Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ilya Ulasov | -- | 2111 | 2022-05-10 12:44:50 | | | |
| 2 | Rita Xu | -3 word(s) | 2108 | 2022-05-11 04:05:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ulasov, I.; Tsibulnikov, S.; , .; Timashev, P. Stem Cells Homing to Glioblastoma. Encyclopedia. Available online: https://encyclopedia.pub/entry/22766 (accessed on 01 March 2026).
Ulasov I, Tsibulnikov S, , Timashev P. Stem Cells Homing to Glioblastoma. Encyclopedia. Available at: https://encyclopedia.pub/entry/22766. Accessed March 01, 2026.
Ulasov, Ilya, Sergey Tsibulnikov, , Peter Timashev. "Stem Cells Homing to Glioblastoma" Encyclopedia, https://encyclopedia.pub/entry/22766 (accessed March 01, 2026).
Ulasov, I., Tsibulnikov, S., , ., & Timashev, P. (2022, May 10). Stem Cells Homing to Glioblastoma. In Encyclopedia. https://encyclopedia.pub/entry/22766
Ulasov, Ilya, et al. "Stem Cells Homing to Glioblastoma." Encyclopedia. Web. 10 May, 2022.
Copy Citation
Multiple efforts are currently underway to develop targeted therapeutic deliveries to the site of glioblastoma progression. The use of carriers represents advancement in the delivery of various therapeutic agents as a new approach in neuro-oncology. Mesenchymal stem cells (MSCs) and neural stem cells (NSCs) are used because of their capability in migrating and delivering therapeutic payloads to tumors.
stem cells
glioblastoma
1. Introduction
Glioblastoma (GBM) is the most common and aggressive primary tumor in adults. According to the classification of the World Health Organization (WHO), glioblastoma belongs to the fourth type of malignancy of intracranial neoplasms. Even though this type of tumor is the most common representative of primary brain tumors, the incidence of the disease in humans is relatively low—3.1 cases per 100,000 population (for comparison, 171.2 and 201.4 cases per 100,000 population were noted for breast and prostate tumors, respectively) [1]. It is well-established that the uncontrolled growth of glial cells stimulates aggressive tumor growth that may lead to the death of patients. Anticancer therapy could increase the survival of patients with neoplasms. A group of scientists demonstrated that the survival rate within five years does not exceed 5%, and the median survival rate reaches 15 months after treatment, including surgery combined with radiation and chemotherapy. As shown earlier [2], the low survival rate of patients with GBM is based on the adaptive response of tumor cells to therapy. An adaptive response is expressed in the activation of the growth of tumor cells by an autocrine mechanism and the induction of cellular factors that cause inflammation and immunosuppression of the tumor [3][4][5]. The standard methods of treatment do not increase the survival rate. Experimental methods of glioblastoma therapy are being actively developed that reach the effectiveness of the existing ones. On the other hand [6], the use of cancer vaccines, which are based on the activation of the acquired immunity of a patient in response to tumor-specific antigens [7], is promising for experimental oncology. A vaccine can be both individual peptides [8][9], which is promising for experimental oncology, and whole autologous antigen-presenting cells [10]. However, the use of a platform based on human and animal viruses seems to be a more effective approach to inducing an immune response in tumors. This is explained by the fact that the virus can be used to deliver and express a library of tumor antigens and succinates the activation of the immune response and the elimination of tumors and metastases. On the other hand, the natural mechanism of viral replication makes it possible to obtain recombinant proteins in significant volumes. There are approaches involving the use of viruses, both as vectors for gene therapy [11] and as oncolytic agents [12], delivering immunogenic proteins or antisense RNAs to tumor proteins that promote carcinogenesis [13]. At the same time, native immune responses can be one of the obstacles that decrease the anticancer efficacy of viral vectors against cancer cells [6]. For example, the pool of the neutralization of antibodies against human adenovirus type 5 decreases the efficacy of CRAd (Conditionally replicated adenoviruses)-based vectors using the human adenovirus type 5 genome [14][15]. Additionally, low diffusion through the tumor mass [16] and the activation of cellular protein kinases [17], for example, modulate the inflammatory immune response and overall decrease the therapeutic response to the selected vectors. To overcome those issues and still specifically deliver a gene therapy vector to the target cells, stem cell carriers can be of interest.
2. General Characteristics of Mesenchymal Stem Cells (MSC)
Mesenchymal stem cells (MSCs) are a heterogeneous population of fibroblast-like cells. MSCs represent a small population (0.01% of all nucleated cells found in bone marrow) [18]. MSCs are multipotent and differentiate into chondrocytes, skeletal myocytes, and neurons, if applicable. As noted earlier, more than 95% of MSCs express a high level of CD73, CD90, and CD105 and a low level of protein II (the major histocompatibility complex (MHC-II)) and CD45, CD34, CD14, CD11b, and CD19 on their surface. MSCs can express other markers in various combinations dependent on the tissue specificity. In 2011, Ode et al. provided a mechanistic insight into the biological significance of surface receptors for MSC migration. CD73/CD29 were among the antigens that were described in the study and implemented in MSC migration. It turns out that MSC migration is controlled by CD73/CD29 and their downstream targets such as Lck, Fyn, and Src-family kinase activation [19].
3. MSC—Cell Vectors for Migration and Delivery of Therapeutic Proteins
MSCs expressing protein molecules have been used in preclinical studies to successfully deliver antigens and induce a therapeutic effect in human and animal glioblastoma cells. As previously stated, stem cells were used to deliver viruses [20][21][22], suicide proteins from herpes simplex viruses like thymidine kinase (TK) [23][24][25], cellular miRNA [26], exosomes [27], apoptosis-induced proteins like TNF (tumor necrosis factor)-related apoptosis-inducing ligand (TRAIL) [28], cytosine deaminase (CD) [29], carboxylesterase 2 (sCE2, a prodrug-activating enzyme) [30], antiangiogenic thrombospondin-1 [31], and chemical agents such as paclitaxel [32] that activates the T-cell response in glioblastomas [33] (Figure 1).
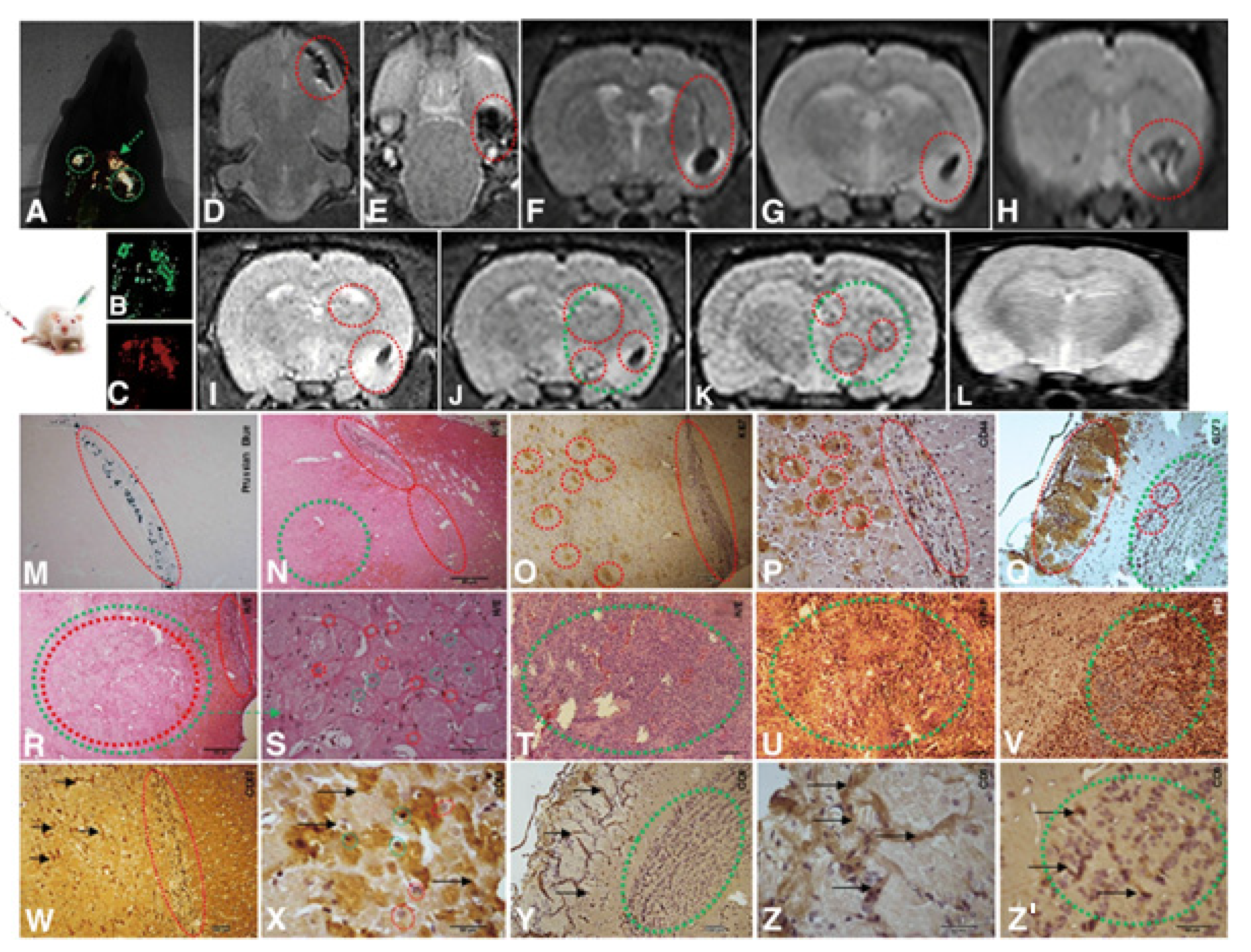
Figure 1. Implantation of 1 × 104 CD133+ GBM cells labeled Qdots (705 nm). After the establishment of the GBM (28 days), there was an infusion in caudal vein 1 × 104 MSCs (MION-Rh); the development of the tumor was followed for 20 days. (A) MSCs labeled MION-Rh and CD133+ GBM cells labeled Qdot 705 nm using combined fluorescence and X-ray detection. (B) CD133+ GBM cells labeled Qdot 705 nm and visualized by fluorescence detection. (C) MSCs labeled MION-Rh and visualized by fluorescence detection. (D–L) MRI (T2*-weighted images) of animal brain monitoring of the process of migration of MSCs, which were able to cross the blood–brain barrier of the animal and migrated to the tumor region, promoting GBM cell proliferation. (L) MRI (T2*-weighted images) of the animal brain without the stereotaxic implantation of cells (control group). The red dot circle shows migration assays of MSCs, and the green dot circle shows tumor propagation. (M) IHC analysis for Prussian blue staining of the MSCs labeled with MION-Rh. (N,R–T) Hematoxylin and eosin staining. (U) IHC analysis for GFAP. (O) IHC analysis for Ki67. (V) IHC analysis for p53. (P) IHC analysis for CD44 staining of the MSCs. (Q) IHC analysis for CD73 staining of the MSCs. (W,X) IHC analysis for CD63 staining of the MSC-derived exosomes. (Y,Z,Z′) IHC analysis for CD9 staining of the MSCs-derived exosomes. Scale, 40 µm. These images are representative of all the collected MSCs and GBM samples. Image and text were copied from the original study by Pavon et al. [34] and distributed under Attribution 4.0 International (CC BY 4.0).
Typically, MSCs are effective in delivering therapeutic proteins into diffuse tumors such as glioblastoma. This fact improves the following activation of the migration and adhesion of genetically modified MSC due to the concentration gradient mediated by SDF-1α, TGF-β1 (transforming growth factor beta), and CCL2 (C-C motif ligand 2) around the tumor. MSC demonstrated an increased migratory potential towards tumor cells [35][36][37]. Thus, MSC migration depends mainly on the expression of the CCR2 and CXCR4 receptors, since their inhibition with specific antibodies leads to a decrease in orchestrated cellular movements [34][38]. At the molecular level, binding ligands to these receptors enhances the expression of transcription factors in MSCs, which is required for stem cell differentiation [39][40]. Furthermore, Pavon et al. [34] demonstrated the endpoint of MSC migration to GBM, which is the area enriched with CD133-positive stem cells (GSC) (Figure 1). It has been shown by multiple investigators that GSCs responsible for tumor growth in vivo [41][42][43] and resistance to therapy [44] are located in anatomical structures. In such a periarteriolar niche [45] with elevated SDF-1α, various cathepsin [46] expressions and, adjacent to the arteriolar tunica adventitia, the CXCR4-enrcihed GSC interact with various cells, including MSC [47] (Figure 2). It has also been proposed that CXCR4, which is highly expressed in MSCs and endothelial cells (EC) [48], upregulates the expression of BDNF and EOMES genes, both of which are important for GSC stemness [48], inhibits the antitumor immune response caused by T-cell exhaustion, and regulates IFN-γ [49] expression. Although, in the glioma niche, the scientific evidence points to MSC-mediated direct interactions with GSC via the paracrine way [50], the presence of immune cells, astrocytes, and/or neural stem/precursor cells (NPC) in GBM [51] might also help to maintain the GSC phenotype and aggressiveness. Considering the localization of GSC in the perialveolar niche surrounded by hypoxic and highly vascularized regions, the targeting and eradication of GSC represent a clinical challenge.
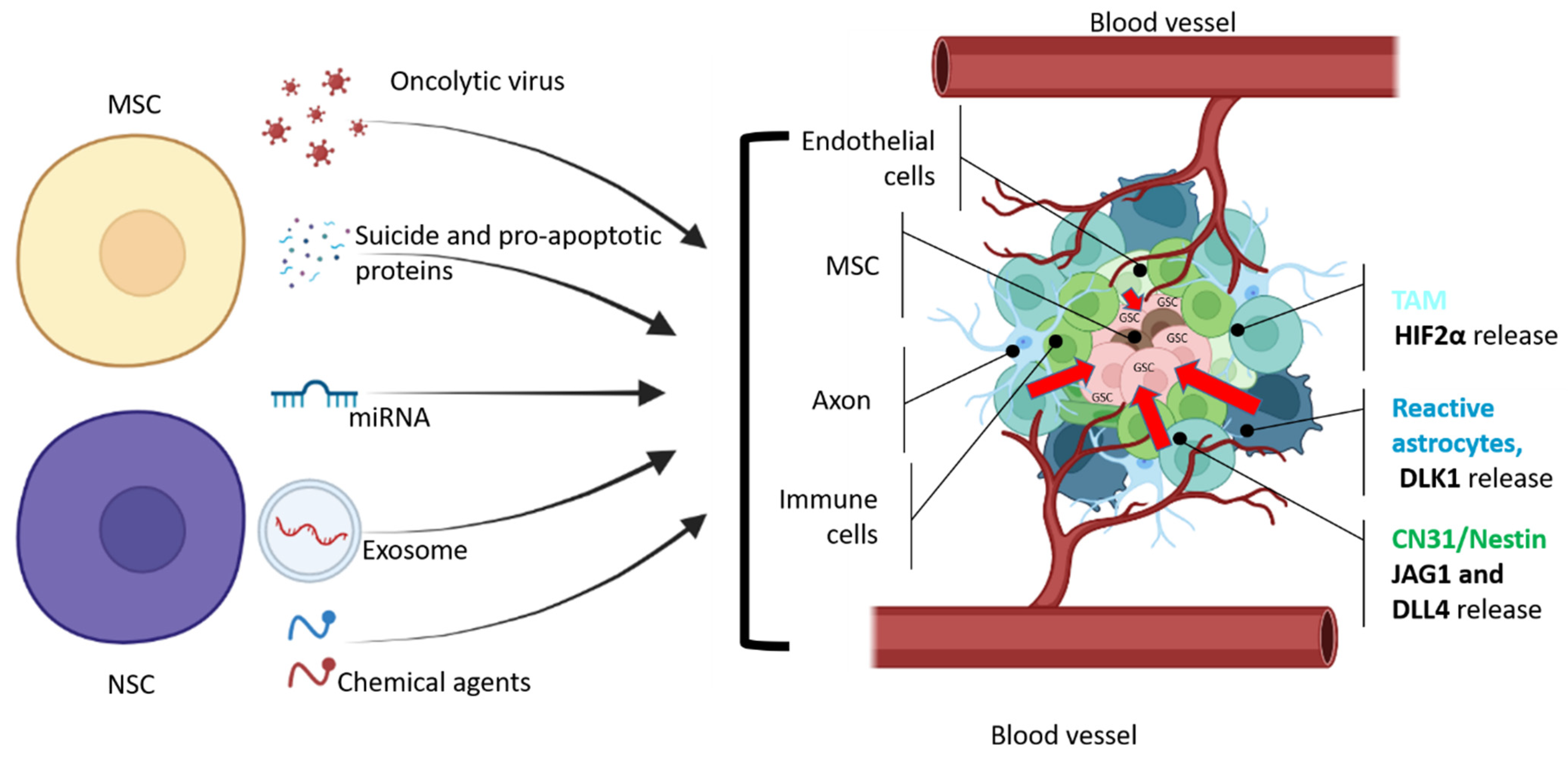
Figure 2. General scheme of therapeutic agents that can be delivered by NSC or MSC cells to glioma stem cells. GSCs are located in the necrotic area of the tumor, which is surrounded by hypoxic and vascularized regions with leaky blood vessels (adapted from Vinogradov et al. [52]). CD31+/Nestin+ and reactive astrocytes produce JAG1, DLL4 [53], and DLK1 [54] to perinecrotic and perivascular tumor regions to maintain GSC stemness and proliferation. According to Kvisten et al., [55] tumor-associated astrocyte (TMA) areas are rare in necrotic areas and more distributed in perivascular and perinecrotic areas of the GBM. Despite the morphological and phenotypical differences, TMA produces HIF-2α [56][57], which also controls the GBM pathobiology. In order to deliver a therapeutic payload, NSCs or MSCs should overcome multiple obstacles that prevent GSCs from being eliminated. Cell microenvironment releases growth factors to maintain GSC (red arrows).
4. Methods and Factors That Increase the Migration Properties of MSCs
Earlier reports suggest that in vitro cultivation conditions can influence the cell surface receptor level [58]. Therefore, the relationship between the types of MSCs of different origins and their ability to migrate into A549 tumors [59], ovarian [60], and colon [61] cancer cells is manifestative. Recently, it has been shown that MSCs have enhanced the tropism towards glioblastoma cells treated with ionizing radiation [62]. This therapy increases the secretion of chemokines and cytokines above the basal level [63]. Even though tumor cells induce SDF-1α [34][58][59] and CCL2, the existing gradient of chemokines and cytokines provides the migration of a small group of MSCs. The findings from earlier research showed that, when MSCs are systemically injected into laboratory animals with intracerebral glioblastoma, less than 1% of the MSCs migrate to GBM [64][65][66], leaving open questions about the fate of most injected MSCs at the injection/implantation site and the possibility of enhancing the migration of all MSCs into the tumor area.
5. Modeling MSC Migration In Vitro
MSC exposure to tissue growth factor-beta (TGF-β) [58] or challenging MSCs with monocyte chemotactic protein-1 (MCP-1), chemokine (C-C motif) ligand 8 (CCL-8), and/or interleukin 8 (IL-8) [67] can increase the migration of MSCs towards the tumor. Another way that increases the migration of MSCs into the tumor is the ability of MSCs to secrete metalloproteinases to cleave the basement membranes of cells and penetrate deep into the tumor. It was experimentally confirmed that cell incubation in a medium containing complement component 1q (C1q) [68], a mixture of erythropoietin and granulocyte colony-stimulating factor (G-CSF) [69], positively affects the expression of metalloproteinases. In this case, the simultaneous addition of erythropoietin and GM-CSF (1 IU/mL and 0.1 ng/mL, respectively) to the medium increased the MMP-2 (matrix metalloproteinase-2) expression, while the number of other metalloproteinases, as well as metalloproteinase inhibitors, remained unchanged. The ability of cells to migrate was also measured if a factor was added to the medium. Cells that were exposed to the medium with the addition of both factors at the same concentration showed the greatest ability to migrate and increase the metalloproteinase expression. At this concentration of substances, the concentration of extracellular signal-regulated kinase (ERK1/2) proteins significantly increased as well. These conditions indicate a possible effect of the ERK1/2 signaling pathway on the migration abilities of MSCs.
The short-term exposure of cells to valproic acid increases the expression of CXCR4, C1q, and MMP2 and improves the migration towards SDF-1 [70]. Additionally, it was shown that the exposure of MSCs to C1q increases the migration activity towards SDF-1 (20 ng/mL)-level migration to SDF-1 at a higher concentration (100 ng/mL). At the same time, CXCR4 expression on the cell surface increased from 1.5% to 9.5%. The study showed that short-term (3 or 6 h) exposure of MSCs to valproic acid at a concentration of 10 mmol increases the expression of CXCR4 mRNA by 40 and 60 times, respectively. The amount of CXCR4 was not increased on the cell surface, which confirmed the results of another study. The majority of CXCR4 was isolated intracellularly and formed heterodimers with CXCR7 [71]. Cells exposed to valproic acid migrated towards low (20 ng/mL) and high (100 ng/mL) SDF-1α gradients two and four times higher, respectively. When using the AMD3100-CXCR4 antagonist, the tropism for SDF-1α almost completely disappeared. The statement that an increased tropism due to cell exposure to valproate is associated with CXCR4 was confirmed. To increase the CXCR4 receptor on the cell surface, insulin-like growth factor 1 (IGF-1); tumor necrosis factor-alpha (TNF-α); and interleukin 1 beta (IL-1β), interferon-gamma (IFN-γ), or glycogen synthase-3 beta (glycogen synthase kinase 3 beta, GSK-3β) were added. It has been shown that the addition of complement 1q to the medium increases cell migration in response to the SDF-1 concentration gradients [68].
References
- Ostrom, Q.; Gittleman, H.; Liao, P.; Rouse, C.; Chen, Y.; Dowling, J.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-Oncology 2014, 16, iv1–iv63.
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013, 310, 1842–1850.
- Bredel, M.; Scholtens, D.M.; Harsh, G.R.; Bredel, C.; Chandler, J.P.; Renfrow, J.J.; Yadav, A.K.; Vogel, O.H.; Scheck, A.; Tibshirani, R.; et al. A network model of a cooperative genetic landscape in brain tumors. JAMA 2009, 302, 261–275.
- Yadav, A.K.; Renfrow, J.J.; Scholtens, D.M.; Xie, H.; Duran, G.E.; Bredel, C.; Vogel, H.; Chandler, J.P.; Chakravarti, A.; Robe, P.A.; et al. Monosomy of chromosome 10 as-sociated with dysregulation of epidermal growth factor signaling in glioblastomas. JAMA 2009, 302, 276–289.
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401.
- Sack, B.K.; Herzog, R.W. Evading the immune response upon in vivo gene therapy with viral vectors. Curr. Opin. Mol. Ther. 2009, 11, 493–503.
- Plotkin, S.A. Vaccines: Past, present and future. Nat. Med. 2005, 11, S5–S11.
- Schuster, J.; Lai, R.K.; Recht, L.D.; Reardon, D.A.; Paleologos, N.A.; Groves, M.D.; Mrugala, M.M.; Jensen, R.; Baehring, J.M.; Sloan, A.; et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: The ACT III study. Neuro-Oncology 2015, 17, 854–861.
- Sampson, J.H.; Heimberger, A.B.; Archer, G.E.; Aldape, K.D.; Friedman, A.H.; Friedman, H.S.; Gilbert, M.R.; Herndon, J.E., 2nd; McLendon, R.E.; Mitchell, D.A.; et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2010, 28, 4722–4729.
- Thomas, A.A.; Ernstoff, M.S.; Fadul, C.E. Immunotherapy for the treatment of glioblastoma. Cancer J. 2012, 18, 59–68.
- Robbins, P.D.; Ghivizzani, S.C. Viral vectors for gene therapy. Pharmacol. Ther. 1998, 80, 35–47.
- Kaufmann, J.K.; Chiocca, E.A. Glioma virus therapies between bench and bedside. Neuro-Oncology 2014, 16, 334–351.
- Rohle, D.; Popovici-Muller, J.; Palaskas, N.; Turcan, S.; Grommes, C.; Campos, C.; Tsoi, J.; Clark, O.; Oldrini, B.; Komisopoulou, E.; et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 2013, 340, 626–630.
- Yang, W.-X.; Zou, X.-H.; Jiang, S.-Y.; Lu, N.-N.; Han, M.; Zhao, J.-H.; Guo, X.-J.; Zhao, S.-C.; Lu, Z.-Z. Prevalence of serum neutralizing antibodies to adenovirus type 5 (Ad5) and 41 (Ad41) in children is associated with age and sanitary conditions. Vaccine 2016, 34, 5579–5586.
- Yu, B.; Zhou, Y.; Wu, H.; Wang, Z.; Zhan, Y.; Feng, X.; Geng, R.; Wu, Y.; Kong, W.; Yu, X. Seroprevalence of neutralizing antibodies to human adenovirus type 5 in healthy adults in China. J. Med. Virol. 2012, 84, 1408–1414.
- Mok, W.; Stylianopoulos, T.; Boucher, Y.; Jain, R.K. Mathematical Modeling of Herpes Simplex Virus Distribution in Solid Tumors: Implications for Cancer Gene Therapy. Clin. Cancer Res. 2009, 15, 2352–2360.
- Gonzalez-Morales, A.; Zabaleta, A.; Garcia-Moure, M.; Alonso, M.M.; Fernandez-Irigoyen, J.; Santamaria, E. Oncolytic adeno-virus Delta-24-RGD induces a widespread glioma proteotype remodeling during autophagy. J. Proteom. 2019, 194, 168–178.
- Park, H.-J.; Shin, J.Y.; Lee, B.R.; Kim, H.O.; Lee, P.H. Mesenchymal stem cells augment neurogenesis in the subventricular zone and enhance differentiation of neural precursor cells into dopaminergic neurons in the substantia nigra of a parkinsonian model. Cell Transplant. 2012, 21, 1629–1640.
- Ode, A.; Kopf, J.; Kurtz, A.; Schmidt-Bleek, K.; Schrade, P.; Kolar, P.; Buttgerei, F.; Lehmann, K.; Hutmacher, D.; Duda, G.; et al. CD73 and CD29 concurrently mediate the mechanically induced decrease of migratory capacity of mesenchymal stromal cells. Eur. Cells Mater. 2011, 22, 26–42.
- Srinivasan, V.M.; Gumin, J.; Camstra, K.M.; Collins, D.E.; Chen, M.M.; Shpall, E.J.; Kerrigan, B.C.P.; Johnson, J.N.; Chen, S.R.; Fueyo, J.; et al. Endovascular Selective Intra-Arterial Infusion of Mesenchymal Stem Cells Loaded with Delta-24 in a Canine Model. Neurosurgery 2020, 88, E102–E113.
- Ahmed, A.U.; Tyler, M.A.; Thaci, B.; Alexiades, N.G.; Han, Y.; Ulasov, I.V.; Lesniak, M.S. A comparative study of neural and mesenchymal stem cell-based carriers for oncolytic adenovirus in a model of malignant glioma. Mol. Pharm. 2011, 8, 1559–1572.
- Yong, R.L.; Shinojima, N.; Fueyo, J.; Gumin, J.; Vecil, G.G.; Marini, F.C.; Bogler, O.; Andreeff, M.; Lang, F.F. Human bone mar-row-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009, 69, 8932–8940.
- Dührsen, L.; Hartfuss, S.; Hirsch, D.; Geiger, S.; Maire, C.L.; Sedlacik, J.; Guenther, C.; Westphal, M.; Lamszus, K.; Hermann, F.G.; et al. Preclinical analysis of human mesenchymal stem cells: Tumor tropism and therapeutic efficiency of local HSV-TK suicide gene therapy in glioblastoma. Oncotarget 2019, 10, 6049–6061.
- Shi, S.; Zhang, M.; Guo, R.; Miao, Y.; Li, B. Bone Marrow–Derived Mesenchymal Stem Cell–Mediated Dual-Gene Therapy for Glioblastoma. Hum. Gene Ther. 2019, 30, 106–117.
- Wei, D.; Hou, J.; Zheng, K.; Jin, X.; Xie, Q.; Cheng, L.; Sun, X. Suicide Gene Therapy Against Malignant Gliomas by the Local Delivery of Genetically Engineered Umbilical Cord Mesenchymal Stem Cells as Cellular Vehicles. Curr. Gene Ther. 2019, 19, 330–341.
- Kurogi, R.; Nakamizo, A.; Suzuki, S.O.; Mizoguchi, M.; Yoshimoto, K.; Amano, T.; Amemiya, T.; Takagishi, S.; Iihara, K. Inhibition of glioblastoma cell invasion by hsa-miR-145-5p and hsa-miR-31-5p co-overexpression in human mesenchymal stem cells. J. Neurosurg. 2018, 130, 44–55.
- Haraszti, R.A.; Didiot, M.-C.; Sapp, E.; Leszyk, J.; Shaffer, S.A.; Rockwell, H.E.; Gao, F.; Narain, N.R.; DiFiglia, M.; Kiebish, M.A.; et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles 2016, 5, 32570.
- Jiang, X.; Fitch, S.; Wang, C.; Wilson, C.; Li, J.; Grant, G.A.; Yang, F. Nanoparticle engineered TRAIL-overexpressing adi-pose-derived stem cells target and eradicate glioblastoma via intracranial delivery. Proc. Natl. Acad. Sci. USA 2016, 113, 13857–13862.
- Chang, D.-Y.; Jung, J.-H.; Kim, A.A.; Marasini, S.; Lee, Y.J.; Paek, S.H.; Kim, S.-S.; Suh-Kim, H. Combined effects of mesenchymal stem cells carrying cytosine deaminase gene with 5-fluorocytosine and temozolomide in orthotopic glioma model. Am. J. Cancer Res. 2020, 10, 1429–1441.
- Yin, J.; Kim, J.K.; Moon, J.H.; Beck, S.; Piao, D.; Jin, X.; Kim, S.H.; Lim, Y.C.; Nam, D.H.; You, S.; et al. hMSC-mediated concurrent delivery of endostatin and carboxylesterase to mouse xenografts suppresses glioma initiation and recurrence. Mol. Ther. 2011, 19, 1161–1169.
- Choi, S.H.; Tamura, K.; Khajuria, R.K.; Bhere, D.; Nesterenko, I.; Lawler, J.; Shah, K. Antiangiogenic variant of TSP-1 targets tumor cells in glioblastomas. Mol. Ther. 2015, 23, 235–243.
- Pacioni, S.; D’Alessandris, Q.G.; Giannetti, S.; Morgante, L.; De Pascalis, I.; Coccè, V.; Bonomi, A.; Pascucci, L.; Alessandri, G.; Pessina, A.; et al. Mesenchymal stromal cells loaded with paclitaxel induce cytotoxic damage in glioblastoma brain xenografts. Stem Cell Res. Ther. 2015, 6, 194.
- Choi, S.H.; Stuckey, D.W.; Pignatta, S.; Reinshagen, C.; Khalsa, J.K.; Roozendaal, N.; Martinez-Quintanilla, J.; Tamura, K.; Keles, E.; Shah, K. Tumor Resection Recruits Effector T Cells and Boosts Therapeutic Efficacy of Encapsulated Stem Cells Expressing IFNbeta in Glioblastomas. Clin. Cancer Res. 2017, 23, 7047–7058.
- Pavon, L.F.; Sibov, T.T.; De Souza, A.V.; da Cruz, E.F.; Malheiros, S.M.F.; Cabral, F.R.; De Souza, J.G.; Boufleur, P.; Oliveira, D.; De Toledo, S.R.C.; et al. Tropism of mesenchymal stem cell toward CD133+ stem cell of glioblastoma in vitro and promote tumor proliferation in vivo. Stem Cell Res. Ther. 2018, 9, 310.
- Chavakis, E.; Urbich, C.; Dimmeler, S. Homing and engraftment of progenitor cells: A prerequisite for cell therapy. J. Mol. Cell. Cardiol. 2008, 45, 514–522.
- Naderi-Meshkin, H.; Matin, M.M.; Heirani-Tabasi, A.; Mirahmadi, M.; Irfan-Maqsood, M.; Edalatmanesh, M.A.; Shahriyari, M.; Ahmadiankia, N.; Moussavi, N.S.; Bidkhori, H.R.; et al. Injectable hydrogel delivery plus preconditioning of mesen-chymal stem cells: Exploitation of SDF-1/CXCR4 axis toward enhancing the efficacy of stem cells’ homing. Cell Biol. Int. 2016, 40, 730–741.
- Huang, W.; Wang, T.; Zhang, D.; Zhao, T.; Dai, B.; Ashraf, A.; Wang, X.; Xu, M.; Millard, R.W.; Fan, G.C.; et al. Mesenchymal stem cells overexpressing CXCR4 attenuate remodeling of postmyocardial infarction by releasing matrix metalloproteinase-9. Stem Cells Dev. 2012, 21, 778–789.
- Liu, H.; Xue, W.; Ge, G.; Luo, X.; Li, Y.; Xiang, H.; Ding, X.; Tian, P.; Tian, X. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1α in MSCs. Biochem. Biophys. Res. Commun. 2010, 401, 509–515.
- Kuan, I.-I.; Lee, C.-C.; Chen, C.-H.; Lu, J.; Kuo, Y.-S.; Wu, H.-C. The extracellular domain of epithelial cell adhesion molecule (EpCAM) enhances multipotency of mesenchymal stem cells through EGFR–LIN28–LET7 signaling. J. Biol. Chem. 2019, 294, 7769–7786.
- Ratajczak, M.Z.; Majka, M.; Kucia, M.; Drukala, J.; Pietrzkowski, Z.; Peiper, S.; Janowska-Wieczorek, A. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells 2003, 21, 363–371.
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401.
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760.
- Okada, M.; Suzuki, S.; Togashi, K.; Sugai, A.; Yamamoto, M.; Kitanaka, C. Targeting Folate Metabolism Is Selectively Cytotoxic to Glioma Stem Cells and Effectively Cooperates with Differentiation Therapy to Eliminate Tumor-Initiating Cells in Glioma Xenografts. Int. J. Mol. Sci. 2021, 22, 11633.
- Lathia, J.D.; Gallagher, J.; Heddleston, J.M.; Wang, J.; Eyler, C.E.; MacSwords, J.; Wu, Q.; Vasanji, A.; McLendon, R.E.; Hjelmeland, A.B.; et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 2010, 6, 421–432.
- Hira, V.V.; Wormer, J.R.; Kakar, H.; Breznik, B.; Van Der Swaan, B.; Hulsbos, R.; Tigchelaar, W.; Tonar, Z.; Khurshed, M.; Molenaar, R.J.; et al. Periarteriolar Glioblastoma Stem Cell Niches Express Bone Marrow Hematopoietic Stem Cell Niche Proteins. J. Histochem. Cytochem. 2018, 66, 155–173.
- Breznik, B.; Stokin, C.L.; Kos, J.; Khurshed, M.; Hira, V.V.V.; Bosnjak, R.; Lah, T.T.; Van Noorden, C.J.F. Cysteine cathepsins B, X and K expression in peri-arteriolar glioblastoma stem cell niches. Histochem. J. 2018, 49, 481–497.
- Hira, V.V.; Breznik, B.; Vittori, M.; de Jong, A.L.; Mlakar, J.; Oostra, R.-J.; Khurshed, M.; Molenaar, R.J.; Lah, T.; Van Noorden, C.J. Similarities Between Stem Cell Niches in Glioblastoma and Bone Marrow: Rays of Hope for Novel Treatment Strategies. J. Histochem. Cytochem. 2019, 68, 33–57.
- Truong, D.; Fiorelli, R.; Barrientos, E.S.; Melendez, E.L.; Sanai, N.; Mehta, S.; Nikkhah, M. A three-dimensional (3D) organotypic microfluidic model for glioma stem cells—Vascular interactions. Biomaterials 2018, 198, 63–77.
- Warrier, N.M.; Agarwal, P.; Kumar, P. Integrative Analysis to Identify Genes Associated with Stemness and Immune Infiltration in Glioblastoma. Cells 2021, 10, 2765.
- Pillat, M.M.; Oliveira-Giacomelli, A.; Oliveira, M.D.N.; Andrejew, R.; Turrini, N.; Baranova, J.; Turnšek, T.L.; Ulrich, H. Mesenchymal stem cell-glioblastoma interactions mediated via kinin receptors unveiled by cytometry. Cytom. Part A 2021, 99, 152–163.
- Uyar, R. Glioblastoma microenvironment: The stromal interactions. Pathol. Res. Pract. 2022, 232, 153813.
- Vinogradov, S.; Wei, X. Cancer stem cells and drug resistance: The potential of nanomedicine. Nanomedicine 2012, 7, 597–615.
- Zheng, Z.Q.; Chen, J.T.; Zheng, M.C.; Yang, L.J.; Wang, J.M.; Liu, Q.L.; Chen, L.F.; Ye, Z.C.; Lin, J.M.; Lin, Z.X. Nestin+/CD31+ cells in the hypoxic perivascular niche regulate glioblastoma chemoresistance by upregulating JAG1 and DLL4. Neuro-Oncology 2021, 23, 905–919.
- Grassi, E.S.; Jeannot, P.; Pantazopoulou, V.; Berg, T.J.; Pietras, A. Niche-derived soluble DLK1 promotes glioma growth. Neoplasia 2020, 22, 689–701.
- Kvisten, M.; Mikkelsen, V.E.; Solheim, O.; Van Der Want, J.; Torp, S.H. Microglia and macrophages in human glioblastomas: A morphological and immunohistochemical study. Mol. Clin. Oncol. 2019, 11, 31–36.
- Wang, Q.; He, Z.; Huang, M.; Liu, T.; Wang, Y.; Xu, H.; Duan, H.; Ma, P.; Zhang, L.; Zamvil, S.S.; et al. Vascular niche IL-6 induces alternative macrophage activation in glioblastoma through HIF-2α. Nat. Commun. 2018, 9, 559.
- Bordji, K.; Grandval, A.; Cuhna-Alves, L.; Lechapt-Zalcman, E.; Bernaudin, M. Hypoxia-inducible factor-2alpha (HIF-2alpha), but not HIF-1alpha, is essential for hypoxic induction of class III beta-tubulin expression in human glioblastoma cells. FEBS J. 2014, 281, 5220–5236.
- Li, M.; Zeng, L.; Liu, S.; Dangelmajer, S.; Kahlert, U.D.; Huang, H.; Han, Y.; Chi, X.; Zhu, M.; Lei, T. Transforming Growth Fac-tor-beta Promotes Homing and Therapeutic Efficacy of Human Mesenchymal Stem Cells to Glioblastoma. J. Neuropathol. Exp. Neurol. 2019, 78, 315–325.
- Layek, B.; Sadhukha, T.; Panyam, J.; Prabha, S. Nano-Engineered Mesenchymal Stem Cells Increase Therapeutic Efficacy of Anticancer Drug Through True Active Tumor Targeting. Mol. Cancer Ther. 2018, 17, 1196–1206.
- Kuroki, L.M.; Jin, X.; Dmitriev, I.P.; Kashentseva, E.A.; Powell, M.A.; Mutch, D.G.; Dietz, A.B.; Curiel, D.T.; Hawkins, W.G.; Spitzer, D. Adenovirus platform enhances transduction efficiency of human mesenchymal stem cells: An opportunity for cel-lular carriers of targeted TRAIL-based TR3 biologics in ovarian cancer. PLoS ONE 2017, 12, e0190125.
- Yu, R.; Deedigan, L.; Albarenque, S.M.; Mohr, A.; Zwacka, R.M. Delivery of sTRAIL variants by MSCs in combination with cytotoxic drug treatment leads to p53-independent enhanced antitumor effects. Cell Death Dis. 2013, 4, e503.
- Thomas, J.G.; Kerrigan, B.C.P.; Hossain, A.; Gumin, J.; Shinojima, N.; Nwajei, F.; Ezhilarasan, R.; Love, P.; Sulman, E.P.; Lang, F.F. Ionizing radiation augments glioma tropism of mesenchymal stem cells. J. Neurosurg. 2018, 128, 287–295.
- Motaln, H.; Turnsek, T.L. Cytokines play a key role in communication between mesenchymal stem cells and brain cancer cells. Protein Pept. Lett. 2015, 22, 322–331.
- Karp, J.M.; Teo, G.S.L. Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell 2009, 4, 206–216.
- Smith, C.L.; Chaichana, K.L.; Lee, Y.M.; Lin, B.; Stanko, K.M.; O’Donnell, T.; Gupta, S.; Shah, S.R.; Wang, J.; Wijesekera, O.; et al. Pre-exposure of human adipose mesenchymal stem cells to soluble factors enhances their homing to brain cancer. Stem Cells Transl. Med. 2015, 4, 239–251.
- Bexell, D.; Gunnarsson, S.; Tormin, A.; Darabi, A.; Gisselsson, D.; Roybon, L.; Scheding, S.; Bengzon, J. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol. Ther. 2009, 17, 183–190.
- Velpula, K.K.; Dasari, V.R.; Rao, J.S. The homing of human cord blood stem cells to sites of inflammation: Unfolding mysteries of a novel therapeutic paradigm for glioblastoma multiforme. Cell Cycle 2012, 11, 2303–2313.
- Qiu, Y.; Marquez-Curtis, L.A.; Janowska-Wieczorek, A. Mesenchymal stromal cells derived from umbilical cord blood migrate in response to complement C1q. Cytotherapy 2012, 14, 285–295.
- Yu, Q.; Chen, L.; You, Y.; Zou, C.; Zhang, Y.; Liu, Q.; Cheng, F. Erythropoietin combined with granulocyte colony-stimulating factor enhances MMP-2 expression in mesenchymal stem cells and promotes cell migration. Mol. Med. Rep. 2011, 4, 31–36.
- Marquez-Curtis, L.A.; Janowska-Wieczorek, A. Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axis. Biomed. Res. Int. 2013, 2013, 561098.
- Zhang, Y.; Foudi, A.; Geay, J.; Berthebaud, M.; Buet, D.; Jarrier, P.; Jalil, A.; Vainchenker, W.; Louache, F. Intracellular localization and constitutive endocytosis of CXCR4 in human CD34+ hematopoietic progenitor cells. Stem Cells 2004, 22, 1015–1029.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
633
Revisions:
2 times
(View History)
Update Date:
11 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





