Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | David Wang | -- | 1532 | 2022-05-09 21:36:41 | | | |
| 2 | Dean Liu | -6 word(s) | 1526 | 2022-05-10 03:29:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, D.; , .; Mohan, C. Design of Gold Nanoparticle Vertical Flow Assays. Encyclopedia. Available online: https://encyclopedia.pub/entry/22726 (accessed on 07 February 2026).
Wang D, , Mohan C. Design of Gold Nanoparticle Vertical Flow Assays. Encyclopedia. Available at: https://encyclopedia.pub/entry/22726. Accessed February 07, 2026.
Wang, David, , Chandra Mohan. "Design of Gold Nanoparticle Vertical Flow Assays" Encyclopedia, https://encyclopedia.pub/entry/22726 (accessed February 07, 2026).
Wang, D., , ., & Mohan, C. (2022, May 09). Design of Gold Nanoparticle Vertical Flow Assays. In Encyclopedia. https://encyclopedia.pub/entry/22726
Wang, David, et al. "Design of Gold Nanoparticle Vertical Flow Assays." Encyclopedia. Web. 09 May, 2022.
Copy Citation
Vertical flow assays (VFAs) or flow-through assays have emerged as an alternate type of paper-based assay due to their faster detection time, larger sample volume capacity, and significantly higher multiplexing capabilities compared to lateral flow assays (LFAs). VFA can be used for detecting important biomarkers in diagnostic medicine, particularly when VFA is paired with gold nanoparticle conjugation.
vertical flow assay
gold nanoparticles
lateral flow assay
1. Introduction
Point-of-care testing (POCT) is a user-friendly, rapid, sensitive diagnostic approach for detecting the presence of targets of interest that is in high demand in resource-limited settings. During the past 50 years, POCT applications in health care, agriculture, food safety, forensic science, animal health, and the military have been developed [1]. Among the different types of POCT, the lateral flow assay is most widely applied across all fields due to its simplicity of operation and rapid reading. In the past ten years, lateral flow assays based on fluorescent nanoparticles (NPs), luminescent NPs, enzymatic reactions, and colorimetric NPs have been developed to meet the requirements for higher sensitivity, accuracy, and multiplexing capability [2]. According to the World Health Organization (WHO) guidelines, POCT should meet the following criteria: affordable, sensitive, specific, user-friendly, rapid, and robust; requiring no complex equipment; and can be delivered efficiently to end-users [3].
Although lateral flow assays are coupled with portable readers such as smartphones and various sensors to obtain quantitative measurements, lateral flow assays (LFAs) are still limited by (i) low multiplexing capacity (<10), (ii) the hook effect (false negative), (iii) low sample volume capacity (<100 µL), and (iv) moderate speed (15–40 min), as summarized in Table 1. For example, rapid commercial tests such as the HIV 1/2 Ag/Ab Combo, the TB LAM Ag test, the Influenza A + B test, the Malaria Pf Test, ImmunoCard STAT! E. coli O157 Plus and the multiplex lateral-flow assay RAIDTM 5 to detect biological threat agents are all designed for the detection of a limited number of targets [1][2][4]. A typical LFA strip consists of four overlapping elements mounted on an adhesive backing. The first element is the sample pad, typically made of cellulose or glass fiber to introduce the sample of interest to the second element, the conjugate pad at a constant rate. The conjugate pad is typically made of cellulose, glass fiber, or polyesters depending on the type of labeled conjugates and the assay’s sensitivity [2][5]. The labeled biomolecules are stored in the conjugate pad and should bind to the analyte in the sample of interest when the sample of interest reaches the conjugate pad. The analyte-conjugate complex laterally flows through the third element, typically the nitrocellulose membrane, where specific biological compounds (typically antibodies, protein, or nucleic acids) are immobilized at pre-defined lines. The analyte, analyte-conjugate complex, and conjugates should react specifically to the compounds dispensed on the membrane. Lastly, the fourth element, the absorbent pad, should absorb any remaining sample of interest and conjugate complex [2][5]. Depending on the molecular weight or structure of the analyte of interest, LFAs can be majorly classified into two categories: one is LFA, where antibodies are used as recognition elements to detect proteins, the other is nucleic acid lateral flow assay (NALFA), where nucleic acids are used as recognition elements to detect amplicons or results of amplification reactions like polymerase chain reaction (PCR) or recombinase polymerase amplification (RPA) [1][2].
Table 1. Comparison between LFAs and VFAs for POCT diagnosis.
| Features | LFAs | VFAs |
|---|---|---|
| Sample flow | Capillary force [6] | External force; Gravity force; Capillary force [7] |
| Flow method | Passive [6] | Passive; Active [7] |
| Sensing response | Moderate [6] | Fast [7] |
| Washing steps | Not required [6] | Mostly yes [7] |
| Timed results | Required [6] | Not required [7] |
| Hook effect | Yes [2][6] | Mostly No [8][9] |
| Sample and conjugate separation | Mostly No [2][6] | Yes [10][11][12] No [8][9][13] |
| Sample volume | <100 µL [2][6] | 10–500 µL [8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33] |
| Sample type | Urine; Serum; Blood; Plasma; Sweat; Mucus; Saliva; Stool; Food; Cerebrospinal fluid [1][2][5] |
Serum; Blood; Plasma [8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33] |
| Reagents volume | <100 µL [2] | <10 mL [8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33] |
| Multiplexing capacity | <10 [34][35] | >30 [16][21][29][31] |
| Detection method | Fluorescent NPs (QD; UCNP); Luminescent; NPs (Phosphors); Enzymatic reaction (HRP); Colorimetric NP (AuNP; CNP/CNT; Latex beads; MNP) [2] | Mostly colorimetric NPs (AgNPs; AuNP; SERS-AuNP) [7]; Enzymatic reaction (HRP; AP) [13] |
| Measurements | Qualitative or Quantitative coupled with portable reader [2][4] |
Mostly qualitative or quantitative coupled with benchtop scanner [7] |
Abbreviations: QD: Quantum Dot, UCNPs: Upconverting Nanoparticles, HRP: Horseradish Peroxidase, CNT/CNT: Carbon Nanoparticle/Carbon Nanotube, MNPs: Magnetic Nanoparticles, SERS: Surface-enhanced Raman Spectroscopy, AP: Alkaline Phosphatase.
Due to the intrinsic nature of antibody-antigen immunoreaction on each test line, the physical distance of multiplexing test lines on a single lateral flow strip has hindered operators from incorporating multiple analytes on one strip [5][6][36]. Additionally, crosstalk or poor sensitivity is usually seen in the antibody-based detection of more than four analytes due to antigenic similarity and compromised buffer conditions. Therefore, it has been particularly challenging to develop multiplexed LFAs on a manufacturing scale into commercial products [2]. Although researchers have explored multiple approaches for the simultaneous detection of up to 10 targets by fabricating a star shaped two-dimensional LFA [34] and have assessed multiplex detection on a single test line [35] by having multiple capture antibodies on a single line, these approaches are limited in how many targets they can interrogate. The hook effect was also observed in LFAs due to the mixing of reporter and sample, causing false negatives [2]. Nevertheless, paper-based lateral flow assays have proven their utility in detecting analytes in a wide range of clinical samples, as shown in Table 1. However, the small volume capacity of such assays has limited the sensitivity, especially for low-level analytes. Clinical samples with high viscosity usually necessitate dilution to facilitate the flow, further diluting the already low levels of each analyte, thus amplifying the difficulty of detecting a signal in the LFA [2].
Another issue with LFAs is their moderate assay response time between 15–40 min. Take for instance LFAs for the COVID-19 pandemic that has struck worldwide since 2020. Compared to the gold standard of real-time PCR, an increasing number of LFA-based diagnostics have earned emergency use authorization (EUA) from the Food and Drug Administration (FDA), under the names COVID Rapid Antigen Test or COVID Serology Test [37][38][39]. These tests, which focus on detecting SARS-CoV-2 nucleoprotein or IgG/IgM against SARS-CoV-2, usually take 15 min to 30 min for a readout. Indeed, these are “rapid” compared to the hours required for PCR, but not rapid enough for diagnosis by health care providers facing incessant waves of infected patients. Additionally, with the discovery of increasing numbers of mutated strains of SARS-CoV-2, it is difficult to identify the early stages of SARS-CoV-2 outbreaks by targeting antigens or antibodies to one or two specific strains, especially in resource-limited settings [40]. In 2021, growing interest in rapidly differentiating the symptoms of fever, cold, and headache caused by COVID-19 from those caused by other inflammatory diseases has created a high demand for an assay capable of rapid multiplex detection [41][42].
An alternative to LFAs is rapid vertical flow assays (VFAs), which offer several advantages, including faster response (1–40 min), no timing requirement (signal maintained for hours after the completion of the assay), high multiplexing capacity (>1000), the absence of a false-negative-inducing hook effect, and high sample volume capacity (>500 µL) [7]. As a result, many next-generation POCT diagnostics are beginning to explore VFAs [43][44]. In recent decades, VFAs with porous membranes have been utilized for the multiplex detection of nucleic acids, proteins, antibodies, polysaccharides, and virus antigens with greatly increased multiplexing capacity in microarray format, as well as increased sensitivity with multi-stacked protein microarrays coupled with filtration. By combining gravity, external forces, and capillary forces, VFA is usually faster than LFA. Furthermore, due to the separation step between sample loading and reporter loading, VFAs avoid the hook effect, which favors the detection of highly concentrated analytes. Finally, VFAs feature high sample volume capacity, potentially increasing the limit of detection of analytes and allowing room for a higher dilution factor of clinical samples [7].
2. VFAs in POCT Diagnostics
Vertical flow assays (VFAs) operate mainly through gravity, where the sample flow is vertical or perpendicular to the paper, and partially through external force and capillary action [7]. On the other hand, lateral flow assays (LFAs) consist of a sample flow parallel to the paper’s surface, permitting wicking only by capillary action [2][6]. This has limited the sensitivity and multiplexing capability of LFAs since the uniform flow rate of LFAs requires pore sizes of several micrometers (5 µm to 15 µm), and rapid response requires a limited length (5 cm to 20 cm) of the membrane [1][2][6]. However, LFAs and VFAs share similar fundamental principles, as they both work by immobilizing a capture substrate onto a reagent pad and applying a sample (in the presence or absence of the target analytes) [7]. First, a typical VFA membrane (NCM) is cut and separated into two zones: the control zone “C” as a fail-safe and the test zone “T” as a signal indicator. Then, the NCM is assembled on top of multiple stacked absorption pads and immobilized with a capture antibody. Next, the NCM is prewetted and loaded with the sample. After absorption, reporters such as gold nanoparticles (GNPs) are added to the NCM, along with several washes. In VFAs, the interactions between the specific antigen, capture antibody, and GNPs result in an immediate and permanent colored dot that can be detected by naked eye or with a smartphone reader. The main biomarkers currently detected in VFA applications are antibodies, protein antigens, and nucleic acids.
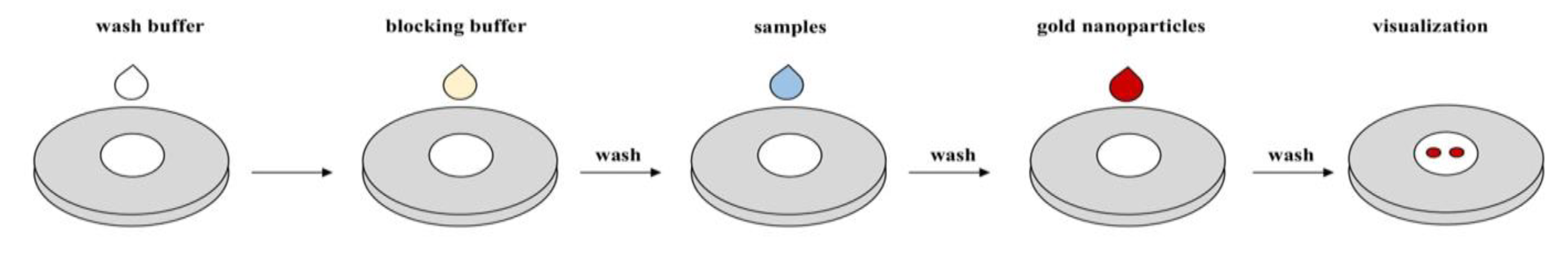
Figure 1. Commonly adopted protocol for vertical flow assays.
References
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251.
- Parolo, C.; Sena-Torralba, A.; Bergua, J.F.; Calucho, E.; Fuentes-Chust, C.; Hu, L.; Rivas, L.; Álvarez-Diduk, R.; Nguyen, E.P.; Cinti, S.; et al. Tutorial: Design and fabrication of nanoparticle-based lateral-flow immunoassays. Nat. Protoc. 2020, 15, 3788–3816.
- Peeling, R.W.; Holmes, K.K.; Mabey, D.; Ronald, A. Rapid tests for sexually transmitted infections (STIs): The way forward. Sex. Transm. Infect. 2006, 82 (Suppl. 5), v1–v6.
- Shen, L.; Hagen, J.A.; Papautsky, I. Point-of-care colorimetric detection with a smartphone. Lab Chip 2012, 12, 4240–4243.
- Lei, R.; Huo, R.; Mohan, C. Current and emerging trends in point-of-care urinalysis tests. Expert Rev. Mol. Diagn. 2020, 20, 69–84.
- Mansfield, M.A. The use of nitrocellulose membranes in lateral-flow assays. In Drugs of Abuse: Body Fluid Testing; Wong, R.C., Tse, H.Y., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 71–85.
- Jiang, N.; Ahmed, R.; Damayantharan, M.; Ünal, B.; Butt, H.; Yetisen, A.K. Lateral and vertical flow assays for point-of-care diagnostics. Adv. Healthc. Mater. 2019, 8, e1900244.
- Park, J.; Park, J.-K. Pressed region integrated 3D paper-based microfluidic device that enables vertical flow multistep assays for the detection of C-reactive protein based on programmed reagent loading. Sens. Actuators B Chem. 2017, 246.
- Oh, Y.K.; Joung, H.A.; Kim, S.; Kim, M.G. Vertical flow immunoassay (VFA) biosensor for a rapid one-step immunoassay. Lab Chip 2013, 13, 768–772.
- Frimpong, R.; Jang, W.; Kim, J.H.; Driskell, J.D. Rapid vertical flow immunoassay on AuNP plasmonic paper for SERS-based point of need diagnostics. Talanta 2021, 223 Pt 2, 121739.
- Prajapati, A.; Verma, N.; Pandya, A. Highly sensitive vertical flow based point-of-care immunokit for rapid and early detection of human CRP as a cardiovascular risk factor. Biomed. Microdevices 2020, 22, 28.
- Nybond, S.; Réu, P.; Rhedin, S.; Svedberg, G.; Alfvén, T.; Gantelius, J.; Svahn, H.A. Adenoviral detection by recombinase polymerase amplification and vertical flow paper microarray. Anal. Bioanal. Chem. 2019, 411, 813–822.
- Kim, S.; Hao, Y.; Miller, E.A.; Tay, D.M.Y.; Yee, E.; Kongsuphol, P.; Jia, H.; McBee, M.; Preiser, P.R.; Sikes, H.D. Vertical flow cellulose-based assays for SARS-CoV-2 antibody detection in human serum. ACS Sens. 2021, 6, 1891–1898.
- HIV—Medmira. Available online: https://medmira.com/hiv/ (accessed on 28 December 2021).
- Clarke, O.J.R.; Goodall, B.L.; Hui, H.P.; Vats, N.; Brosseau, C.L. Development of a SERS-based rapid vertical flow assay for point-of-care diagnostics. Anal. Chem. 2017, 89, 1405–1410.
- Ramachandran, S.; Singhal, M.; McKenzie, K.; Osborn, J.; Arjyal, A.; Dongol, S.; Baker, S.; Basnyat, B.; Farrar, J.; Dolecek, C.; et al. A rapid, multiplexed, high-throughput flow-through membrane immunoassay: A convenient alternative to ELISA. Diagnostics 2013, 3, 244–260.
- Joung, H.-A.; Ballard, Z.S.; Ma, A.; Tseng, D.; Teshome, H.; Burakowski, S.; Garner, O.B.; Di Carlo, D.; Ozcan, A. Paper-based multiplexed vertical flow assay for point-of-care testing. Lab Chip 2019, 19, 1027–1034.
- Shi, F.; Sun, Y.; Wu, Y.; Zhu, M.; Feng, D.; Zhang, R.; Peng, L.; Chen, C. A novel, rapid and simple method for detecting brucellosis based on rapid vertical flow technology. J. Appl. Microbiol. 2019, 128, 794–802.
- Reveal COVID-19. Available online: https://revealcovid19.com/ (accessed on 28 December 2021).
- Reuterswärd, P.; Gantelius, J.; Andersson Svahn, H. An 8-minute colorimetric paper-based reverse phase vertical flow serum microarray for screening of hyper IgE syndrome. Analyst 2015, 140, 7327–7334.
- Chinnasamy, T.; Segerink, L.I.; Nystrand, M.; Gantelius, J.; Andersson Svahn, H. Point-of-care vertical flow allergen microarray assay: Proof of concept. Clin. Chem. 2014, 60, 1209–1216.
- Chen, R.; Liu, B.; Ni, H.; Chang, N.; Luan, C.; Ge, Q.; Dong, J.; Zhao, X. Vertical flow assays based on core-shell SERS nanotags for multiplex prostate cancer biomarker detection. Analyst 2019, 144, 4051–4059.
- Chen, P.; Gates-Hollingsworth, M.; Pandit, S.; Park, A.; Montgomery, D.; AuCoin, D.; Gu, J.; Zenhausern, F. Paper-based Vertical Flow Immunoassay (VFI) for detection of bio-threat pathogens. Talanta 2019, 191, 81–88.
- Chen, R.; Du, X.; Cui, Y.; Zhang, X.; Ge, Q.; Dong, J.; Zhao, X. Vertical flow assay for inflammatory biomarkers based on nanofluidic channel array and SERS nanotags. Small 2020, 16, e2002801.
- Mehta, S.M.; Mehta, S.; Muthurajan, H.; D’Souza, J.S. Vertical flow paper-based plasmonic device for cysteine detection. Biomed. Microdevices 2019, 21, 55.
- Berger, A.G.; Restaino, S.M.; White, I.M. Vertical-flow paper SERS system for therapeutic drug monitoring of flucytosine in serum. Anal. Chim. Acta 2017, 949, 59–66.
- Schonhorn, J.E.; Fernandes, S.C.; Rajaratnam, A.; Deraney, R.N.; Rolland, J.P.; Mace, C.R. A device architecture for three-dimensional, patterned paper immunoassays. Lab Chip 2014, 14, 4653–4658.
- Bhardwaj, J.; Sharma, A.; Jang, J. Vertical flow-based paper immunosensor for rapid electrochemical and colorimetric detection of influenza virus using a different pore size sample pad. Biosens. Bioelectron. 2019, 126, 36–43.
- Cretich, M.; Torrisi, M.; Daminelli, S.; Gagni, P.; Plavisch, L.; Chiari, M. Flow-through, viral co-infection assay for resource-limited settings. Talanta 2015, 132, 315–320.
- Yee, E.H.; Lathwal, S.; Shah, P.P.; Sikes, H.D. Detection of biomarkers of periodontal disease in human saliva using stabilized, vertical flow immunoassays. ACS Sens. 2017, 2, 1589–1593.
- Rivas, L.; Reuterswärd, P.; Rasti, R.; Herrmann, B.; Mårtensson, A.; Alfvén, T.; Gantelius, J.; Andersson-Svahn, H. A vertical flow paper-microarray assay with isothermal DNA amplification for detection of Neisseria meningitidis. Talanta 2018, 183, 192–200.
- Serhan, M.; Jackemeyer, D.; Abi Karam, K.; Chakravadhanula, K.; Sprowls, M.; Cay-Durgun, P.; Forzani, E. A novel vertical flow assay for point of care measurement of iron from whole blood. Analyst 2021, 146, 1633–1641.
- Moumita, M.; Shankar, K.M.; Abhiman, P.B.; Shamasundar, B.A. Development of a sandwich vertical flow immunogold assay for rapid detection of oxytetracycline residue in fish tissues. Food Chem. 2019, 270, 585–592.
- Fenton, E.M.; Mascarenas, M.R.; López, G.P.; Sibbett, S.S. Multiplex lateral-flow test strips fabricated by two-dimensional shaping. ACS Appl. Mater. Interfaces 2009, 1, 124–129.
- Danthanarayana, A.N.; Finley, E.; Vu, B.; Kourentzi, K.; Willson, R.C.; Brgoch, J. A multicolor multiplex lateral flow assay for high-sensitivity analyte detection using persistent luminescent nanophosphors. Anal. Methods 2020, 12, 272–280.
- Zhang, G.; Guo, J.; Wang, X. Immunochromatographic lateral flow strip tests. Methods Mol. Biol. 2009, 504, 169–183.
- Luminostics. Available online: https://luminostics.com/ (accessed on 28 December 2021).
- INDICAID COVID-19 Rapid Antigen Test—Instructions for Use. Available online: https://www.fda.gov/media/151215/download (accessed on 28 December 2021).
- Rapid COVID-19 IgM/IgG Combo Test Kit—Instructions for Use. Available online: https://www.fda.gov/media/140297/download (accessed on 28 December 2021).
- SARS-CoV-2 Viral Mutations: Impact on COVID-19 Tests|FDA. Available online: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-viral-mutations-impact-covid-19-tests (accessed on 28 December 2021).
- Ragnesola, B.; Jin, D.; Lamb, C.C.; Shaz, B.H.; Hillyer, C.D.; Luchsinger, L.L. COVID19 antibody detection using lateral flow assay tests in a cohort of convalescent plasma donors. BMC Res. Notes 2020, 13, 372.
- Lei, R.; Mohan, C. Immunological biomarkers of COVID-19. Crit. Rev. Immunol. 2020, 40, 497–512.
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A.; Luong, J.H.T. Emerging technologies for next-generation point-of-care testing. Trends Biotechnol. 2015, 33, 692–705.
- St. John, A.; Price, C.P. Existing and emerging technologies for point-of-care testing. Clin. Biochem. Rev. 2014, 35, 155–167.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
10 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




