Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nicolás Pedrini | -- | 1633 | 2022-05-10 16:55:09 | | | |
| 2 | Dean Liu | -1 word(s) | 1632 | 2022-05-11 03:33:54 | | | | |
| 3 | Dean Liu | -3 word(s) | 1629 | 2022-05-13 06:05:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pedrini, N. Beauveria bassiana Secondary Metabolite Gene Expression in Insects. Encyclopedia. Available online: https://encyclopedia.pub/entry/22779 (accessed on 07 February 2026).
Pedrini N. Beauveria bassiana Secondary Metabolite Gene Expression in Insects. Encyclopedia. Available at: https://encyclopedia.pub/entry/22779. Accessed February 07, 2026.
Pedrini, Nicolás. "Beauveria bassiana Secondary Metabolite Gene Expression in Insects" Encyclopedia, https://encyclopedia.pub/entry/22779 (accessed February 07, 2026).
Pedrini, N. (2022, May 10). Beauveria bassiana Secondary Metabolite Gene Expression in Insects. In Encyclopedia. https://encyclopedia.pub/entry/22779
Pedrini, Nicolás. "Beauveria bassiana Secondary Metabolite Gene Expression in Insects." Encyclopedia. Web. 10 May, 2022.
Copy Citation
Entomopathogenic fungi are extensively used for the control of insect pests worldwide. Among them, Beauveria bassiana (Ascomycota: Hypocreales) produce a plethora of toxic secondary metabolites that either facilitate fungal invasion or act as immunosuppressive compounds.
entomopathogenic fungi
virulence
nonribosomal peptides synthetases (NRPS)
polyketides synthetases(PKS)
1. Introduction
The fungus Beauveria bassiana (Ascomycota: Hypocreales) is a generalist (broad-spectrum host range) insect pathogen able to infect nearly 1000 insect species [1]. As for other entomopathogenic fungi of the order Hypocreales, the main route of infection for B. bassiana is the penetration of the insect cuticle, which represents the first encounter and barrier between the fungus and host [2]. Upon the adhesion to and recognition of the insect surface, B. bassiana deploys a combination of biochemical and mechanical tools to make its way through the insect integument into the hemocoel [3] (Figure 1). Once the fungus reaches this nutrient-rich environment, the mycelium switches to a specialized yeast-like cell phenotype; in invertebrate pathology, they are often referred as hyphal bodies or blastospores when they are produced artificially in culture media. At this stage, the insect host has very little chance of surviving the fungal infection despite the activation of the immune response (humoral and cellular) as a last-ditch attempt to overcome the fungus [4]. A successful fungal infection will then depend on the concerted combination of several events, one of the main ones being the production of a plethora of toxic secondary metabolites [5] that can either facilitate the fungal invasion [6] or act as immunosuppressive compounds, fighting against host defenses [7]. These secondary metabolites can have many different chemical natures, and include nonribosomal peptides and polyketides. Metarhizium spp. mainly produce destruxins (cyclic hexadepsipeptides), and Beauveria spp. synthesize beauvericin and bassianolide (cyclooligomeric nonribosomal peptides), a variety of beauverolides (cyclic peptides), oosporein (dibenzoquinone), bassiatin (diketomorpholine), and tenellin (2-pyridone) [5] (Figure 1). Even though the precise role of secondary metabolites is poorly understood, they are usually linked to the virulence of fungal strains [2][4][5][7]. Researchers aim to summarize the information available from transcriptomics (RNA-seq) and quantitative PCR (qPCR) studies related to the expression of B. bassiana genes involved in toxin production inside different insect orders as the infection progresses, to help understand how these toxins participate as virulence factors, antimicrobials, or immunosuppressives within the context of a fungus–insect interaction.
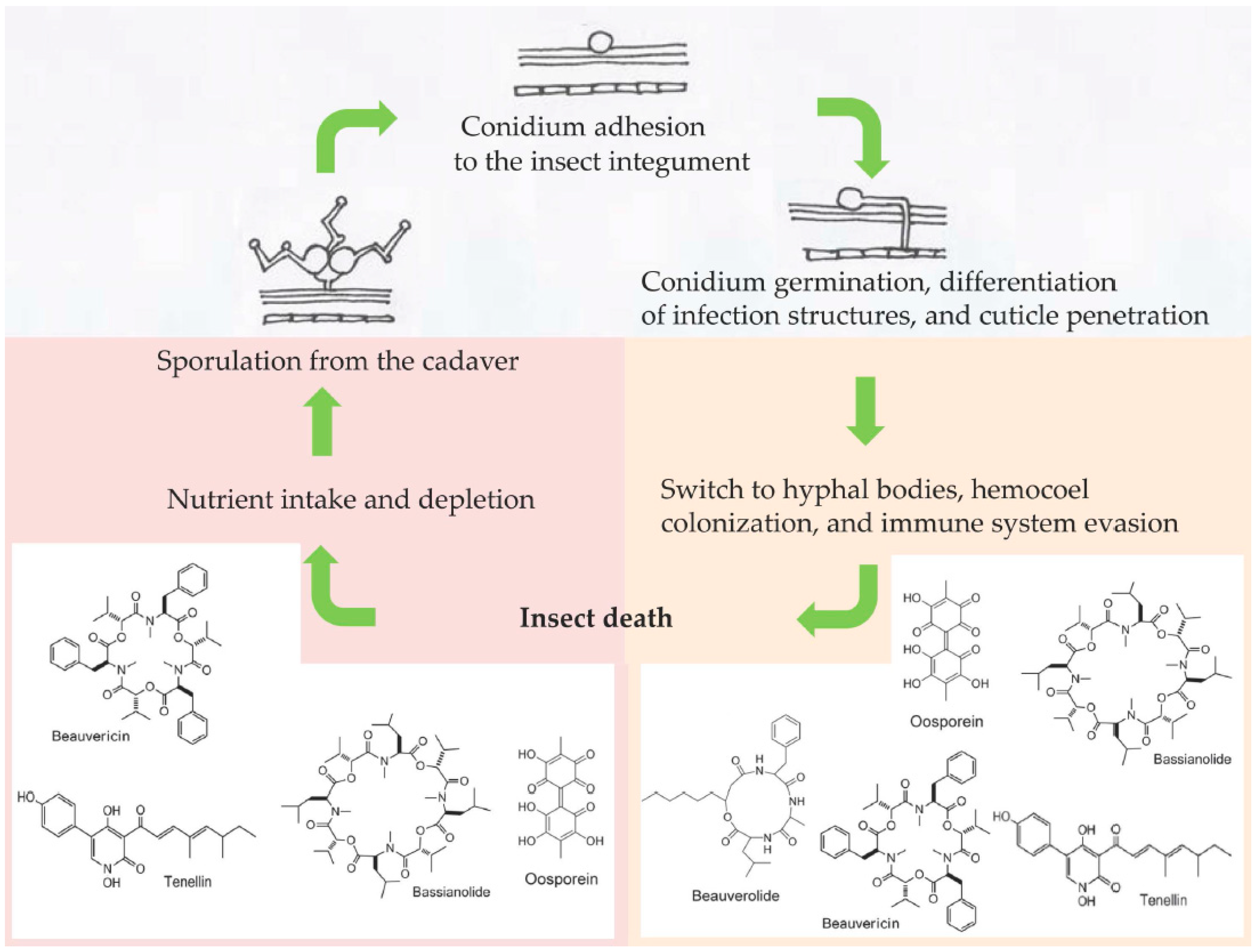
Figure 1. Schematic showing some of the secondary metabolites produced by Beauveria bassiana during the infection process in an insect host.
2. Role of Secondary Metabolites as Virulence Factors
Searching the information available reveals some clues, but no conclusive evidence, that entomopathogenic fungal secondary metabolites are produced within the insect as part of the infection process. A metabolomics approach found differences in secondary metabolite production on both live and dead tissues, assigning different purposes to different compounds; i.e., beauverolides are involved in killing the host, and destruxins mainly function as antimicrobials [8]. Oosporein also acts as an antimicrobial compound [9], but also promotes infection, probably by reducing the number of insect hemocytes, with the consequent alteration of the humoral immune system [10]. Entomopathogenic fungal secondary metabolites have been isolated from mycelia cakes, free-cell cultures [11][12], and also pooled insects infected with either Metarhizium spp. [13][14] or Beauveria spp. [15]. However, the analysis of individual mycosed insects rather than pooled samples is essential to provide an indication of the expression pattern of secondary metabolites during the time course of fungal infection [16]. A major hurdle to this is that the sensitivity of current analytical techniques does not permit the detection of the few fungal molecules that are expected to be produced inside the insect hemocoel. In this regard, analyzing the expression of genes involved in their biosynthesis when the fungus grows within its insect host could help to better elucidate their roles in pathogenesis.
Secondary metabolites are synthesized from gene clusters, including nonribosomal peptide synthetases (NRPSs), polyketide synthetases (PKSs), and hybrid NRPS–PKS genes [17][18]. Their induction is achieved when entomopathogenic fungi are confronted with whole insects or insect tissues, but several of the known clustered genes have no secondary metabolite assigned, and vice versa. In this regard, over 80% of the putative secondary-metabolite-associated genes identified in Metarhizium ssp. and B. bassiana have no identified specific products, and their sequences are unique to entomopathogenic fungi [19]. Although bassiacridin and beauverolides are secondary metabolites of currently unknown origin, there are many characterized metabolites with well-known biosynthetic pathways in B. bassiana, e.g., tenellin, beauvericin, oosporein, and bassianolide. Functional studies targeting B. bassiana NRPS and PKS have assigned very important functions in fungal development and virulence against insect hosts. PKSs are linked to asexual development and cell wall integrity; for example, the Bbpks11 gene acts in responses to oxidation, high temperature, and UV irradiation [20], and Bbpks15 is necessary for the formation of conidia and blastospores [21]. Regarding their roles in virulence, the bassianolide synthetase gene (BbbslS) is very important [22], the beauvericin synthetase gene (BbbeaS) participates, but its role is not key [23], the tenellin synthetase gene (BbtenS) does not contribute to virulence [24], and oosporein polyketide synthase (BbopS1) seems to directly participate in the evasion of insect immunity, and facilitates fungal growth [25]. More detailed information is available for oosporein expression; the zinc finger protein encoded by BbSmr1 negatively regulates oosporin production [26] by binding to the promoter region of the BbbrlA gene [27]. Using label-free quantitative proteomics, an increased level of oosporein biosynthetic enzymes was reported after the addition of exogenous oosporein into B. caledonica cultures [10]. The direct injection of oosporein did not kill the insects, but increased their susceptibility to subsequent fungal infection [10]; similar effects were described for beauverolides and destruxins [16]. For other secondary metabolites, individual applications did not cause significant mortality or macroscopical alterations in insects; however, this does not mean that they are not important, as they may act concertedly, and their exact roles are yet to be understood [16]. The key to unlocking the potential of the secondary metabolites is directly related to understanding and manipulating the complex regulatory networks controlling gene expression in fungi [16]. The biosynthesis of secondary metabolites is an energy-intensive process, and it occurs only under specific ecological conditions, e.g., when an insect’s immune system is attacking the pathogen [28]. Little is known about the environmental or host signals that are responsible for the induction of secondary-metabolite-synthesizing genes over the course of host infection, and there is still a lack of conclusive knowledge about their ecological function. For B. bassiana, it was proposed that the environmental signal for NRPS expression mainly comes from the insect host, and thus, there is a group of fast-evolving NRPSs (classified as group II, see below) that are closely associated with pathogenesis [29]. In summary, the scarcity of information about the biosynthetic pathways and products at the molecular level might be the main reason there is no extensive use of secondary metabolite genes from entomopathogenic fungi as a tool for controlling recombinant strains [30][31], although group II NRPS would be the best candidates to test in this regard.
3. Expression of Nonribosomal Peptide Synthetases (NRPSs) and Polyketide Synthetases (PKSs) during Pathogenesis
The B. bassiana genome harbors 21 NRPS or NRPS-like genes [32]. A comparative genomics study reported a high level of genetic diversity related to virulence between fungal isolates, and some of them show unique NRPS and PKS gene clusters [33]. Phylogenetics combined with comparative genomics also offers a tool useful for predicting the potential roles of B. bassiana’s secondary metabolites in effecting the fungus’ fitness and virulence. Of the 21 predicted NRPSs, two of them seem to have unknown functions, and the rest have been putatively categorized into two functional groups: seven NRPSs might be important during both saprophytic and pathogenic lifestyles and, thus, be related to basic metabolism (group I), and 12 NRPSs are likely to be involved in pathogenicity (group II), which includes the well-characterized genes BbbslS and BbbeaS [29]. In a dual transcriptomics approach between B. bassiana ARSEF 2860 and the diamondback moth Plutella xylostella, these group II NRPS genes were not found to be expressed [34]. However, the same interacting system (same fungal isolate and host) was assayed by qPCR [29], and all 12 group II NRPS genes were detected in three conditions: fungal growth at 6 or 9 days on artificial complete media, and 6 days after the infection of the diamondback moth. Based on the transcript levels during the last condition, half of the group II NRPSs were likely to be involved in infection, including BbbeaS, BbbslS, and BbtenS, as well as a gene that is speculated to encode a beauverolide synthetase (BBA_08222) [29]. Another group II NRPS (BBA_07611) has been identified as highly expressed in the hemocoel of the diamondback moth [29] and by transcriptomics in larvae of the wax moth Galleria mellonella [35]. On the other hand, the expression levels of PKAs are much less studied; the oosporein polyketide synthase BbopS1 is mainly expressed in insect cadavers at 24–48 h after death, reinforcing oosporein’s proposed role as an antimicrobial [26].
The first detailed study on the expression of fungal genes involved in the biosynthetic pathways for secondary metabolites inside insects, as well as the host response, was developed by Lobo et al. [36]. This absolute quantification using dual qPCR allowed them to observe that B. bassiana’s expression of toxin genes peaked during the first days of infection, with the toxins perhaps functioning as virulence factors, and later in moribund insects and/or cadavers to protect them from competitive microorganisms [36]. The type of NRPS genes expressed by B. bassiana is also related to the fungal isolate: with the same insect as the host (the kissing bug Triatoma infestans), the strain GHA mainly expresses BbbeaS [36], but the strain Bb-C001 mainly expresses BbbslS, with a lower levels of BbbeaS and BbtenS [37]. Both studies were performed at different time points after insect treatment with various concentrations of propagules, either by immersing the insects in conidial suspensions or by injecting them with blastospores, and the toxin profile was the same within each isolate. Despite this difference in toxic signature, the infections with both strains developed with similar virulence. This early evidence allows speculation that different fungal isolates can express different secondary metabolites during insect infection, perhaps in an overlapping manner, but their effects are the same in the general scheme of the infection process, i.e., culminating in host death. However, more case studies are needed to confirm these observations.
References
- Araújo, J.P.; Hughes, D.P. Diversity of entomopathogenic fungi: Which groups conquered the insect body? Adv. Genet. 2016, 94, 1–39.
- Pedrini, N. Molecular interactions between entomopathogenic fungi (Hypocreales) and their insect host: Perspectives from stressful cuticle and hemolymph battlefields and the potential of dual RNA sequencing for future studies (Review). Fungal Biol. 2018, 122, 538–545.
- Pedrini, N.; Crespo, R.; Juárez, M.P. Biochemistry of insect epicuticle degradation by entomopathogenic fungi. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 124–137.
- Butt, T.M.; Coates, C.J.; Dubovskiy, I.M.; Ratcliffe, N.A. Entomopathogenic fungi: New insights into host-pathogen interactions. Adv. Genet. 2016, 94, 307e364.
- Zhang, L.; Fasoyin, O.E.; Molnár, I.; Xu, Y. Secondary metabolites from hypocrealean entomopathogenic fungi: Novel bioactive compounds. Nat. Prod. Rep. 2020, 37, 1181–1206.
- Altimira, F.; Arias-Aravena, M.; Jian, L.; Real, N.; Correa, P.; González, C.; Godoy, S.; Castro, J.F.; Zamora, O.; Vergara, C.; et al. Genomic and experimental analysis of the insecticidal factors secreted by the entomopathogenic fungus Beauveria pseudobassiana RGM 2184. J. Fungi 2022, 8, 253.
- Lu, H.-L.; St Leger, R.J. Insect Immunity to Entomopathogenic Fungi. Adv. Genet. 2016, 94, 251–285.
- de Bekker, C.; Smith, P.B.; Patterson, A.D.; Hughes, D.P. Metabolomics reveals the heterogeneous secretome of two ento-mopathogenic fungi to ex vivo cultured insect tissues. PLoS ONE 2013, 8, e70609.
- Brewer, D.; Jen, W.-C.; Jones, G.A.; Taylor, A. The antibacterial activity of some naturally occurring 2,5-dihydroxy-l,4-benzoquinones. Can. J. Microbiol. 1984, 30, 1068–1072.
- Mc Namara, L.; Dolan, S.K.; Walsh, J.M.D.; Stephens, J.C.; Glare, T.R.; Kavanagh, K.; Griffin, C.T. Oosporein, an abundant metabolite in Beauveria caledonica, with a feedback induction mechanism and a role in insect virulence. Fungal Biol. 2019, 123, 601–610.
- Gupta, S.; Montllor, C.; Hwang, Y.-S. Isolation of Novel Beauvericin Analogues from the Fungus Beauveria bassiana. J. Nat. Prod. 1995, 58, 733–738.
- Xu, Y.; Zhan, J.; Wijeratne, E.M.K.; Burns, A.M.; Gunatilaka, A.A.L.; Molnár, I. Cytotoxic and Antihaptotactic Beauvericin Analogues from Precursor-Directed Biosynthesis with the Insect Pathogen Beauveria bassiana ATCC 7159. J. Nat. Prod. 2007, 70, 1467–1471.
- Amiri-Besheli, B.; Khambay, B.P.S.; Cameron, S.; Deadman, M.; Butt, T.M. Inter and intra-specific variation in destruxin production by insect pathogenic Metarhizium spp., and its significance to pathogenesis. Mycol. Res. 2000, 104, 447–452.
- Skrobek, A.; Shah, F.A.; Butt, T.M. Destruxin production by the entomogenous fungus Metarhizium anisopliae in insects and factors influencing their degradation. BioControl 2008, 53, 361–373.
- Strasser, H.; Abendstein, D.; Stuppner, H.; Butt, T.M. Monitoring the distribution of secondary metabolites produced by the entomogenous fungus Beauveria brongniartii with particular reference to oosporein. Mycol. Res. 2000, 104, 1227–1233.
- Molnár, I.; Gibson, D.M.; Krasnoff, S.B. Secondary metabolites from entomopathogenic Hypocrealean fungi. Nat. Prod. Rep. 2010, 27, 1241–1275.
- Süssmuth, R.; Muller, J.; von Dohren, H.; Molnar, I. Fungal cyclooligomer depsipeptides: From classical biochemistry to com-binatorial biosynthesis. Nat. Prod. Rep. 2011, 28, 99–124.
- Zhang, L.; Yue, Q.; Wang, C.; Xu, Y.; Molnár, I. Secondary metabolites from hypocrealean entomopathogenic fungi: Genomics as a tool to elucidate the encoded parvome. Nat. Prod. Rep. 2020, 37, 1164–1180.
- Gibson, D.M.; Donzelli, B.G.G.; Krasnoff, S.B.; Keyhani, N.O. Discovering the secondary metabolite potential encoded within entomopathogenic fungi. Nat. Prod. Rep. 2014, 31, 1287–1305.
- Meng, X.; Liao, Z.; Liu, T.; Hussain, K.; Chen, J.; Fang, Q.; Wang, J. Vital roles of Pks11, a highly reducing polyketide synthase, in fungal conidiation, antioxidant activity, conidial cell wall integrity, and UV tolerance of Beauveria bassiana. J. Invertebr. Pathol. 2021, 181, 107588.
- Udompaisarn, S.; Toopaang, W.; Sae-Ueng, U.; Srisuksam, C.; Wichienchote, N.; Wasuwan, R.; Nahar, N.A.; Tanticharoen, M.; Amnuaykanjanasin, A. The polyketide synthase PKS15 has a crucial role in cell wall formation in Beauveria bassiana. Sci. Rep. 2020, 10, 12630.
- Xu, Y.; Orozco, R.; Wijeratne, E.M.K.; Espinosa-Artiles, P.; Gunatilaka, A.A.L.; Stock, S.P.; Molnar, I. Biosynthesis of the cy-clooligomer depsipeptide bassianolide, an insecticidal virulence factor of Beauveria bassiana. Fungal Genet. Biol. 2009, 46, 353–364.
- Xu, Y.; Orozco, R.; Wijeratne, E.M.K.; Gunatilaka, A.A.L.; Stock, S.P.; Molnár, I. Biosynthesis of the Cyclooligomer Depsipeptide Beauvericine, a Virulence Factor of the Entomopathogenic Fungus Beauveria bassiana. Chem. Biol. 2008, 15, 898–907.
- Eley, K.L.; Halo, L.M.; Song, Z.; Powles, H.; Cox, R.J.; Bailey, A.M.; Lazarus, C.M.; Simpson, T.J. Biosynthesis of the 2-Pyridone Tenellin in the Insect Pathogenic FungusBeauveria bassiana. ChemBioChem 2007, 8, 289–297.
- Feng, P.; Shang, Y.; Cen, K.; Wang, C. Fungal biosynthesis of the bibenzoquinone oosporein to evade insect immunity. Proc. Natl. Acad. Sci. USA 2015, 112, 11365–11370.
- Fan, Y.; Liu, X.; Keyhani, N.O.; Tang, G.; Pei, Y.; Zhang, W.; Tong, S. Regulatory cascade and biological activity of Beauveria bassiana oosporein that limits bacterial growth after host death. Proc. Natl. Acad. Sci. USA 2017, 114, E1578–E1586.
- Chen, J.-F.; Liu, Y.; Tang, G.-R.; Jin, D.; Chen, X.; Pei, Y.; Fan, Y.-H. The secondary metabolite regulator, BbSmr1, is a central regulator of conidiation via the BrlA-AbaA-WetA pathway in Beauveria bassiana. Environ. Microbiol. 2021, 23, 810–825.
- Rohlfs, M.; Churchill, A.C.L. Fungal secondary metabolites as modulators of interactions with insects and other arthropods. Fungal Genet. Biol. 2011, 48, 23–34.
- Liu, H.; Xie, L.; Wang, J.; Guo, Q.; Yang, S.; Liang, P.; Wang, C.; Lin, M.; Xu, Y.; Zhang, L. The Stress-Responsive and Host-Oriented Role of Nonribosomal Peptide Synthetases in an Entomopathogenic Fungus, Beauveria bassiana. J. Microbiol. Biotechnol. 2017, 27, 439–449.
- Zhao, H.; Lovett, B.; Fang, W. Genetically Engineering Entomopathogenic Fungi. Adv. Genet. 2016, 94, 137–163.
- Lovett, B.; Leger, R.J.S. Genetically engineering better fungal biopesticides. Pest Manag. Sci. 2018, 74, 781–789.
- Xiao, G.; Ying, S.H.; Zheng, P.; Wang, Z.L.; Zhang, S.; Xie, X.Q.; Feng, M.G. Genomic perspectives on the evolution of fungal entomopatho-genicity in Beauveria bassiana. Sci. Rep. 2012, 2, 483.
- Valero-Jiménez, C.A.; Faino, L.; Veld, D.; Smit, S.; Zwaan, B.J.; Van Kan, J.A.L. Comparative genomics of Beauveria bassiana: Uncovering signatures of virulence against mosquitoes. BMC Genom. 2016, 17, 986.
- Chu, Z.-J.; Wang, Y.-J.; Ying, S.-H.; Wang, X.-W.; Feng, M.-G. Genome-Wide Host-Pathogen Interaction Unveiled by Transcriptomic Response of Diamondback Moth to Fungal Infection. PLoS ONE 2016, 11, e0152908.
- Dong, W.-X.; Ding, J.-L.; Gao, Y.; Peng, Y.-J.; Feng, M.-G.; Ying, S.-H. Transcriptomic insights into the alternative splic-ing-mediated adaptation of the entomopathogenic fungus Beauveria bassiana to host niches: Autophagy-related gene 8 as an example. Environ. Microbiol. 2017, 19, 4126–4139.
- Lobo, L.S.; Luz, C.; Fernandes, É.K.; Juárez, M.P.; Pedrini, N. Assessing gene expression during pathogenesis: Use of qRT-PCR to follow toxin production in the entomopathogenic fungus Beauveria bassiana during infection and immune response of the insect host Triatoma infestans. J. Invertebr. Pathol. 2015, 128, 14–21.
- Baldiviezo, L.V.; Pedrini, N.; Santana, M.; Mannino, M.C.; Nieva, L.B.; Gentile, A.; Cardozo, R.M. Isolation of Beauveria bassiana from the Chagas Disease Vector Triatoma infestans in the Gran Chaco Region of Argentina: Assessment of Gene Expression during Host–Pathogen Interaction. J. Fungi 2020, 6, 219.
More
Information
Subjects:
Mycology; Entomology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
3 times
(View History)
Update Date:
13 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




