Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Péter Hajdinák | -- | 2025 | 2022-05-11 11:19:34 | | | |
| 2 | Nora Tang | Meta information modification | 2025 | 2022-05-13 04:06:35 | | | | |
| 3 | Nora Tang | + 12 word(s) | 2037 | 2022-05-13 08:38:34 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hajdinák, P.; Szarka, A.; Lőrincz, T. The Dual Role of Reactive Oxygen Species. Encyclopedia. Available online: https://encyclopedia.pub/entry/22803 (accessed on 10 March 2026).
Hajdinák P, Szarka A, Lőrincz T. The Dual Role of Reactive Oxygen Species. Encyclopedia. Available at: https://encyclopedia.pub/entry/22803. Accessed March 10, 2026.
Hajdinák, Péter, Andras Szarka, Tamás Lőrincz. "The Dual Role of Reactive Oxygen Species" Encyclopedia, https://encyclopedia.pub/entry/22803 (accessed March 10, 2026).
Hajdinák, P., Szarka, A., & Lőrincz, T. (2022, May 11). The Dual Role of Reactive Oxygen Species. In Encyclopedia. https://encyclopedia.pub/entry/22803
Hajdinák, Péter, et al. "The Dual Role of Reactive Oxygen Species." Encyclopedia. Web. 11 May, 2022.
Copy Citation
The phrase “reactive oxygen species” (ROS) is commonly used to describe the highly reactive free radicals and molecules originating from molecular oxygen. This so-called bi-radical state of oxygen explains its reactivity: one of its electrons can be paired with an external electron with an antiparallel spin, resulting in the production of the highly reactive superoxide radical (O2·−). Since the superoxide radical is weakly basic and highly soluble in water at physiological pH, cellular membranes are relatively impermeable to it. However, O2·− can be converted into membrane-permeable H2O2 by superoxide dismutase (SOD) or protonated to hydroperoxyl radicals (HOO·). Furthermore, O2·− reacts with H2O2 through the Haber–Weiss reaction using iron catalysis, resulting in the formation of highly reactive and cytotoxic hydroxyl radicals (HO·). Furthermore, during the hydroperoxide and polyunsaturated fatty acid metabolism, other types of ROS, namely the peroxyl (ROO·) and alkoxyl (RO·) radicals, are formed as intermediates. On the one hand, high amounts of ROS may damage biomolecules due to their high reactivity. On the other hand—at strictly regulated levels—ROS are essential to maintaining the redox homeostasis of the cells, and they are engaged in many cellular signalling pathways, so their total elimination by the antioxidant system is not expedient.
reactive oxygen species
oxidative stress
cell death
1. Cellular Sources and Roles of ROS
ROS can be formed in many compartments of the cell (Figure 1). Among all compartments, mitochondria are the primary sources of ROS, since they are known to produce around 90% of the cellular ROS under physiological conditions [1]. This high ROS production rate is due to the involvement of mitochondria in oxidative phosphorylation, in which molecular oxygen is reduced to water. The mitochondrial electron transport chain may leak electrons, which results in the partial reduction of oxygen to O2·−. It has been reported that 0.2–2.0% of the molecular oxygen consumed by mitochondria is reduced to O2·− [1][2]. Overall, 70–80% of O2·− is generated in the Q cycle of complex III, but complex I is also considered to be a major source of mitochondrial ROS [3]. Glycerol 3-phosphate dehydrogenase, Q oxidoreductase, pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase may also generate O2·− [4][5][6]. Superoxide radicals are quickly transformed into H2O2 by SOD, which can be reduced to water by catalase or glutathione peroxidase, to avoid the formation of HO· [5][6][7].
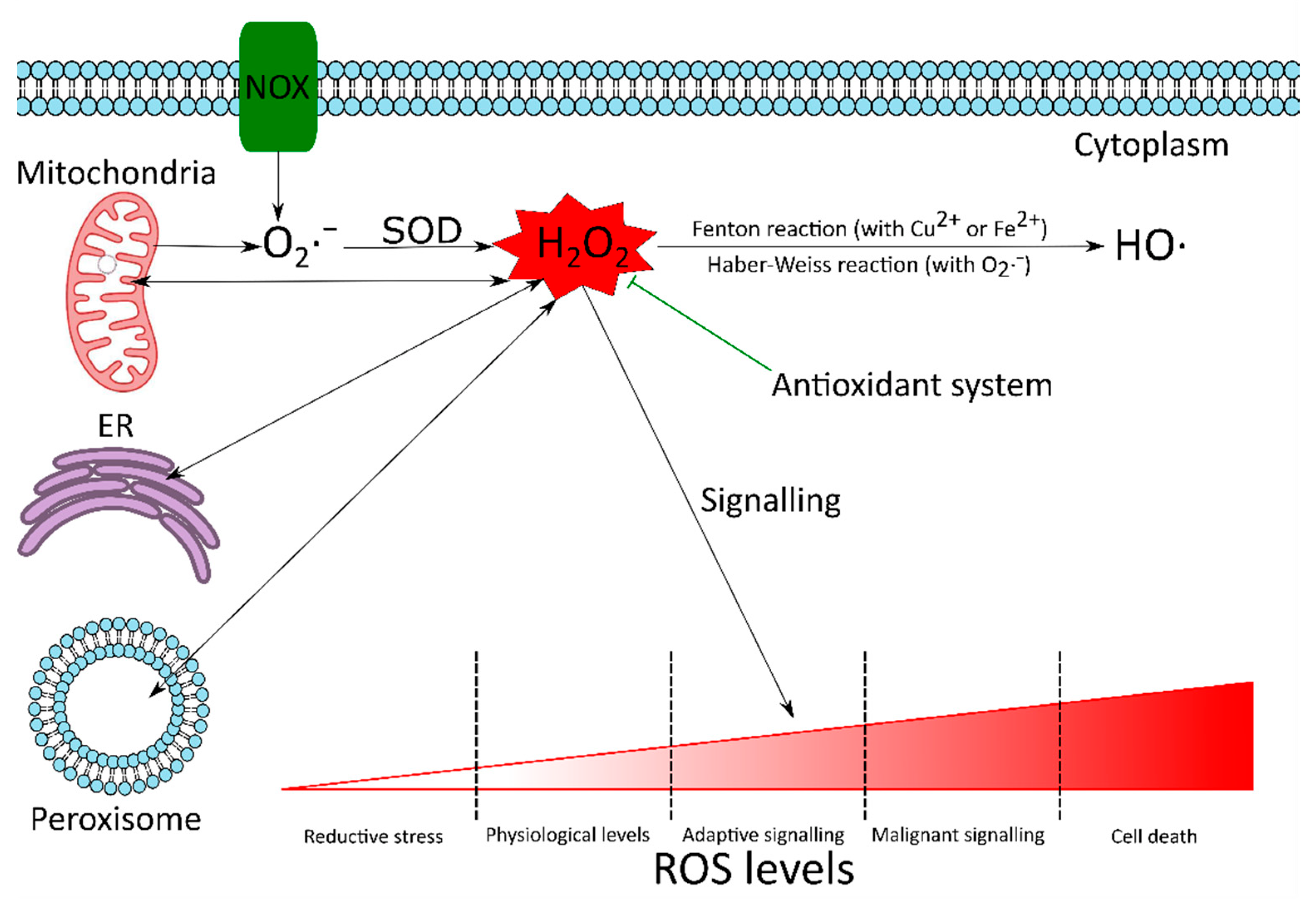
Figure 1. The dual role of ROS. There are many reactive oxygen species (ROS) sources in the cell. Although mitochondria are known to produce around 90% of the cellular ROS under physiological conditions, there are other notable sources too. These include NADPH oxidases (NOX), endoplasmic reticulum (ER) and peroxisomes. The superoxide radical (O2·−) produced in the cell is converted by superoxide dismutase (SOD) to hydrogen peroxide (H2O2). H2O2 can be converted to highly reactive and cytotoxic hydroxyl radical (HO·), via the Haber–Weiss (Equation (1)) or Fenton reactions (Equation (2)). Although ROS can cause oxidative damage to biomolecules, at strictly regulated levels they are required to maintain the redox homeostasis of the cell and are involved in adaptive signalling to overcome various stresses. If the antioxidant system fails to keep ROS under control, high ROS concentrations can initiate malignant signalling or cell death. Since H2O2 is relatively stable and can cross biological membranes, it is considered to be the most important redox signalling molecule.
O2·− + H2O2 → O2 + HO· + OH−
Fe2+ + H2O2 → Fe3+ + HO· + OH−
There are various sources of ROS with cytoplasmic origin. The most significant are the members of the NADPH oxidase (NOX) family [6][8]. The NOX family has seven members: NOX1–5 and dual oxidase 1 and 2 (DUOX1 and 2). These membrane-bound enzymes transfer an electron from NADPH across a biological membrane to reduce oxygen to superoxide radical, which is then spontaneously or enzymatically dismutated to H2O2 (Figure 1) [4][5][6][9].
ROS are also produced in the peroxisomes and the endoplasmic reticulum (Figure 1). Peroxisomes are one of the primary sources of H2O2, which is produced as a by-product of the abundant peroxisomal oxidases, such as acyl-CoA oxidases, xanthine oxidase and D-amino acid oxidase [4][6][10]. In the presence of transition metal ions, H2O2 can be rapidly converted to highly reactive, cytotoxic HO· via the Fenton reaction (Equation (2)) [4][11][12][13]. The endoplasmic reticulum (ER) plays an important role in many cellular functions, such as protein synthesis and folding, posttranslational modifications, regulation of the secretory pathway and calcium storage [6][11][14]. Disturbances in the redox homeostasis or folding apparatus can lead to the accumulation of unfolded and misfolded proteins, which activate a survival mechanism, called unfolded protein response (UPR). UPR aims to help the cells to restore homeostasis, but it also induces ROS production, which may promote ER stress further, resulting in additional ROS production. If the balance is not restored and UPR is prolonged, it may trigger apoptosis [4][6][7][14].
Cells have developed various antioxidant and repair mechanisms to prevent the oxidative damage caused by ROS. If the systemic production of ROS exceeds the cell’s ability to restore the redox homeostasis, then the increasing ROS levels may have harmful effects on cellular structures and functions, resulting in so-called oxidative stress. Oxidative stress has been associated with the development of various degenerative processes, diseases, syndromes and other pathologies. Furthermore, oxidative stress has a role in tumour initiation, progression and resistance to therapy (Figure 1) [4][7][9][13][15].
However, at strictly regulated levels, ROS are involved in many physiological redox signalling pathways (Figure 1). These include chemotaxis, immune response, cytoskeletal remodelling, calcium homeostasis, growth, differentiation, cell cycle progression and cell death. Thus, the antioxidant system does not aim to eliminate ROS totally, but to keep them under control [4][5][6][10][13][16][17][18][19].
H2O2 is often considered to be the most important redox signalling molecule because it is relatively stable in vivo and can cross biological membranes. Nevertheless, the dipole moment of H2O2 is higher than that of water, which severely limits its passive diffusion across biological membranes. The efficient transport of this molecule requires specific channel proteins, namely the aquaporins [10][11]. These give the cells the capability to spatially separate H2O2-producing and -consuming reactions and form intracellular H2O2 gradients to regulate the response of redox-sensitive systems finely [10].
In addition to normal physiological processes, ROS signalling may be involved in malicious processes, depending on cell type, environment and ROS source [6]. The involvement of ROS in malignant signalling also highlights the need for the elements of the antioxidant system to act orchestrated. For example, SOD increases the levels of H2O2. Without sufficient activity of enzymes that catalyse the conversion of H2O2, increased H2O2-dependent signalling may occur, resulting in various pathologies [20].
2. ROS in Cancer
Elevated ROS production can be observed in cancer cells compared to normal cells [4][7][11][12][15]. Multiple factors can have a role in increased ROS generation, such as enhanced metabolic activity, mitochondrial dysfunction, peroxisome activity, increased cellular receptor signalling, oncogene activity, and increased activity of ROS producing enzymes such as oxidases, cyclooxygenases, and lipoxygenases [11][21]. ROS in cancer cells may amplify the tumorigenic behaviour and facilitate the development of additional mutations promoting metastasis [15].
The oncogene signals that boost ROS production in cancer cells also reprogram the antioxidant system to withstand the constant oxidative stress and keep ROS production at nontoxic levels [8][21]. Accordingly, increased catalase levels were reported in breast cancer tissues, malignant mesothelioma and colorectal carcinoma. Furthermore, in addition to peroxisomal catalase, malignant cells may acquire membrane-associated catalase to increase their survival under oxidative stress. At the same time, several other studies found the downregulation of catalase [8][11][21].
ROS also increase the expression of genes under the control of nuclear factor erythroid 2 (Nrf2). These genes encode enzymes that take part in the biosynthesis, utilization and regeneration of glutathione, thioredoxin and NADPH. Furthermore, the expression of other antioxidant enzymes under the control of activator protein 1 (AP-1), nuclear factor κB (NF-κB), hypoxia-inducible transcription factor 1α (HIF-1α) and p53 is also increased [5][8][21].
Autophagy is also induced by ROS. Autophagy enhances cancer cell survival by increasing stress tolerance and supplying nutrients to meet the high metabolic and energetic demands of proliferating cancer cells by recycling biomolecules [8][22].
The induction of antioxidant systems and autophagy provides an interconnected and finely adjustable mechanism for cancer cells to survive and maintain the oxidative stress required for tumour development and progression [8][23].
However, it must be noted in general that the responses to ROS are highly complex and dependent on multiple factors, including the origin, the environment and stage of the tumours, and the types, levels, localisation and persistence of ROS (for an extensive review, see [20]).
3. ROS in Ischemia-Reperfusion Injury
Ischemia-reperfusion injury (IRI) occurs when the blood flow to an organ is interrupted (ischemia) and then re-established (reperfusion). IRI has a central role in the pathology of major cardiovascular diseases, such as stroke and myocardial infarction, and in organ injuries after their transplantation [24][25][26][27].
IRI is mediated by several factors, including ROS, produced in great amounts after reperfusion [24][25]. However, the main source of ROS during this oxidative burst is controversial, and it probably depends on tissue type. For example, mitochondria seem to be the primary ROS sources in the metabolically highly active brain and heart, while xanthine oxidase in the intestine. Furthermore, the physiological status of the tissue may also influence the relative contribution of enzymes to ROS production; for example, cytokines may up-regulate the expression of certain ROS-producing enzymes in inflamed tissues [27].
The mechanisms involved in the pathogenesis of IRI are multifactorial, complex and highly integrated [25]. During reperfusion, increased ROS production and mitochondrial calcium overload were observed. These are believed to play a critical role in the opening of mitochondrial permeability transition pore (mPTP), which is a crucial element of reperfusion damage [24][25][28]. mPTP is a non-selective channel across the inner and outer mitochondrial membranes, which allows the exchange of solutes between the mitochondrial matrix and the cytoplasm up to 1500 Da. The opening of mPTP is associated with the drastic and sustained depolarization of mitochondria and a burst of ROS production. The increased O2·− production due to mPTP opening may lead to further mPTP opening in adjacent mitochondria and eventually to cell death [24][27][29].
ROS may also act as signalling messengers to protect the cells and tissues against lethal oxidative stress induced by ischemia and subsequent reperfusion. During ischemic preconditioning (IPC), short, non-lethal cycles of ischemia and reperfusion are applied [24][25][30][31][32]. The moderate amounts of ROS produced during IPC activate signal transduction pathways by posttranslational modification of redox-sensitive proteins. The inhibition of mPTP opening is considered to be the final step of these pathways [24]. IPC also triggers further adaptive changes in the cells and tissues, including better electrolyte and acid-base homeostasis, lower ROS production, reduced inflammatory responses and improved microcirculatory perfusion [31]. All these changes protect the preconditioned tissues against severe IRI [24][25][30][31][32].
4. ROS in the Defence against Pathogens
High concentrations of ROS are not only harmful to the cell producing them but also to invading pathogens. Phagocytes such as macrophages, neutrophils, and dendritic cells generate large amounts of ROS in the phagolysosomes to kill microbes during the earliest stages of pathogen interactions [9][15][33][34][35].
The multi-subunit NOX2 mediates the phagocyte oxidative burst. After its assembly on the phagolysosomal membrane, NOX2 pumps electrons into the compartment to reduce molecular oxygen to O2·−, which is then dismutated to membrane-permeable H2O2. Furthermore, highly cytotoxic HO· is also formed via the Haber–Weiss reaction. In addition, the myeloperoxidase, found in the granules of neutrophils, catalyses the formation of further oxidants, such as hypochlorous acid, hypothiocyanous acid and hypobromous acid from H2O2 [9][15][36]. These compounds kill the engulfed microbes by oxidizing their biomolecules, including their DNA [33][35].
ROS produced by NOX2 also play a role in the formation of neutrophil extracellular traps (NETs) by activating granular proteases. NETs are extracellular structures composed of DNA and granule proteins, such as histones, neutrophil elastase, myeloperoxidase and cathepsin G. In these traps, extracellular pathogens are caught and killed [9][34][37].
Many loss-of-function alleles have been described for NOX2 NADPH oxidase subunits in humans, making NOX2 unable to produce ROS. The inability of phagocytes to produce ROS renders the innate immune system inefficient and results in chronic granulomatous disease. Patients with this life-threatening primary immunodeficiency suffer from recurrent bacterial and fungal infections and granuloma formation [9][15][33][34][38]. ROS are not only required in defence against pathogens, but to limit inflammation and immune response. Accordingly, the lack of NOX2-produced ROS is also associated with severe autoimmune diseases, such as autoimmune arthritis [38][39][40].
References
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 398.
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z. Bin Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15.
- Rigoulet, M.; Yoboue, E.D.; Devin, A. Mitochondrial ROS generation and its regulation: Mechanisms involved in H(2)O(2) signaling. Antioxid. Redox Signal. 2011, 14, 459–468.
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2019, 2019, 6175804.
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965.
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902.
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta-Mol. Cell Res. 2016, 1863, 2977–2992.
- Sznarkowska, A.; Kostecka, A.; Meller, K.; Bielawski, K.P. Inhibition of cancer antioxidant defense by natural compounds. Oncotarget 2017, 8, 15996–16016.
- Hoffmann, M.H.; Griffiths, H.R. The dual role of Reactive Oxygen Species in autoimmune and inflammatory diseases: Evidence from preclinical models. Free Radic. Biol. Med. 2018, 125, 62–71.
- Lismont, C.; Revenco, I.; Fransen, M. Peroxisomal Hydrogen Peroxide Metabolism and Signaling in Health and Disease. Int. J. Mol. Sci. 2019, 20, 3673.
- Szarka, A.; Kapuy, O.; Lőrincz, T.; Bánhegyi, G. Vitamin C and Cell Death. Antioxid. Redox Signal. 2021, 34, 831–844.
- Tóth, S.Z.; Lőrincz, T.; Szarka, A. Concentration Does Matter: The Beneficial and Potentially Harmful Effects of Ascorbate in Humans and Plants. Antioxid. Redox Signal. 2018, 29, 1516–1533.
- Gammella, E.; Recalcati, S.; Cairo, G. Dual Role of ROS as Signal and Stress Agents: Iron Tips the Balance in favor of Toxic Effects. Oxid. Med. Cell. Longev. 2016, 2016, 8629024.
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016, 73, 79–94.
- Bardaweel, S.K.; Gul, M.; Alzweiri, M.; Ishaqat, A.; ALSalamat, H.A.; Bashatwah, R.M. Reactive Oxygen Species: The Dual Role in Physiological and Pathological Conditions of the Human Body. Eurasian J. Med. 2018, 50, 193–201.
- Czobor, Á.; Hajdinák, P.; Szarka, A. Rapid ascorbate response to bacterial elicitor treatment in Arabidopsis thaliana cells. Acta Physiol. Plant. 2017, 39, 62.
- Czobor, Á.; Hajdinák, P.; Németh, B.; Piros, B.; Németh, Á.; Szarka, A. Comparison of the response of alternative oxidase and uncoupling proteins to bacterial elicitor induced oxidative burst. PLoS ONE 2019, 14, e0210592.
- Verbon, E.H.; Post, J.A.; Boonstra, J. The influence of reactive oxygen species on cell cycle progression in mammalian cells. Gene 2012, 511, 1–6.
- Hajdinák, P.; Czobor, Á.; Szarka, A. The potential role of acrolein in plant ferroptosis-like cell death. PLoS ONE 2019, 14, e0227278.
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297.
- Marengo, B.; Nitti, M.; Furfaro, A.L.; Colla, R.; De Ciucis, C.; Marinari, U.M.; Pronzato, M.A.; Traverso, N.; Domenicotti, C. Redox Homeostasis and Cellular Antioxidant Systems: Crucial Players in Cancer Growth and Therapy. Oxid. Med. Cell. Longev. 2016, 2016, 6235641.
- Yun, C.; Lee, S. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466.
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; LLeonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390.
- Cadenas, S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89.
- Sabet Sarvestani, F.; Azarpira, N.; Al-Abdullah, I.H.; Tamaddon, A.M. microRNAs in liver and kidney ischemia reperfusion injury: Insight to improve transplantation outcome. Biomed. Pharmacother. 2021, 133, 110944.
- Dar, W.A.; Sullivan, E.; Bynon, J.S.; Eltzschig, H.; Ju, C. Ischaemia reperfusion injury in liver transplantation: Cellular and molecular mechanisms. Liver Int. 2019, 39, 788–801.
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551.
- Garcia-Dorado, D.; Ruiz-Meana, M.; Inserte, J.; Rodriguez-Sinovas, A.; Piper, H.M. Calcium-mediated cell death during myocardial reperfusion. Cardiovasc. Res. 2012, 94, 168–180.
- Li, Y.; Sun, J.; Wu, R.; Bai, J.; Hou, Y.; Zeng, Y.; Zhang, Y.; Wang, X.; Wang, Z.; Meng, X. Mitochondrial MPTP: A Novel Target of Ethnomedicine for Stroke Treatment by Apoptosis Inhibition. Front. Pharmacol. 2020, 11, 352.
- Donato, M.; Evelson, P.; Gelpi, R.J. Protecting the heart from ischemia/reperfusion injury. Curr. Opin. Cardiol. 2017, 32, 784–790.
- Livingston, M.J.; Wang, J.; Zhou, J.; Wu, G.; Ganley, I.G.; Hill, J.A.; Yin, X.-M.; Dong, Z. Clearance of damaged mitochondria via mitophagy is important to the protective effect of ischemic preconditioning in kidneys. Autophagy 2019, 15, 2142–2162.
- Pan, T.; Jia, P.; Chen, N.; Fang, Y.; Liang, Y.; Guo, M.; Ding, X. Delayed Remote Ischemic Preconditioning ConfersRenoprotection against Septic Acute Kidney Injury via Exosomal miR-21. Theranostics 2019, 9, 405–423.
- Graham, D.B.; Becker, C.E.; Doan, A.; Goel, G.; Villablanca, E.J.; Knights, D.; Mok, A.; Ng, A.C.Y.; Doench, J.G.; Root, D.E.; et al. Functional genomics identifies negative regulatory nodes controlling phagocyte oxidative burst. Nat. Commun. 2015, 6, 7838.
- Moghadam, Z.M.; Henneke, P.; Kolter, J. From Flies to Men: ROS and the NADPH Oxidase in Phagocytes. Front. Cell Dev. Biol. 2021, 9, 618.
- Slauch, J.M. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol. Microbiol. 2011, 80, 580–583.
- Love, D.T.; Barrett, T.J.; White, M.Y.; Cordwell, S.J.; Davies, M.J.; Hawkins, C.L. Cellular targets of the myeloperoxidase-derived oxidant hypothiocyanous acid (HOSCN) and its role in the inhibition of glycolysis in macrophages. Free Radic. Biol. Med. 2016, 94, 88–98.
- Li, T.; Zhang, Z.; Li, X.; Dong, G.; Zhang, M.; Xu, Z.; Yang, J. Neutrophil Extracellular Traps: Signaling Properties and Disease Relevance. Mediat. Inflamm. 2020, 2020, 9254087.
- Zhong, J.; Olsson, L.M.; Urbonaviciute, V.; Yang, M.; Bäckdahl, L.; Holmdahl, R. Association of NOX2 subunits genetic variants with autoimmune diseases. Free Radic. Biol. Med. 2018, 125, 72–80.
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659.
- Martner, A.; Aydin, E.; Hellstrand, K. NOX2 in autoimmunity, tumor growth and metastasis. J. Pathol. 2019, 247, 151–154.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
3 times
(View History)
Update Date:
13 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





