Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sunil Pareek | -- | 2871 | 2022-05-10 10:34:22 | | | |
| 2 | Vivi Li | -1 word(s) | 2870 | 2022-05-11 04:13:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pareek, S.; Maurya, V.; Shakya, A.; , .; Bohn, T. β-Carotene within Loaded Delivery Systems in Food. Encyclopedia. Available online: https://encyclopedia.pub/entry/22747 (accessed on 13 January 2026).
Pareek S, Maurya V, Shakya A, , Bohn T. β-Carotene within Loaded Delivery Systems in Food. Encyclopedia. Available at: https://encyclopedia.pub/entry/22747. Accessed January 13, 2026.
Pareek, Sunil, Vaibhav Maurya, Amita Shakya, , Torsten Bohn. "β-Carotene within Loaded Delivery Systems in Food" Encyclopedia, https://encyclopedia.pub/entry/22747 (accessed January 13, 2026).
Pareek, S., Maurya, V., Shakya, A., , ., & Bohn, T. (2022, May 10). β-Carotene within Loaded Delivery Systems in Food. In Encyclopedia. https://encyclopedia.pub/entry/22747
Pareek, Sunil, et al. "β-Carotene within Loaded Delivery Systems in Food." Encyclopedia. Web. 10 May, 2022.
Copy Citation
Nanotechnology has opened new opportunities for delivering bioactive agents. Their physiochemical characteristics, i.e., small size, high surface area, unique composition, biocompatibility and biodegradability, make these nanomaterials an attractive tool for β-carotene delivery. Delivering β-carotene through nanoparticles does not only improve its bioavailability/bioaccumulation in target tissues, but also lessens its sensitivity against environmental factors during processing. Regardless of these benefits, nanocarriers have some limitations, such as variations in sensory quality, modification of the food matrix, increasing costs, as well as limited consumer acceptance and regulatory challenges.
beta-carotene
bioavailability
delivery system
encapsulation
engineered nanomaterial
SLNs
NLCs
1. Introduction
Vitamin A deficiency is one of the most diagnosed micronutrient deficiency disorders worldwide, especially in developing countries. However, its magnitude is more widespread in the vegetarian population [1]. Across the globe, approximately 250 million preschool children are estimated to be affected by vitamin A deficiency [2]. Furthermore, occurrence of disease has an intimate relationship with a low antioxidant load in the daily diet. Furthermore, lifestyle (exercise, smoking, drinking and high consumption of meat-based and processed foods), environment (emotional and social stress), and cultural constraints trigger the expression of housekeeping genes to adopting genes to retain the cellular, organ or body homeostasis [3]. The aforesaid stimuli also cause the generation of reactive oxygen species (ROS), resulting in oxidative homoeostasis imbalance at cellular and tissue levels, thus generating oxidative stress [4]. Oxidative stress can be defined as a phenomenon triggered by an imbalance between the generation and accumulation of ROS. In general, ROS, including organic hydro peroxides, hydrogen peroxide, nitric oxide, hydroxyl radicals and superoxide, are generated as by-products of oxygen metabolism; in addition, these environmental stimuli (UV, pollutants, heavy metals, and xenobiotics (including antiblastic drugs, antiallergic drugs, immunosuppressant drugs) equally contribute to ROS production, thus causing oxidative stress [5]. Accruing scientific evidence is accumulating on the involvement of oxidative stress in the occurrence of several health complications, which are attributed to inactivation of metabolic enzymes and damage vital cellular components, oxidization the nucleic acids, resulting in eye disorders, atherosclerosis, cardiovascular diseases, joint and bone disorders, neurological diseases (amyotrophic lateral sclerosis, Parkinson’s disease and Alzheimer’s disease) and misfunctioning of different organ including lung, kidney, liver and reproductive system [6]. ROS are primarily generated in mitochondria under both pathological as well as physiological conditions [7]. Cells activate an antioxidant defensive system which primarily includes enzymatic components such as superoxide dismutase, glutathione peroxidase, and catalase in order to minimize the oxidative stress cell [8].
1.1. Oxidative Stress and Antioxidants
ROS generation is attributed to both nonenzymatic and enzymatic reactions. Enzymatic processes that have intricate involvement in the respiratory chain, phagocytosis, prostaglandins biosynthesis, and cytochrome P450 system are responsible for ROS generation. Superoxide radicals produced as the result of enzymatic action of NADPH oxidase, peroxidases and xanthine oxidase initiate the chain reaction for ROS formation including hydrogen peroxide, hydroxyl radicals, peroxynitrite, hypochlorous acid and so on [9]. Hydroxyl radicals (•OH) are considered as the most reactive among all ROS in vivo and are produced as a result of catalysis of H2O2 in the presence of Fe2+ or Cu+ (Fenton reactions).
In addition, some nonenzymatic processes also contribute to ROS generation, especially when oxygen is either exposed to ionizing radiations or reacts with organic compounds. ROS are produced due to exogenous and endogenous sources. Exogenous sources of ROS include inflammation, immune cell activation, infection, ischemia, cancer, mental stress, excessive exercise and aging [4][10]. Exogeneous ROS generation relies on exposure to radiation, heavy metals [11], environmental pollutants [12], certain drugs (bleomycin, cyclosporine, gentamycin, tacrolimus) [13], toxic chemical and solvents [13], food processing (used oil and fat and smoked meat) [14], cigarette smoking and alcohol consumption, among other [10]. ROS are essential part of several biological processes when they remain at low or moderate concentrations. For instance, these ROS are obligatory for synthesis of some cellular structures, which have vital role in the host defense system, i.e. in the defence of pathogens [14][15]. In fact, macrophages synthesize and store ROS to kill pathogenic microbes [16]. The critical role of ROS in the immune system is well recognized as patients unable to produce ROS are more prone to pathological infections [17]. In addition, ROS are also integrated in an array of cellular signaling pathways as they play a regulatory role in intracellular signaling cascades, including endothelial cells, fibroblasts, cardiac myocytes, vascular smooth muscle cells and thyroid tissue. Nitric oxide (NO) is considered as a key cell-to-cell messenger, which plays a vital role in cell signaling and is intricately involved in several processes, such as blood flow modulation, thrombosis and normal neural functioning [18]. Nitric oxide also demonstrates close association with nonspecific host defense in eliminating the tumor cells, as well as intracellular pathogens [19]. In addition to beneficial effects, ROS also pose several negative impacts by affecting cellular structure, including plasma membrane, proteins, lipoprotein, proteins and nucleic acids (deoxyribonucleic acid, DNA; ribonucleic acid, RNA). Oxidative stress is a result of ROS imbalance between its rate of generation and rate of clearance within the cell [20]. These excess ROS thus cause damage in the plasma membrane by lipid peroxidation and form malondialdehyde and conjugated dienes which are cytotoxic and mutagenic in nature. Being a chain reaction cascade, lipid peroxidation spreads very rapidly, damaging a significant number of lipids, proteins and nucleic acids, hence hampering their functionalities [21]. In summary, ROS impart beneficial effects when they are maintained at low or moderate concentrations while they negatively affect several cellular structures at higher concentrations.
The human body adopts several strategies to combat the negative effects generated due to oxidative stress, including enzymatic (superoxide dismutase, glutathione peroxidase and catalase) or nonenzymatic (L-arginine, glutathione, coenzyme Q10 and lipoic acid) antioxidant molecules. In addition to the aforesaid molecules, several exogenous antioxidants molecules from animal or plant origins are deliberately incorporated, i.e. fortified, into the diet [5].
1.2. Mode of Action of β-Carotene against Oxidative Stress
β-Carotene, a key member of the carotenoid family, is recognized as one of the most potent antioxidants [22] and the major provitamin A carotenoid available in the human diet. The health benefits of β-carotene are attributed to its given biological properties [21]: (a) as antioxidants that scavenge and quench ROS of oxidative metabolism, (b) as provitamin A compounds that activate retinol-mediated pathways, (c) as electrophiles that boost endogenous antioxidant systems, (d) by hampering inflammation-related processes mediated by nuclear factor κ-light-chain-enhancer of activated B cell (NF-κB) pathway, and/or (e) by directly binding nuclear receptors (NRs) and other transcription factors in target cells.
Retinoic acid acts as ligand for the retinoid X receptors (RXRs) and canonical retinoid acid receptors (RARs), which influence the expression of a number of responsive genes and have intimate relationships with fatty acid, cholesterol, Ca2+ and phosphate homeostasis [23]. β-Carotene also demonstrated tumor cell suppression activity and enhanced intercellular communication at gap junctions [3]. It is believed that consumption of β-carotene may cause low incidence of hepatic oxidative stress and lipid oxidation. The assumption was supported by a mice model study where expression of 1207 genes (approximately 4% genes) of a total of 30,855 genes in a hepatic transcriptome was influenced when mice were fed with β-carotene as compared to control mice [24]. Remarkably, numerous differentially expressed genes were intimately involved in energy metabolism, lipid metabolism, and mitochondrial redox homeostasis.
β-Carotene is the main contributor to vitamin A in human beings, if preformed vitamin A intake is insufficient. It acts as a precursor of vitamin A, with the potential to yield two retinal molecules following cleavage by beta-carotene oxygenase 1 in the intestine, as compared to other carotenoids which generally yield only one retinal molecule. Despite its indispensable role in vision, it may furthermore play a role as a bioactive compound, due to its potential antioxidant effects [25], and its interaction with nuclear receptors, mainly RAR/RXR, which is important for cell differentiation and immunity [26]. These properties make β-carotene one of the most investigated biological molecules, both in academia and industry. Though its multifunctionality in humans is yet to be fully understood, several epidemiologic studies have demonstrated its relationship to a decreased incidence of chronic diseases such as blindness [27], xerophthalmia [28], cancer [29], cardiovascular diseases [30], diabetes [31] and premature death [32] and found to have an antioxidant component.
2. Delivery Systems for β-Carotene
β-Carotene is often used as a natural colorant and additive in food in spite of having poor water solubility, a high melting point, susceptibility to environmental conditions, chemical instability, heterogenous distribution in food matrices, and low bioavailability—all factors that limit its potential for the food industry. In this regard, encapsulation techniques have allowed researchers to develop a range of delivery systems with desired functionalities, such as enhanced stability, high dispersibility, improved solubility and targeted/controlled release and improved bioavailability [33][34].
Delivery system is the technology where a bioactive ingredient is enclosed in nano-/microstructure not only to protect bioactive compounds against environmental degradation (oxidation, pH and enzyme), but also to release them at a particular target site in a defined rate [35]. At present, the most investigated delivery systems adopted for β-carotene can primarily be categorized into two groups: polymer-based delivery systems (PBDSs) and lipid-based delivery systems (LBDSs).
2.1. Polymer-Based Delivery Systems
Polymer-based delivery systems use the intrinsic diversity of polymers to develop encapsulating bioactive compounds in nanodelivery with improved functionalities. The long-term health risks of PBDSs either fabricated with a synthetic polymer or made up of natural polymers, such as proteins and carbohydrates, are regarded as minimal. However, the latter are either hard to scale-up as they require several heat and often complex treatments which are hard to control or result in porous micro-/nanoparticles, thereby not achieving the objective of encapsulation. A range of PBDSs have been reported in the literature. In the present entry, researchers have included only those PBDSs which are derived from either natural food grade materials or are generally recognized as safe polymers. Typical PBDSs include nano-/microspheres, nano-/microcapsules, hydrogel micelles, colloidal nano-/microemulsions and nanofibers, all of which mainly consist of synthetic or natural polymers (Figure 1A,B).
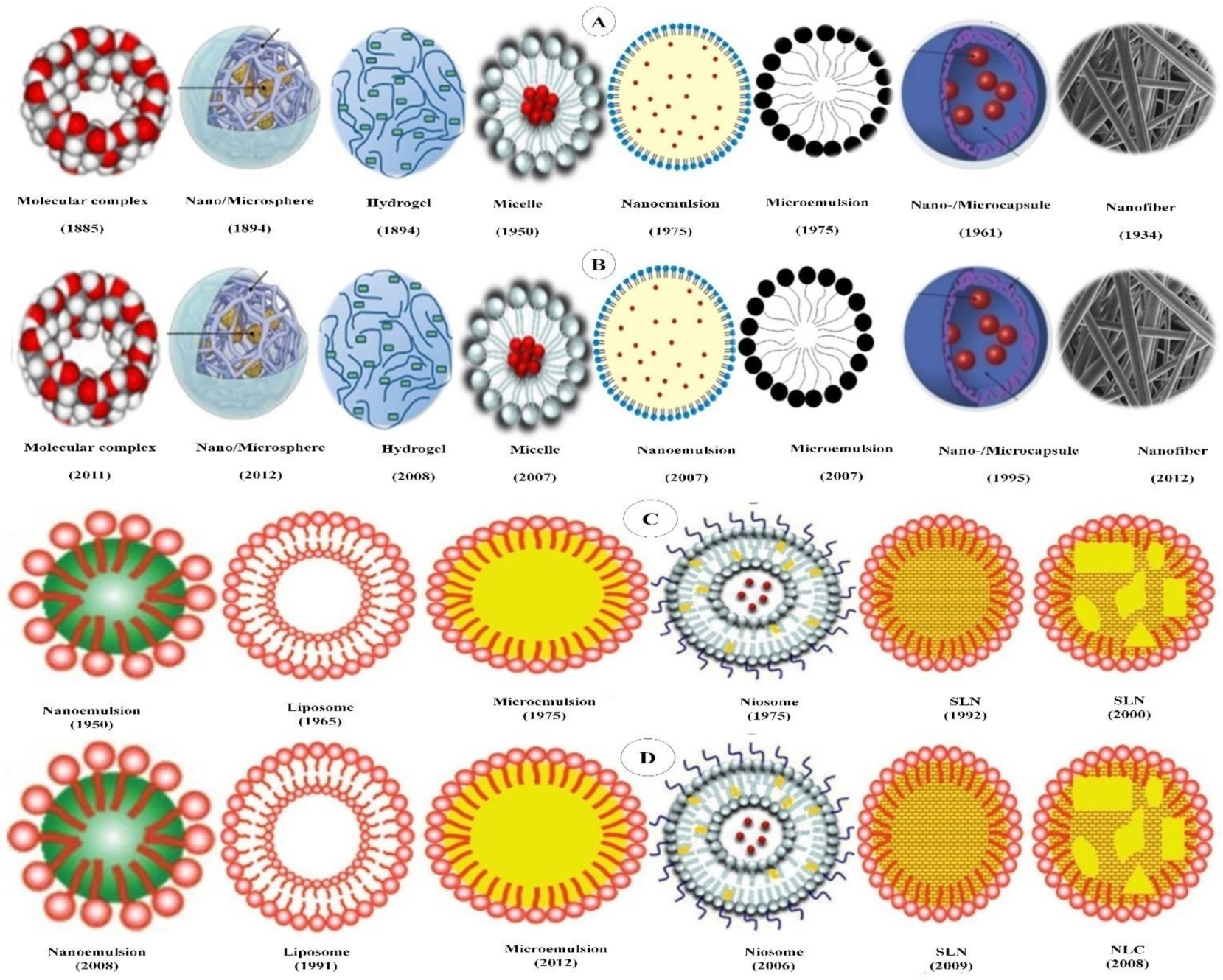
Figure 1. (A) Historical event in the evolution of polymer-based delivery systems; (B) historical event in the application of polymer-based delivery system for encapsulating β-carotene; (C) historical event in the evolution of lipid-based delivery systems; (D) historical event for applying lipid-based delivery system for encapsulating β-carotene.
2.2. Lipid-Based Delivery Systems
Lipid-based delivery systems (LBDSs) involve delivery systems which are principally composed of physiological lipid analogs such as surfactants as stabilizers (Figure 1A,B). LBDSs have been recognized for their promising biocompatibility, competency in GIT penetration, easy to scale-up and broad application [33][36]. LBDSs have been admired for their potential for drug delivery through various administration routes, particularly for the oral delivery of lipophilic drugs, because of their competence to mimic the food lipids during the digestive process [37][38]. With their properties, lipid-based delivery systems offer an array of advantages over polymer-based systems as shown in Table 1. Some of these advantages of lipid-based nanodelivery systems entail: (i) biocompatibility and use of nontoxic excipients [36][39]; (ii) high drug payload [40]; (iii) viability of incorporating both lipophilic and hydrophilic bioactives [36]; (iv) prospect of controlled release and drug targeting; (v) improved drug stability [41]; (vi) averting of organic solvents [42]; (vii) cost-effectiveness [43]; (viii) ease of scale-up during production and sterilization [44]. Over the course of time, a range of lipid-based delivery systems have been developed for encapsulating bioactive compounds such as micelles, micro- and nanoemulsions, liposomes, niosomes, solid lipid carriers, nanostructured lipid carriers, bilosomes, cubosomes, etc. [45]. However, in the present entry, the emphasis has given those LBDSs which have been adopted for encapsulation β-carotene are discussed in the following sections.
Table 1. Various factors that need to be considered prior to selecting a delivery system for encapsulating any bioactive agent.
| ENMS | Class of DeliverySystem | Subclass of Delivery System | Ability to Deliver Lipophilic and Lipophobic BA | Physical Stability | Biological Stability | Biocompatibility | Drug Targeting | Drug Loading | Feasibility to be Delivery System for β-Carotene |
|---|---|---|---|---|---|---|---|---|---|
| Lipid-derived delivery system | Self-assembled delivery system | Liposome | Yes | poor | Poor | Good | Moderate | Low to moderate | Poor |
| Niosome | Yes | moderate | Poor | Moderate | Moderate | Moderate | Poor | ||
| Particulate | Solid lipid nanoparticles | Only lipophilic | Good | Moderate | Good | Moderate | Moderate | Moderate | |
| Nanostructured lipid carriers | Only lipophilic | Good | High | Good | Moderate | High | Good | ||
| Emulsion | Microemulsion | Yes | Moderate | Moderate | Good | Poor | High | Good | |
| Nanoemulsion | Yes | poor | Moderate | Good | Poor | High | Poor | ||
| Polymer-derived delivery system | Self-assembled delivery system | Starch-based Micelle | Yes | Good | Good | Moderate | Poor | Poor | Good |
| Protein-based micelles | Yes | Poor | Good | Moderate | Moderate | Poor | Good | ||
| Carbohydrate | Poor | ||||||||
| Hydrogel | Yes | Good | Good | Poor | Poor | Poor | Good | ||
| Colloidal nanoemulsion | Yes | Moderate | Moderate | Good | Poor | High | moderate | ||
| Nanoemulsion | Yes | poor | Moderate | Good | Poor | High | Poor | ||
| Molecular complexes | Only lipophilic | Good | Moderate | Poor | Poor | Low | Poor | ||
| Particulate | Protein inclusion complexes | Yes | Good | Moderate | Moderate | Moderate | Low | Poor | |
| Nanosphere | Yes | Good | Moderate | Moderate | Moderate | Moderate | Poor | ||
| Microsphere | Yes | Good | Moderate | Moderate | Moderate | Low | Moderate | ||
| Fibrous | Nanofiber | Yes | Good | Moderate | Moderate | Moderate | Low | Poor | |
| Capsular | Microcapsule | Yes | Good | Moderate | Moderate | Moderate | Low | Poor | |
| Nanosphere | Yes | Good | Moderate | Moderate | Moderate | Moderate | Poor |
3. Safety Compliance and Risks of β-Carotene Nanoparticles
The customized properties of the discussed delivery systems, including the potential for bioavailability, better absorption and controlled release kinetics of the encapsulated bioactive compounds, may also impart unseen risks to biological systems [43][46]. It is assumed that utilization of biodegradable or natural materials may curtail the health hazards as compared to polymeric nanoparticles which are either derived from synthetic polymers or involve toxic organic solvents during their fabrication processes [46]. Due to the ambiguity on long- or short-term effects of direct or indirect employed nanoparticles in food systems, it is paramount to evaluate the impacts of nanoparticles on human health [47]. With regard to food safety, the FDA has listed certain strategies in conjunction with nanoparticle-based food and food components for mass production [48]. Regardless of the potential health concern, at present no standardized legislation for incorporation of nanoparticles in food systems, particularly for nanoparticles encapsulating β-carotene, are available. Nevertheless, several agencies and governmental bodies insist that people embrace the safety concerns of nanoparticle-based food products in legislative guidelines [49]. The European Food Safety Authority (EFSA) has published an excellent report on the topic (https://www.efsa.europa.eu/en/efsajournal/pub/5327, accessed on 20 December 2020). This guideline provides an overview on the required information about physico-chemical characterization and the other data requirements. It also states about the performance of risk assessment of nanomaterials in the food and feed area including novel food, FCMs, food/feed additives and pesticides. This lack of universal legislations compelled duty-bound policymakers to outline a guideline specifically dealing with the nanoscale materials in the food system [50].
The potentially tailored bioavailability of encapsulated bioactive compounds in delivery systems is a key safety concern, specifically for bioactive compounds, or the nanodelivery systems which may become toxic beyond a certain dose. To scrutinize the safety aspects, the bioavailability of bioactive compounds needs to be revaluated when it is encapsulated within nanodelivery vehicles, and reflections on alterations of the Recommended Daily Allowance (RDA) as well as the Tolerable Upper Intake Level (UL) of encapsulated bioactives are needed [51].
In addition, food scientists may also need to conduct studies addressing the safety concerns associated with nanoparticles, with special attention regarding: (i) the physiochemical characterization constraints of nanoparticles utilized in food items such as food additives, enzymes, flavorings, food contact materials (FCMs), novel foods, feed additives and pesticides [52]; (ii) development of the testing strategies to determine and characterize hazards transmitted via the engineered nanomaterials (ENMs)—i.e., assays for in vitro genotoxicity, absorption, distribution, metabolism and excretion and repeated-dose trials to study toxicity in test animals such as rodents [53].
In addition, the interactions between food items and nanodelivery systems should also be debated, which may result in producing radical oxygen species, photoreactions, etc. In December 2014, EU legislative bodies have insisted that food industries mention relevant information on the label if nano-food products are sold [50]. According to this guideline, particles have one or more dimensions of either 100 nm or less and agglomerates above 100 nm exhibiting ENM characteristics and should be considered as ENMs. In conjunction with this, the FDA has drafted guidelines which clearly define ENM-derived foods as (i) agents or products having particle sizes within the range of 1 to 100 nm with at least one dimension being within the nanoscale; (ii) agents or products exerting biological, chemical and physical characteristic associated with nanoscale materials and that are also on the nanoscale even though they are not nanosized.
In addition to legislative guidelines, there are several moral responsibilities of the food processing manufacturers, including: (i) evaluation of the changes imparted on the food materials—i.e., impurities and physiochemical properties; (ii) evaluation of the safety of food materials after modifications; (iii) submission of the regulatory assessment reporting to the legislative bodies such as FDA, FSSAI, EU, FASSAI, etc.; (iv) identification and a statement about the regulatory concern due to the ingestion of the nanoparticle-derived food items.
Apart from the US-FDA, several other regulatory authorities from various countries including Australia, New Zealand (FSANS) and Korea (MFDS) have issued their own guidelines [54]. These agencies counseled to conduct safety experiments (in vitro as well as in vivo) to evaluate the effect of nanoparticle-containing foods and publish the data, as well as to establish guidelines before releasing these nanoparticle containing foods to the food supply chain. Nevertheless, there is a lack of specific guidelines regarding nanoparticles containing foods, thus it is high time that the legislative bodies should come together to frame a more universal guideline for nanomaterial-derived food products which can then be applied or further tailored to different countries.
References
- McLaren, D.S.; Kraemer, K. Manual on Vitamin A Deficiency Disorders (VADD); Karger Medical and Scientific Publishers: Basel, Switzerland, 2012.
- Unit, N.; World Health Organization. Global Prevalence of Vitamin A Deficiency; World Health Organization: Geneva, Switzerland, 1995.
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93.
- Shibata, M.; Sato, H.; Shimizu, T.; Shibata, S.; Toriumi, H.; Kuroi, T.; Ebine, T.; Iwashita, T.; Funakubo, M.; Akazawa, C. Differential cellular localization of antioxidant enzymes in the trigeminal ganglion. J. Headache Pain 2013, 14, P83.
- Adwas, A.A.; Elsayed, A.; Azab, A.E.; Quwaydir, F.A. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47.
- Giustarini, D.; Dalle-Donne, I.; Tsikas, D.; Rossi, R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit. Rev. Clin. 2009, 46, 241–281.
- Adam-Vizi, V.; Chinopoulos, C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol. Sci. 2006, 27, 639–645.
- Lenaz, G. Mitochondria and reactive oxygen species. Which role in physiology and pathology? Adv. Mitochondrial. Med. 2012, 93–136.
- Auten, R.L.; Davis, J.M. Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatr. Res. 2009, 66, 121–127.
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694.
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and health effects of heavy metal toxicity in humans. In Poisoning in the Modern World-New Tricks for an Old Dog? IntechOpen: London, UK, 2019; pp. 77–100.
- Leni, Z.; Künzi, L.; Geiser, M. Air pollution causing oxidative stress. Curr. Opin. Toxicol. 2020, 20, 1–8.
- Klawitter, J.; Klawitter, J.; Pennington, A.; Kirkpatrick, B.; Roda, G.; Kotecha, N.C.; Thurman, J.M.; Christians, U. Cyclophilin D knockout protects the mouse kidney against cyclosporin A-induced oxidative stress. Am. J. Physiol. Ren. Physiol. 2019, 317, F683–F694.
- Wu, S.-C.; Yen, G.-C. Effects of cooking oil fumes on the genotoxicity and oxidative stress in human lung carcinoma (A-549) cells. Toxicol. In Vitro 2004, 18, 571–580.
- Bardaweel, S.K.; Gul, M.; Alzweiri, M.; Ishaqat, A.; Alsalamat, H.A.; Bashatwah, R.M. Reactive oxygen species: The dual role in physiological and pathological conditions of the human body. Eurasian J. Med. 2018, 50, 193.
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species: Key regulators in vascular health and diseases. Br. J. Pharmacol. 2018, 175, 1279–1292.
- Franchina, D.G.; Dostert, C.; Brenner, D. Reactive oxygen species: Involvement in T cell signaling and metabolism. Trends Immunol. 2018, 39, 489–502.
- Martina, A.; Jana, P.; Anna, S.; Tomas, B. Nitric oxide—Important messenger in human body. J. Mol. Integr. Physiol. 2012, 2, 98–106.
- Hezel, M.P.; Weitzberg, E. The oral microbiome and nitric oxide homoeostasis. Oral Dis. 2015, 21, 7–16.
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523.
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929.
- Kawata, A.; Murakami, Y.; Suzuki, S.; Fujisawa, S. Anti-inflammatory activity of β-carotene, lycopene and tri-n-butylborane, a scavenger of reactive oxygen species. In Vivo 2018, 32, 255–264.
- Evans, R.M.; Mangelsdorf, D.J. Nuclear receptors, RXR, and the big bang. Cell 2014, 157, 255–266.
- Palczewski, G.; Widjaja-Adhi, M.A.; Amengual, J.; Golczak, M.; Von Lintig, J. Genetic dissection in a mouse model reveals interactions between carotenoids and lipid metabolism. J. Lipid Res. 2016, 57, 1684–1695.
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005, 26, 459–516.
- Czarnewski, P.; Das, S.; Parigi, S.M.; Villablanca, E.J. Retinoic acid and its role in modulating intestinal innate immunity. Nutrients 2017, 9, 68.
- Al Binali, H.A.H. Night blindness and ancient remedy. Heart Views 2014, 15, 136.
- Biradar, S.T.; Ankitha, C.S.; Pasha, S.M. An Ayurvedic insight to keratoconus-A case report. J. Ayurveda Integr. Med. 2020, 5, 412–416. Available online: https://www.jaims.in/index.php/jaims/article/view/1312 (accessed on 26 February 2021).
- Balaji, S.; Roy, A. Beta-carotene--A versatile antioxidant in oral cancer: A review. Drug Invent. Today 2020, 13, 398–403.
- Miller, A.P.; Coronel, J.; Amengual, J. The role of β-carotene and vitamin A in atherogenesis: Evidences from preclinical and clinical studies. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 158635.
- Csepanyi, E.; Czompa, A.; Szabados-Furjesi, P.; Lekli, I.; Balla, J.; Balla, G.; Tosaki, A.; Bak, I. The effects of long-term, low-and high-dose beta-carotene treatment in Zucker diabetic fatty rats: The role of HO-1. Int. J. Mol. Sci. 2018, 19, 1132.
- Stevens, G.A.; Bennett, J.E.; Hennocq, Q.; Lu, Y.; De-Regil, L.M.; Rogers, L.; Danaei, G.; Li, G.; White, R.A.; Flaxman, S.R. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: A pooled analysis of population-based surveys. Lancet Glob. Health 2015, 3, e528–e536.
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Control. Release 2019, 298, 38–67.
- Maurya, V.K.; Aggarwal, M.; Ranjan, V.; Gothandam, K.M. Improving bioavailability of vitamin A in food by encapsulation: An update. In Nanoscience in Medicine Vol. 1; Springer: Cham, Switzerland, 2020; pp. 117–145.
- Donhowe, E.G.; Kong, F. Beta-carotene: Digestion, microencapsulation, and in vitro bioavailability. Food Bioprocess Technol. 2014, 7, 338–354.
- Barroso, L.; Viegas, C.; Vieira, J.; Pego, C.; Costa, J.; Fonte, P. Lipid-based carriers for food ingredients delivery. J. Food. Eng. 2020, 295, 110451.
- Chaudhari, V.S.; Murty, U.S.; Banerjee, S. Lipidic nanomaterials to deliver natural compounds against cancer: A review. Environ. Chem. Lett. 2020, 18, 1–10.
- Sarkar, A.; Mackie, A.R. Engineering oral delivery of hydrophobic bioactives in real world scenarios. Curr. Opin. Colloid Interface Sci. 2020, 48, 40–52.
- Shukla, D.; Chakraborty, S.; Singh, S.; Mishra, B. Lipid-based oral multiparticulate formulations–advantages, technological advances and industrial applications. Expert Opin. Drug Deliv. 2011, 8, 207–224.
- Yao, M.; McClements, D.J.; Xiao, H. Improving oral bioavailability of nutraceuticals by engineered nanoparticle-based delivery systems. Curr. Opin. Food Sci. 2015, 2, 14–19.
- Mozafari, M.R.; Flanagan, J.; Matia-Merino, L.; Awati, A.; Omri, A.; Suntres, Z.E.; Singh, H. Recent trends in the lipid-based nanoencapsulation of antioxidants and their role in foods. J. Sci. Food Agric. 2006, 86, 2038–2045.
- Akhavan, S.; Assadpour, E.; Katouzian, I.; Jafari, S.M. Lipid nano scale cargos for the protection and delivery of food bioactive ingredients and nutraceuticals. Trends Food Sci. Technol. 2018, 74, 132–146.
- de Souza Simões, L.; Madalena, D.A.; Pinheiro, A.C.; Teixeira, J.A.; Vicente, A.A.; Ramos, O.L. Micro-and nano bio-based delivery systems for food applications: In vitro behavior. Adv. Colloid Interface Sci. 2017, 243, 23–45.
- Borba, C.M.; Tavares, M.N.; Macedo, L.P.; Araújo, G.S.; Furlong, E.B.; Dora, C.L.; Burkert, J.F.M. Physical and chemical stability of β-carotene nanoemulsions during storage and thermal process. Food Res. Int. 2019, 121, 229–237.
- Fathi, M.; Mozafari, M.R.; Mohebbi, M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Technol. 2012, 23, 13–27.
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability of nutraceuticals: Role of the food matrix, processing conditions, the gastrointestinal tract, and nanodelivery systems. Compr. Rev. Food Sci. 2020, 19, 954–994.
- Dowling, A.P. Development of nanotechnologies. Mater. Today 2004, 7, 30–35.
- Chau, C.-F.; Wu, S.-H.; Yen, G.-C. The development of regulations for food nanotechnology. Trends Food Sci. Technol. 2007, 18, 269–280.
- Amenta, V.; Aschberger, K.; Arena, M.; Bouwmeester, H.; Moniz, F.B.; Brandhoff, P.; Gottardo, S.; Marvin, H.J.; Mech, A.; Pesudo, L.Q. Regulatory aspects of nanotechnology in the agri/feed/food sector in EU and non-EU countries. Regul. Toxicol. Pharmacol. 2015, 73, 463–476.
- Committee, E.S. Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain. EFSA J. 2011, 9, 2140.
- Livney, Y.D. Nanostructured delivery systems in food: Latest developments and potential future directions. Curr. Opin. Food Sci. 2015, 3, 125–135.
- Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; Ricci, A. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA J. 2018, 16.
- Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. In vivo assays for evaluating the release of nanoencapsulated food ingredients. In Release and Bioavailability of Nanoencapsulated Food Ingredients; Elsevier: Gorgan, Iran, 2020; pp. 179–207.
- Luo, Y.; Wang, Q.; Zhang, Y. Biopolymer-based nanotechnology approaches to deliver bioactive compounds for food applications: A perspective on the past, present, and future. J. Agric. Food Chem. 2020, 68, 12993–13000.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
991
Revisions:
2 times
(View History)
Update Date:
11 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




