V.; Sati, H.C.; Gupta, V. A comparative hospital-based observational study of mono- and co-infections of malaria, dengue virus and scrub typhus causing acute undifferentiated fever. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 705–711.
- Mondal, R.; Devi, N.P.; Jauhari, R.K. Studies on Symptomatic Profiles of Dengue Fever (DF) vis-a-vis Non-Dengue Fever (NDF) in District Dehradun, Uttarakhand. J. Commun. Dis. 2016, 48, 15–20.
- Sudhan, S.S.; Sharma, M.; Gupta, R.K.; Sambyal, S.S. Sero-Epidemiological trends of Dengue Fever in Jammu Province of J&K State. Int. J. Med. Res. Health Sci. 2016, 5, 1–6.
- Dev, V.; Mahanta, N.; Baruah, B.K. Dengue, an emerging arboviral infection in assam, northeast India. Trop. Biomed. 2015, 32, 796–799.
- Singh, R.; Singh, S.P.; Ahmad, N. A study of etiological pattern in an epidemic of acute febrile illness during monsoon in a tertiary health care institute of Uttarakhand, India. J. Clin. Diagnostic Res. 2014, 8, 1–3.
- Khan, S.A.; Dutta, P.; Topno, R.; Soni, M.; Mahanta, J. Dengue outbreak in a hilly state of Arunachal Pradesh in Northeast India. Sci. World J. 2014, 2014, 1–6.
- Khan, S.A.; Dutta, P.; Borah, J.; Chowdhury, P.; Doloi, P.K.; Mahanta, J. Dengue outbreak in an Indo-Myanmar boarder area: Epidemiological aspects and risk factors. Trop. Biomed. 2013, 30, 1–8.
- Ahmad, S.; Dhar, M.; Srivastava, S.; Bhat, N.K.; Shirazi, N.; Biswas, D.; Kadian, M.; Ghai, S. Dengue hepatitis sans dysfunction: Experience of a single tertiary referral centre in the north Indian state of Uttarakhand. Trop. Doct. 2013, 43, 62–65.
- Sankari, T.; Hoti, S.L.; Singh, T.B.; Shanmugavel, J. Outbreak of dengue virus serotype-2 (DENV-2) of Cambodian origin in Manipur, IndiaߞAssociation with meteorological factors. Indian J. Med. Res. 2012, 136, 649–655.
- Dutta, P.; Khan, S.A.; Khan, A.M.; Borah, J.; Chowdhury, P.; Mahanta, J. First evidence of chikungunya virus infection in Assam, Northeast India. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 355–357.
- Taraphdar, D.; Sarkar, A.; Bhattacharya, M.K.; Chatterjee, S. Sero diagnosis of dengue activity in an unknown febrile outbreak at the Siliguri Town, District Darjeeling, West Bengal. Asian Pac. J. Trop. Med. 2010, 3, 364–366.
- Agarwal, J.P.; Bhattacharyya, P.C.; Das, S.K.; Sharma, M.; Gupta, M. Dengue encephalitis. Southeast Asian J. Trop. Med. Public Health 2009, 40, 54–55.
- Baruah, H.C.; Mohapatra, P.K.; Kire, M.; Pegu, D.; Mahanta, J. Haemorrhagic Manifestations Associated with Dengue Virus Infection in Nagaland. J. Commun. Dis. 1996, 4, 301–303.
- Mathew, T.; Suri, J.C.; Suri, N.K.; Bhola, S.R.; Arora, R.R.; Lal, P.; Raichaudhari, A.N.; Mathur, K.K.; Gupta, J.P. Investigation on an epidemic of dengue in jammu, 1974. Indian J. Med. Res. 1977, 65, 613–622.
- Cecilia, D. Current status of dengue and chikungunya in India. WHO South-East Asia J. Public Health 2014, 3, 1–6.
- Paul, R.E.; Patel, A.Y.; Mirza, S.; Fisher-Hoch, S.P.; Luby, S.P. Expansion of epidemic dengue viral infections to Pakistan. Int. J. Infect. Dis. 1998, 2, 197–201.
- Ali, J. Dengue fever in Pakistan: Challenges, priorities and measures. J. Coast. Life Med. 2015, 3, 834–837.
- Hasan, Z.; Atkinson, B.; Jamil, B.; Samreen, A.; Altaf, L.; Hewson, R. Short report: Diagnostic testing for hemorrhagic fevers in Pakistan: 2007-2013. Am. J. Trop. Med. Hyg. 2014, 91, 1243–1246.
- Sutherst, R.W. Global Change and Human Vulnerability to Vector-Borne Diseases. Clin. Microbiol. Rev. 2004, 17, 136–173.
- Wesolowski, A.; Qureshi, T.; Boni, M.F.; Sundsøy, P.R.; Johansson, M.A.; Rasheed, S.B.; Engø-Monsen, K.; Buckee, C.O. Impact of human mobility on the emergence of dengue epidemics in Pakistan. Proc. Natl. Acad. Sci. USA 2015, 112, 11887–11892.
- Dhimal, M.; Ahrens, B.; Kuch, U. Climate change and spatiotemporal distributions of vector-borne diseases in Nepal - A systematic synthesis of literature. PLoS ONE 2015, 10, e0129869.
- Peters, W.; Dewar, S. A preliminary record of the megarhine and culicine mosquitoes of Nepal with notes on their taxonomy (Diptera: Culicidae). Indian J. Malariol. 1956, 1, 37–51.
- Malla, S.; Thakur, G.D.; Shrestha, S.K.; Banjeree, M.K.; Thapa, L.B.; Gongal, G.; Ghimire, P.; Upadhyay, B.P.; Gautam, P.; Khanal, S.; et al. Identification of all dengue serotypes in Nepal. Emerg. Infect. Dis. 2008, 14, 1669–1670.
- Gautam, I.; Dhimal, M.N.; Shrestha, S.R.; Tamrakar, A.S. First Record of Aedes aegypti (L.) Vector of Dengue Virus from Kathmandu, Nepal. J. Nat. Hist. Mus. 2009, 24, 156–164.
- Dhimal, M.; Gautam, I.; Kreß, A.; Müller, R.; Kuch, U. Spatio-Temporal Distribution of Dengue and Lymphatic Filariasis Vectors along an Altitudinal Transect in Central Nepal. PLoS Negl. Trop. Dis. 2014, 8, 1–30.
- Sahak, M.N. Dengue fever as an emerging disease in Afghanistan: Epidemiology of the first reported cases. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 20, 124658.
- Thaung, U.; Ming, K.C.; Swe, T.; Thein, S. Epidemiological features of dengue and chikungunya infections in Burma. Southeast Asian J. Trop. Med. Public Health 1975, 6, 276–283.
- Dutta, P.; Khan, S.A.; Phukan, A.C.; Hazarika, S.; Hazarika, N.K.; Khan, A.M.; Kaur, H. Surveillance of Chikungunya virus activity in some North-eastern states of India. Asian Pac. J. Trop. Med. 2019, 12, 19–25.
- Dutta, P.; Khan, S.; Chetry, S.; Apum, B. First report of Chikungunya virus circulation during a dengue outbreak in Arunachal Pradesh, a Northeastern state of India. Trop. Biomed. 2018, 35, 59–66.
- Dutta, P.; Khan, S.A.; Hazarika, N.K.; Chetry, S. Molecular and Phylogenetic Evidence of Chikungunya Virus Circulating in Assam, India. Indian J. Med. Microbiol. 2017, 35, 389–393.
- Khan, S.A.; Dutta, P.; Topno, R.; Borah, J.; Chowdhury, P.; Mahanta, J. Chikungunya outbreak in Garo Hills, Meghalaya: An epidemiological perspective. Indian J. Med. Res. 2015, 141, 591–597.
- Pandey, K.; Pandey, B.D.; Chaurasiya, R.R.; Thakur, M.; Neupane, B.; Shah, Y.; Tun, M.M.N.; Morita, K. Evidence of Chikungunya virus circulation in the Terai region of Nepal in 2014 and 2015. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 294–299.
- Pandey, B.D.; Neupane, B.; Pandey, K.; Tun, M.M.N.; Morita, K. Detection of Chikungunya Virus in Nepal. Am. J. Trop. Med. Hyg. 2015, 93, 697–700.
- Pun, S.B.; Bastola, A.; Shah, R. First Report of Chikungunya Virus Infection in Nepal. J. Infect. Dev. Ctries. 2014, 8, 790–792.
- Wangchuk, S.; Chinnawirotpisan, P.; Dorji, T.; Tobgay, T.; Dorji, T.; Yoon, I.K.; Fernandez, S. Chikungunya fever outbreak, Bhutan, 2012. Emerg. Infect. Dis. 2013, 19, 1681–1684.
- Acharya, B.K.; Cao, C.; Chen, W.; Pandit, S. Spatiotemporal Distribution and Geospatial Diffusion Patterns of 2013 Dengue Outbreak in Jhapa. Asia Pacific J. Public Health 2018, 30, 396–405.
- Khetan, R.P.; Stein, D.A.; Chaudhary, S.K.; Rauniyar, R.; Upadhyay, B.P.; Gupta, U.P.; Gupta, B.P. Profile of the 2016 dengue outbreak in Nepal. BMC Res. Notes 2018, 11, 1–6.
- Dumre, S.P.; Bhandari, R.; Shakya, G.; Shrestha, S.K.; Cherif, M.S.; Ghimire, P.; Klungthong, C.; Yoon, I.K.; Hirayama, K.; Na-Bangchang, K.; et al. Dengue Virus Serotypes 1 and 2 Responsible for Major Dengue Outbreaks in Nepal: Clinical, Laboratory, and Epidemiological Features. Am. J. Trop. Med. Hyg. 2017, 4, 1062–1069.
- Thapa, S.; Pant, N.D.; Shrestha, R.; Gc, G.; Shrestha, B.; Pandey, B.D.; Gautam, I. Prevalence of dengue and diversity of cultivable bacteria in vector Aedes aegypti (L.) from two dengue endemic districts, Kanchanpur and Parsa of Nepal. J. Health. Popul. Nutr. 2017, 36, 1–5.
- Gupta, B.P.; Singh, S.; Kurmi, R.; Malla, R.; Sreekumar, E.; Manandhar, K. Das. Re-emergence of dengue virus serotype 2 strains in the 2013 outbreak in Nepal. Indian J. Med. Res. 2015, 142, 1–6.
- Pandey, B.D.; Pandeya, K.; Neupane, B.; Shah, Y.; Adhikary, K.P.; Gautam, I.; Hagge, D.A.; Morita, K. Persistent dengue emergence: The 7 years surrounding the 2010 epidemic in Nepal. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 775–782.
- Pun, R.; Pant, K.P.; Bhatta, D.R.; Pandey, B.D. Acute Dengue Infection in the Western Terai Region of Nepal. J. Nepal Med. Assoc. 2011, 51, 11–14.
- Sah, O.P.; Subedi, S.; Morita, K.; Inone, S.; Kurane, I.; Pandey, B.D. Serological study of dengue virus infection in Terai region, Nepal. Nepal Med. Coll. J. 2009, 11, 104–106.
- Rosa-freitas, M.G.; Tsouris, P.; Sibajev, A.; Ferreira, R.L.; Luitgards-moura, J.F. Exploratory Temporal and Spatial Distribution Analysis of Dengue Notifications in Boa Vista, Roraima, Brazilian Amazon, 1999–2001. Dengue Bull. 2001, 27, 1999–2001.
- Wongkoon, S.; Jaroensutasinee, M.; Jaroensutasinee, K. Development of temporal modeling for prediction of dengue infection in Northeastern Thailand. Asian Pac. J. Trop. Med. 2012, 5, 249–252.
- Dev, V.; Khound, K.; Tewari, G. Dengue vectors in urban and suburban Assam, India: Entomological observations. WHO South-East Asia J. Public Health 2014, 3, 51–59.
- Lambrechts, L.; Paaijmans, K.P.; Fansiri, T.; Carrington, L.B.; Kramer, L.D.; Thomas, M.B.; Scott, T.W. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl. Acad. Sci. USA 2011, 1–6.
- Liu, Z.; Zhang, Z.; Lai, Z.; Zhou, T.; Jia, Z.; Gu, J.; Wu, K.; Chen, X.G. Temperature increase enhances Aedes albopictus competence to transmit dengue virus. Front. Microbiol. 2017, 8, 1–7.
- Woodward, A.; Smith, K.R.; Campbell-Lendrum, D.; Chadee, D.D.; Honda, Y.; Liu, Q.; Olwoch, J.; Revich, B.; Sauerborn, R.; Chafe, Z.; et al. Climate change and health: On the latest IPCC report. Lancet 2014, 383, 1185–1189.
- Rocklöv, J.; Tozan, Y. Climate change and the rising infectiousness of dengue. Emerg. Top. Life Sci. 2019, 3, 133–142.
- Dhimal, M.; Ahrens, B.; Kuch, U. Species composition, seasonal occurrence, habitat preference and altitudinal distribution of malaria and other disease vectors in eastern Nepal. Parasit. Vectors 2014, 7, 1–11.