Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Héctor Mora-Montes | -- | 3146 | 2022-05-05 16:14:52 | | | |
| 2 | Camila Xu | Meta information modification | 3146 | 2022-05-06 03:20:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mora-Montes, H.; García Carnero, L. Mucormycosis and COVID-19-Associated Mucormycosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/22626 (accessed on 07 February 2026).
Mora-Montes H, García Carnero L. Mucormycosis and COVID-19-Associated Mucormycosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/22626. Accessed February 07, 2026.
Mora-Montes, Héctor, Laura García Carnero. "Mucormycosis and COVID-19-Associated Mucormycosis" Encyclopedia, https://encyclopedia.pub/entry/22626 (accessed February 07, 2026).
Mora-Montes, H., & García Carnero, L. (2022, May 05). Mucormycosis and COVID-19-Associated Mucormycosis. In Encyclopedia. https://encyclopedia.pub/entry/22626
Mora-Montes, Héctor and Laura García Carnero. "Mucormycosis and COVID-19-Associated Mucormycosis." Encyclopedia. Web. 05 May, 2022.
Copy Citation
Mucormycosis, previously known as zygomycosis, is a rare opportunistic, invasive, and deadly fungal infection that has increased in incidence in the last few years, as a secondary infection in patients with debilitating diseases, such as diabetes, cancer, and organ transplantation, and during natural disasters, such as tsunamis and earthquakes.
mucormycosis
CAM
immunosuppression
hyperglycemia
1. Mucormycosis
Mucormycosis, previously known as zygomycosis, is a rare opportunistic, invasive, and deadly fungal infection that has increased in incidence in the last few years, as a secondary infection in patients with debilitating diseases, such as diabetes, cancer, and organ transplantation, and during natural disasters, such as tsunamis and earthquakes [1]. Despite the availability of treatment options for mucormycosis, mortality rates range between 50% and 100% [2], which highlights the importance of this emerging disease.
Although precise data about the prevalence of this mycosis are unknown, due to the lack of diagnosis, according to epidemiological data of autopsy reports on invasive fungal diseases from 2008 to 2013, the etiological agents of mucormycosis were the fourth most common cause of invasive mycoses in the general population and the third most common cause in the onco-hematological and stem cell transplant populations, with a high prevalence of severe cases (around 70%) [3]. This infection is widely found in developing countries, being highly reported in India with uncontrolled diabetes as the main cause, while in developed countries, it is mainly found in patients with hematologic malignancies and hematopoietic stem cell transplantation [4][5].
The transmission route for these fungi can be through spores inhalation, contaminated food ingestion, traumatic inoculation with contaminated materials, or implantation in already abraded skin [6][7], which results in the development of several forms of the disease, including rhino-cerebral/rhino-orbital, pulmonary, gastrointestinal, cutaneous, and other disseminated forms. The disseminated disease is caused by the fungal angioinvasive capacity [8], with the rhino-cerebral form being the most common one in developing countries [4]. Regardless of the clinical form, patients show hemorrhage, thrombosis, infarction, and tissue necrosis [9], and the mortality rate depends on the organ affected, the causative agent, the patient’s health condition, and early diagnosis and treatment [10].
The mucormycosis causative agents are fast-growing thermotolerant fungi belonging to the Mucorales order, under the subphylum Mucormycotina and the phylum Glomeromycota [11][12]. Rhizopus is the genus most frequently associated with the disease, followed by Mucor and Lichtheimia, while Zygomycetes genera, including Saksenae, Cunninghamella, Apophysomyces, and Rhizomucor, are less common [7][12][13]. These organisms form a heterogenous group and are saprobes or facultative parasites, found worldwide growing on decaying organic matter, agricultural and forest soils, and animal feces, all with similar morphologies characterized by large aseptate/pauci-septated, ribbon-like hyaline hyphae with irregular or right-angle branching [8][10][14]. Most of these species are heterothallic [15][16] and reproduce asexually forming nonmotile mitospores of 3–11µm in diameter, produced in multi- or few-spored sporocarps, but there are a few species reported as homothallic, which are self-fertile [6][8].
Although all Mucorales share many unique characteristics, there are important differences among species in their epidemiology, pathogenesis, virulence, susceptibilities to the host immune response and antifungals, disease severity, and outcome [17][18][19]. However, most of the research has focused on Rhizopus oryzae, which is the most common mucormycosis causative agent [8].
1.1. Risk Factors
As mentioned before, these fungi are ubiquitously found in the environment, and their ability to cause disease in immunocompetent hosts is anecdotic but possible [20]. In the majority of the cases, the patients have underlying health conditions [12][21][22]. These patients have comorbidities or certain conditions that cause impairment of the host immune defenses and immunosuppression directly or indirectly [1][21], such as:
- -
- -
- -
- -
- -
- -
- -
1.2. Pathogenesis
Mucorales have many traits that contribute to their ability to cause such an aggressive disease, including thermotolerance [27][28], rapid growth and angioinvasive nature [27][29], cell wall remodeling to endure hostile environments [29], iron uptake from the host [6][8][30], ability to bind to glucose-regulated proteins on endothelial cells [31], downregulation of host genes involved in the immune response and tissue repair [22][32], and resistance to most available antifungals [33].
The fungus might enter the host through different vias, including spore inhalation, skin inoculation, or ingestion through the gastrointestinal tract [22]. Independently of the inoculation route, the establishment of the infection depends on several steps: inoculation of spores, immune response evasion, attachment to the endothelium, endocytosis, germination into hyphae, endothelial damage, and hematogenous dissemination [13][22][28]. Thus far, the most studied Mucorales virulence factors that participate in the infection process are the attachment to endothelial cells and iron uptake. Mucorales are vasotropic; therefore, the interaction between the fungus and endothelial cells around blood vessels is an indispensable step in mucormycosis pathogenesis [1][12], which explains the angioinvasive nature of these organisms.
Once in the host, spores attach to the basement membrane extracellular matrix proteins, laminin and collagen IV, and secrete proteolytic enzymes [34][35][36], subtilases [37], and lipolytic/glycosidic enzymes [38], which contribute to the destruction of the host tissue, which could have been already damaged by other host conditions, such as hyperglycemia or chemotherapy [8]. This interaction with the endothelial cells is specific and is through the spore-coat homolog (CotH) proteins [39], which bind to the receptor glucose-regulator protein 78 (GRP-78) [31]. It is well-reported that the expression levels of the CotH proteins determine the fungal virulence degree since high-virulent genera, such as Rhizopus, Mucor, and Lichtheimia, have three to seven CotH copies expressed, while low-virulent genera, such as Apophysomyces, Cunninghamella, Saksenaea, and Syncephalastrum, express only one to two copies, and Entomophthorales isolates, previously considered close to Mucorales, have no CotH genes and are avirulent [40].
The GRP-78 recognition by CotH proteins causes host cell death by the induction of endothelial cell-mediating endocytosis, initiating the fungal invasion of the endothelium [40]. The expression of both CotH and GRP-78 is highly enhanced in a hyperglycemic environment and with high concentrations of iron in acid pH, which is the reason diabetes and ketoacidosis are important risk factors for the development of mucormycosis [8][31]. GRP-78 binding to CotH proteins has been recognized as a unique mechanism for the Mucorales order since other opportunistic pathogens, such as Candida albicans and Aspergillus fumigatus, do not bind this endothelial cell receptor [31]. However, it is not the only factor involved in Mucorales binding to the host’s cells since blockage or suppression of GRP-78 does not completely abolish endothelial invasion [1]. In this regard, the platelet-derived growth factor receptor B (PDGFRB), found in the transcriptome of endothelial cells interacting with Rhizopus delemar, R. oryzae, and Mucor circinelliodes [40], also contributes to the fungus growth and angioinvasion since inhibition of PDGFRB phosphorylation partially reduces Mucorales-mediated endothelial damage in vitro [40].
When talking about fungal growth and replication, nutrition is a very important factor, and iron is essential for these processes. In Mucorales, iron uptake is performed by three mechanisms: low molecular weight iron chelators or siderophores, high-affinity iron permeases, and heme-oxygenases [6][8]. In healthy individuals, iron is bound to the serum proteins, but in diabetic patients with uncontrolled high glucose levels and ketoacidosis, iron is found unbound and in elevated concentrations, improving R. oryzae growth and causing phagocytosis defects [41][42]. In hyperglycemia conditions, excessive iron sequestering proteins glycation, such as transferrin, ferritin, and lactoferrin, is induced, which reduces iron affinity and increases free iron levels [1] while increasing CotH and GRP-78 expression [31][43]. Under acidic conditions due to the accumulation of ketone bodies, such as β-hydroxy butyrate, the transferrin capacity of chelating iron is impaired [24], and the GRP-78 expression is also increased [43]. Mucorales have an intrinsic siderophore known as rhizoferrin, which supplies the fungus with unbound iron through a receptor-mediated process [44][45] that, however, is incapable of obtaining iron bound to serum proteins [45][46], which is thought to be the main mechanism for iron uptake under hyperglycemic and acidic conditions. However, for iron uptake, Rhizopus is capable of using external siderophores, such as DFO, an iron chelator used as a treatment in patients with increased iron overload [8][46]. DFO chelates iron from transferrin and forms the complex iron-DFO, known as ferroxamine, which binds to the fungal cell surface receptors Fob1 and Fob2, where the iron from the complex is reduced [45][46][47], reoxidized, and transported intracellularly by the high-affinity iron permease FTR1 [30][45]. The use of DFO plays an important predisposing role in the development of highly lethal and disseminated mucormycosis [46]. FTR1 permease has been proved to be important for fungal pathogenicity since during infection, R. oryzae expresses FTR1, which, when inhibited or reduced, causes fungal virulence reduction [30].
Finally, hemoglobin can also be used as a source of ferric iron by Mucorales, to which they have access due to their angioinvasive ability [8]. Once the fungus transports hemoglobin intracellularly, the heme-oxygenases in the cytoplasm, through the reductase-permease system, enable the iron uptake from the host hemoglobin [29][30][45]. It is thought that during this process, FTR1 acts as a cytoplasmic membrane permease that facilitates intracellular heme uptake [6].
1.3. Immune Response during Mucormycosis
The main host defense against the Mucorales spores germination and dissemination are innate immune cells, consisting of circulating neutrophils, mononuclear cells, and macrophages [48]. Tissue and alveolar macrophages phagocytose the spores and kill them, and in the case where any spore survives and germinates, hyphae then induce neutrophils chemotaxis, which eliminate them through reactive oxygen metabolites, cationic peptides, perforin, and the production of proinflammatory cytokines, such as TNFα, INFγ, and IL-1β, which recruit and activate other immune cells [6][48][49][50]. However, other reports suggest that, although capable of phagocytosing R. oryzae spores and inhibiting their germination, macrophages cannot kill them due to the inhibition of LC3-associated phagocytosis (LAP), an important antifungal pathway that participates in the host defense regulation [51]. It was observed that melanin in the Rhizopus spore cell wall blocks phagosome responses, completely inhibiting phagosome maturation and allowing fungal intracellular persistence [48]. This fungistatic activity of macrophages and other phagocytes is thought to promote Mucorales virulence, prolonging fungal survival in the lungs and serving as transport of spores to other organs [51][52]. Therefore, it is suggested that macrophages’ fungicidal activity is dependent on the spores development stage since lack of cell wall remodeling and germination, which results in the retention of the cell wall melanin, inhibits LAP and therefore causes intracellular persistence [51][53]. In addition, recognition and phagocyte binding of Mucorales seem to be dependent on the spore wall composition [51], and the receptors participating in this process include Toll-like receptors, especially TLR-2, which plays an important role in Rhizopus recognition by neutrophils [48][49] and activation of proinflammatory cytokines such as IL-1β and TNFα [49].
Platelets also participate in the host immune response during this infection, as they strongly bind to Mucorales spores and hyphae. Once the platelets bind the pathogen and are activated, they damage hyphae in a time-dependent manner through the secretion of granules with pro- and anti-inflammatory cytokines and chemokines with antifungal properties [50][54] as well as increasing their tendency to aggregate, which enhances clot formation and suppresses hyphal growth and dissemination [19][50][54]. It was even observed that platelet antifungal activity is greater than that of polymorphonuclear leucocytes [54][55]. Additionally, these cells express membrane-bound molecules that bind to endothelial cells, monocytes, and dendritic cells, activating them and enhancing the immune response against the fungus [56].
Natural killer (NK) cells are also considered to have an important role in the immune response against Mucorales. It has been observed that NK cells are activated by R. oryzae hyphae and damage this morphology, mainly by the protein perforin [57][58] and depending on the amount of fungal biomass [59]. However, direct contact of R. oryzae hyphae with these cells decreases secretion of IFNγ and RANTES (Regulated upon Activation, Normal T-Cell Expressed, and Presumably Secreted), which are important molecules that improve the fungicidal activity of macrophages and the host defense against fungi [57][59]. Therefore, these cells’ activity is more effective in the early stage of the infection [58][59].
It has been demonstrated that Mucorales are capable of down-regulating genes involved in pathogen recognition, innate immune responses, and tissue repair mechanisms, facilitating fungal growth [60].
Although the knowledge regarding the adaptative response against Mucorales is scarce, it is already known that greater resistance to pulmonary mucormycosis is associated with an early Th-1 response, mediated by IFNγ and IL-2, while the infection control is mediated by the Th-17 response, with increased production of IL-17 and IL-2 by the spleen [61]. Just like in the pulmonary infection, in disseminated mucormycosis, IL-17 signaling and the Th1- response, through IFNγ signaling, are crucial for fungal clearance and thus control of the infection [61].
Finally, the patient’s health conditions, such as diabetes and acidosis, alter the immune response against Mucorales. It has been widely reported that hyperglycemia causes phagocyte dysfunction [62] and suppresses T lymphocyte induction and IFNγ production [8], while ketoacidosis impairs chemotaxis and neutrophils functions [6][32][38], all of them factors that favor the fungus growth and dissemination.
2. COVID-19-Associated Mucormycosis
Although the first report of mucormycosis in the literature dates from 1855 [63], and despite having extremely high mortality rates [2], this infection has not received enough attention, as much information about the disease and its etiological agents is still unknown. However, with the surge of the COVID-19 pandemic, a rise in mucormycosis cases has been reported [64][65], highlighting the severity and importance of both diseases, especially when combined. Before the COVID-19 pandemic, mucormycosis had a mortality rate of 50%, but now, the mortality of CAM has increased to 85% in India not only due to the COVID-19 infection but also due to crowed hospitals and inadequate infrastructure, ineffective healthcare resources, lack of healthcare workers, poor diagnosis, and lack of awareness [66][67]. Currently, CAM represents 0.3% of fungal COVID-19 coinfections [68].
The increase in CAM development has been mainly observed during the second wave of the COVID-19 pandemic, which might suggest a more effective association between mucormycosis and the SARS-CoV-2 delta variant, probably because it is more contagious and resistant to vaccines, with a higher risk of hospitalization and predisposition for the rhino-cerebral form, and also because of this variant’s ability to affect the pancreas, predisposing to hyperglycemia [69].
The majority of CAM cases reported worldwide are from India, followed by the United States, Egypt, Iran, Brazil, and Chile, and a few cases have also been reported in the United Kingdom, France, Italy, Austria, and Mexico [70].
According to epidemiological reports, it has been observed that, just like COVID-19, CAM is mainly present in male patients with diabetes, hypertension, or treated with glucocorticoids [64][70][71][72][73][74][75][76][77][78]. Furthermore, in concordance with what has been observed in mucormycosis, the main clinical form of CAM is the rhino-orbital/cerebral, followed by pulmonary, cutaneous, disseminated, and gastrointestinal forms, with the most common etiological agents being Rhizopus spp. [64][65][70][78].
CAM has been reported in patients with active SARS-CoV-2 infection but also in patients already recovered, and although the majority of CAM cases were reported in severe COVID-19 patients, the mycosis has also been found in mild/moderate SARS-CoV-2 infections [70]. The first evidence of CAM is usually found 15 days after COVID-19 diagnosis, but it can take up to 90 days to detect the earliest symptoms [79][80].
To cause such an aggressive and lethal infection, the causative mucormycosis fungi take advantage of the many alterations that SARS-CoV-2 infection generates on the host, such as (Figure 1):
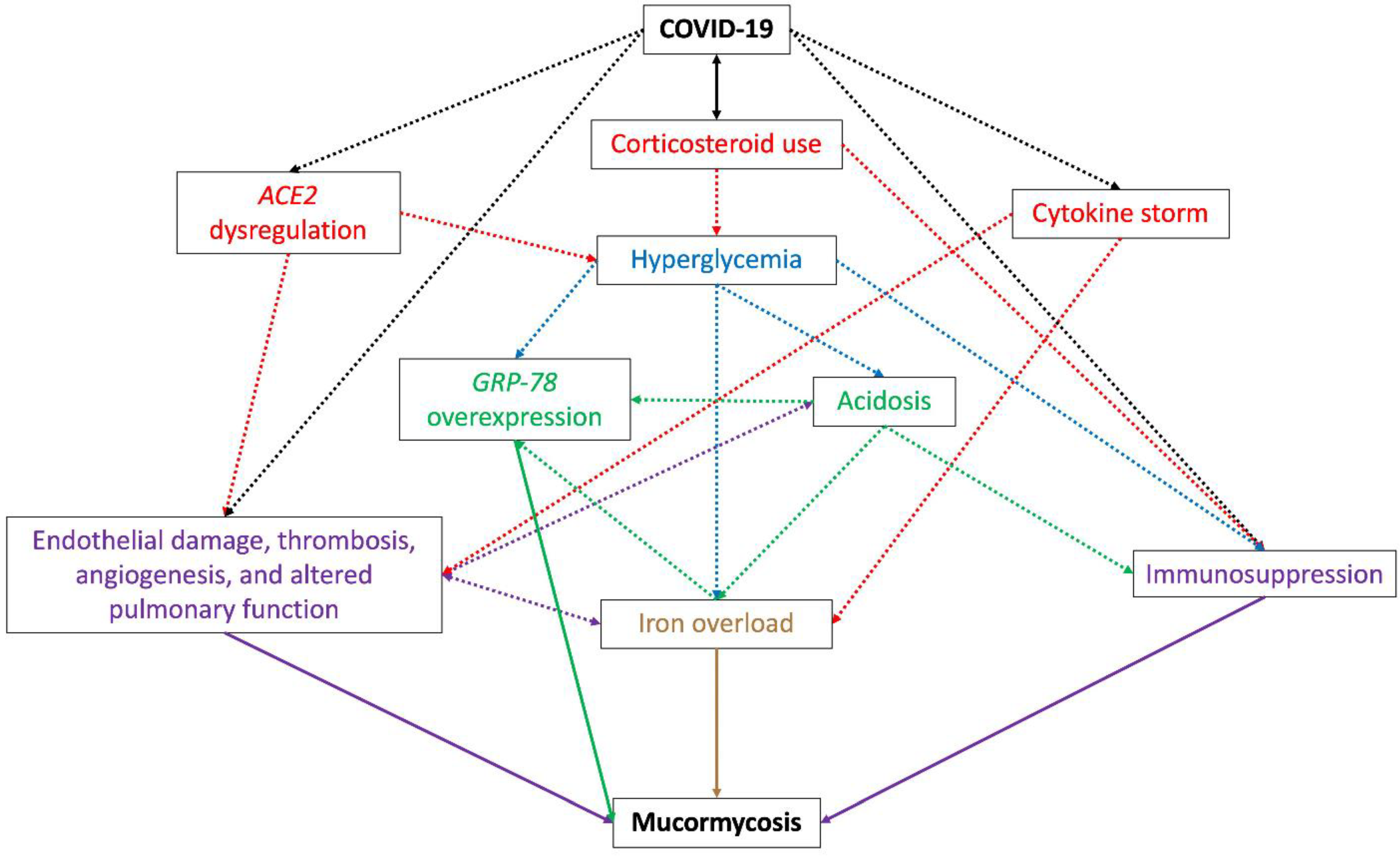 Figure 1. Schematic diagram representing the environment promoted by COVID-19 that predisposes the development of mucormycosis. The use of corticosteroids for COVID treatment and the dysregulation of ACE2 caused by SARS-CoV-2 create a hyperglycemic status in the patient, which causes overexpression of GRP-78, acidosis, and immunosuppression, this last also promoted by corticosteroid use, acidosis, and the COVID-19 itself. GRP-78 overexpression and acidosis are closely related since the presence of one promotes the development of the other, both causing iron overload; this last is also promoted by the cytokine storm observed during COVID-19. ACE2 dysregulation is related to the development of endothelial damage, thrombosis, angiogenesis, and altered pulmonary function, which can also be caused by the SARS-CoV-2 replication and the cytokine storm. Iron overload, GRP-78 overexpression, immunosuppression, endothelial damage, thrombosis, angiogenesis, and altered pulmonary function are directly related to mucormycosis development during COVID-19. Double black arrow: a risk factor for COVID-19 and mucormycosis infection. Dotted black lines: alterations directly caused by SARS-CoV-2 and COVID-19. Dotted red, blue, green, and purple lines: alterations caused by COVID-19. Purple, green, and brown lines: factors directly related to the development of mucormycosis.
Figure 1. Schematic diagram representing the environment promoted by COVID-19 that predisposes the development of mucormycosis. The use of corticosteroids for COVID treatment and the dysregulation of ACE2 caused by SARS-CoV-2 create a hyperglycemic status in the patient, which causes overexpression of GRP-78, acidosis, and immunosuppression, this last also promoted by corticosteroid use, acidosis, and the COVID-19 itself. GRP-78 overexpression and acidosis are closely related since the presence of one promotes the development of the other, both causing iron overload; this last is also promoted by the cytokine storm observed during COVID-19. ACE2 dysregulation is related to the development of endothelial damage, thrombosis, angiogenesis, and altered pulmonary function, which can also be caused by the SARS-CoV-2 replication and the cytokine storm. Iron overload, GRP-78 overexpression, immunosuppression, endothelial damage, thrombosis, angiogenesis, and altered pulmonary function are directly related to mucormycosis development during COVID-19. Double black arrow: a risk factor for COVID-19 and mucormycosis infection. Dotted black lines: alterations directly caused by SARS-CoV-2 and COVID-19. Dotted red, blue, green, and purple lines: alterations caused by COVID-19. Purple, green, and brown lines: factors directly related to the development of mucormycosis.-
Hyperglycemia, caused by the use of corticosteroids to treat COVID-19 [81] and dysregulation of the host receptor ACE2 (angiotensin-converting enzyme 2) observed during the viral infection [82][83] (explained in vii). Pre-existing diabetes is the main risk factor in most CAM cases [64] and is also related to an increase in the severity of SARS-CoV-2 infection [84];
-
Free iron availability, caused by hyperglycemia, the COVID-19 cytokine storm, or by acidosis. Hyperglycemia and diabetic ketoacidosis cause dissociation of iron from ferritin and lactoferrin, thus elevating free iron concentrations [90], and some cytokines, mainly IL-6, stimulate ferritin synthesis and decrease iron export, increasing intracellular iron storage [91][92][93] and causing tissue damage and thus the release of iron into circulation [94]. Therapeutic intervention with lactoferrin has been suggested to revert iron availability [95][96][97];
-
Overexpression of endothelial cells GRP-78, caused by hyperglycemia, acidosis, and iron availability, which enables angioinvasion, hematogenous dissemination, and tissue necrosis [31];
-
Dysregulation of ACE2 by SARS-CoV-2. By being present in many organs and tissues, the alteration of the receptor ACE2 expression causes a suitable environment for the development of mucormycosis. During COVID-19, a downregulation of ACE2 has been observed in the lungs [100], which causes inflammation, leukocytes exudation, and altered pulmonary function and therefore poor oxygenation [82]. Additionally, the effect of the virus on ACE2 in the pancreatic beta cells causes hyperglycemia, while dysregulation of the receptor in the vascular endothelium causes endothelial damage and vascular thrombosis, leading to vascular endothelial injury and venous stasis, causing an increase in serum iron due to hemolysis [82][83];
In addition, the use of industrial oxygen in COVID-19 patients due to a shortage of medical oxygen and the re-use of oxygen masks is suggested to be related to the development of CAM, especially in third-world countries. Unlike medical oxygen, industrial oxygen is not purified nor stored in disinfected cylinders [78][101].
References
- Baldin, C.; Ibrahim, A.S. Molecular mechanisms of mucormycosis—The bitter and the sweet. PLOS Pathog. 2017, 13, e1006408.
- Gleissner, B.; Schilling, A.; Anagnostopolous, I.; Siehl, I.; Thiel, E. Improved Outcome of Zygomycosis in Patients with Hematological Diseases? Leuk. Lymphoma 2004, 45, 1351–1360.
- Dignani, M.C. Epidemiology of invasive fungal diseases on the basis of autopsy reports. F1000Prime Rep. 2014, 6, 81.
- Chakrabarti, A.; Singh, R. The emerging epidemiology of mould infections in developing countries. Curr. Opin. Infect. Dis. 2011, 24, 521–526.
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867.
- Ibrahim, A.S.; Spellberg, B.; Walsh, T.J.; Kontoyiannis, D.P. Pathogenesis of Mucormycosis. Clin. Infect. Dis. 2012, 54, S16–S22.
- Park, H.-R.; Voigt, K. Interaction of Zygomycetes with innate immune cells reconsidered with respect to ecology, morphology, evolution and infection biology: A mini-review. Mycoses 2014, 57, 31–39.
- Challa, S. Mucormycosis: Pathogenesis and Pathology. Curr. Fungal Infect. Rep. 2019, 13, 11–20.
- Spellberg, B.; Edwards, J., Jr.; Ibrahim, A. Novel Perspectives on Mucormycosis: Pathophysiology, Presentation, and Management. Clin. Microbiol. Rev. 2005, 18, 556–569.
- Hassan, M.A.; Voigt, K. Pathogenicity patterns of mucormycosis: Epidemiology, interaction with immune cells and virulence factors. Med Mycol. 2019, 57, S245–S256.
- Kwon-Chung, K.J. Taxonomy of Fungi Causing Mucormycosis and Entomophthoramycosis (Zygomycosis) and Nomenclature of the Disease: Molecular Mycologic Perspectives. Clin. Infect. Dis. 2012, 54, S8–S15.
- Petrikkos, G.; Skiada, A.; Lortholary, O.; Roilides, E.; Walsh, T.J.; Kontoyiannis, D.P. Epidemiology and Clinical Manifestations of Mucormycosis. Clin. Infect. Dis. 2012, 54 (Suppl. 1), S23–S34.
- Farmakiotis, D.; Kontoyiannis, D.P. Mucormycoses. Infect. Dis. Clin. North Am. 2016, 30, 143–163.
- Sun, H.-Y.; Singh, N. Mucormycosis: Its contemporary face and management strategies. Lancet Infect. Dis. 2011, 11, 301–311.
- Richardson, M. The ecology of the Zygomycetes and its impact on environmental exposure. Clin. Microbiol. Infect. 2009, 15, 2–9.
- Idnurm, A. Sex Determination in the First-Described Sexual Fungus. Eukaryot. Cell 2011, 10, 1485–1491.
- Katragkou, A.; Walsh, T.J.; Roilides, E. Why is mucormycosis more difficult to cure than more common mycoses? Clin. Microbiol. Infect. 2014, 20, 74–81.
- Spellberg, B. Mucormycosis pathogenesis: Beyond Rhizopus. Virulence 2017, 8, 1481–1482.
- Schulze, B.; Rambach, G.; Schwartze, V.U.; Voigt, K.; Schubert, K.; Speth, C.; Jacobsen, I.D. Ketoacidosis alone does not predispose to mucormycosis by Lichtheimia in a murine pulmonary infection model. Virulence 2017, 8, 1657–1667.
- Chandra, S.; Sharma, S.; Vats, R.; Pandey, S. Isolated cerebral mucormycosis masquerading as a tumor in an immunocompetent patient. Autops. Case Rep. 2020, 11, e2020233.
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and Outcome of Zygomycosis: A Review of 929 Reported Cases. Clin. Infect. Dis. 2005, 41, 634–653.
- Petrikkos, G.; Tsioutis, C. Recent Advances in the Pathogenesis of Mucormycoses. Clin. Ther. 2018, 40, 894–902.
- Bonifaz, A.; Tirado-Sánchez, A.; Hernández-Medel, M.L.; Araiza, J.; Kassack, J.J.; del Angel-Arenas, T.; Moisés-Hernández, J.F.; Paredes-Farrera, F.; Gómez-Apo, E.; Treviño-Rangel, R.D.J.; et al. Mucormycosis at a tertiary-care center in Mexico. A 35-year retrospective study of 214 cases. Mycoses 2020, 64, 372–380.
- Artis, W.M.; Fountain, J.A.; Delcher, H.K.; Jones, H.E. A Mechanism of Susceptibility to Mucormycosis in Diabetic Ketoacidosis Transferrin and Iron Availability. Diabetes 1982, 31, 1109–1114.
- Lamaris, G.A.; Ben-Ami, R.; Lewis, R.; Chamilos, G.; Samonis, G.; Kontoyiannis, D.P. Increased Virulence of Zygomycetes Organisms Following Exposure to Voriconazole: A Study Involving Fly and Murine Models of Zygomycosis. J. Infect. Dis. 2009, 199, 1399–1406.
- Benedict, K.; Park, B.J. Invasive Fungal Infections after Natural Disasters. Emerg. Infect. Dis. 2014, 20, 349–355.
- Sugar, A.M. Mucormycosis. Clin. Infect. Dis. 1992, 14, S126–S129.
- Lewis, R.E.; Kontoyiannis, D.P. Epidemiology and treatment of mucormycosis. Futur. Microbiol. 2013, 8, 1163–1175.
- Ma, L.-J.; Ibrahim, A.S.; Skory, C.; Grabherr, M.G.; Burger, G.; Butler, M.; Elias, M.; Idnurm, A.; Lang, B.F.; Sone, T.; et al. Genomic Analysis of the Basal Lineage Fungus Rhizopus oryzae Reveals a Whole-Genome Duplication. PLoS Genet. 2009, 5, e1000549.
- Ibrahim, A.S.; Gebremariam, T.; Lin, L.; Luo, G.; Husseiny, M.I.; Skory, C.D.; Fu, Y.; French, S.W.; Edwards, J.E., Jr.; Spellberg, B. The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis. Mol. Microbiol. 2010, 77, 587–604.
- Liu, M.; Spellberg, B.; Phan, Q.T.; Fu, Y.; Fu, Y.; Lee, A.; Edwards, J.E.; Filler, S.G.; Ibrahim, A.S. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J. Clin. Investig. 2010, 120, 1914–1924.
- Binder, U.; Maurer, E.; Lass-Flörl, C. Mucormycosis–from the pathogens to the disease. Clin. Microbiol. Infect. 2014, 20, 60–66.
- Tissot, F.; Agrawal, S.; Pagano, L.; Petrikkos, G.; Groll, A.H.; Skiada, A.; Lass-Flörl, C.; Calandra, T.; Viscoli, C.; Herbrecht, R. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 2016, 102, 433–444.
- Wang, H.L.; Hesseltine, C.W. Studies on the extracellular proteolytic enzymes of rhizopus oligosporus. Can. J. Microbiol. 1965, 11, 727–732.
- Staib, F. Extracellular proteolysis by Mucoraceae in serum-albumin agar tested by the agar block method. Mycoses 2009, 34, 231–233.
- Schoen, C.; Reichard, U.; Monod, M.; Kratzin, H.D.; Rüchel, R. Molecular cloning of an extracellular aspartic proteinase fromRhizopus microsporusand evidence for its expression during infection. Med Mycol. 2002, 40, 61–71.
- Spreer, A.; Rüchel, R.; Reichard, U. Characterization of an extracellular subtilisin protease ofRhizopus microsporusand evidence for its expression during invasive rhinoorbital mycosis. Med Mycol. 2006, 44, 723–731.
- Ribes, J.A.; Vanover-Sams, C.L.; Baker, D.J. Zygomycetes in Human Disease. Clin. Microbiol. Rev. 2000, 13, 236–301.
- Gebremariam, T.; Liu, M.; Luo, G.; Bruno, V.; Phan, Q.T.; Waring, A.J.; Edwards, J.E.; Filler, S.G.; Yeaman, M.R.; Ibrahim, A.S. CotH3 mediates fungal invasion of host cells during mucormycosis. J. Clin. Investig. 2013, 124, 237–250.
- Chibucos, M.C.; Soliman, S.; Gebremariam, T.; Lee, H.; Daugherty, S.; Orvis, J.; Shetty, A.; Crabtree, J.; Hazen, T.H.; Etienne, K.A.; et al. An integrated genomic and transcriptomic survey of mucormycosis-causing fungi. Nat. Commun. 2016, 7, 12218.
- Cantinieaux, B.; Janssens, A.; Boelaert, J.; Lejeune, M.; Vermylen, C.; Kerrels, V.; Cornu, G.; Winand, J.; Fondu, P. Ferritin-associated iron induces neutrophil dysfunction in hemosiderosis. J. Lab. Clin. Med. 1999, 133, 353–361.
- Shirazi, F.; Kontoyiannis, D.P.; Ibrahim, A.S. Iron starvation induces apoptosis in rhizopus oryzae in vitro. Virulence 2015, 6, 121–126.
- Gebremariam, T.; Lin, L.; Liu, M.; Kontoyiannis, D.P.; French, S.; Edwards, J.E.; Filler, S.G.; Ibrahim, A.S. Bicarbonate correction of ketoacidosis alters host-pathogen interactions and alleviates mucormycosis. J. Clin. Investig. 2016, 126, 2280–2294.
- Thieken, A.; Winkelmann, G.; Winkelmann, G. Rhizoferrin: A complexone type siderophore of the mocorales and entomophthorales (Zygomycetes). FEMS Microbiol. Lett. 1992, 94, 37–41.
- De Locht, M.; Boelaert, J.R.; Schneider, Y.-J. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of rhizopus microsporus. Biochem. Pharmacol. 1994, 47, 1843–1850.
- Boelaert, J.R.; De Locht, M.; Van Cutsem, J.; Kerrels, V.; Cantinieaux, B.; Verdonck, A.; Van Landuyt, H.W.; Schneider, Y.-J. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. J. Clin. Investig. 1993, 91, 1979–1986.
- Liu, M.; Lin, L.; Gebremariam, T.; Luo, G.; Skory, C.D.; French, S.W.; Chou, T.-F.; Edwards, J.E., Jr.; Ibrahim, A.S. Fob1 and Fob2 Proteins Are Virulence Determinants of Rhizopus oryzae via Facilitating Iron Uptake from Ferrioxamine. PLoS Pathog. 2015, 11, e1004842.
- Roilides, E.; Kontoyiannis, D.P.; Walsh, T.J. Host Defenses Against Zygomycetes. Clin. Infect. Dis. 2012, 54, S61–S66.
- Chamilos, G.; Lewis, R.E.; Lamaris, G.; Walsh, T.J.; Kontoyiannis, D.P. Zygomycetes Hyphae Trigger an Early, Robust Proinflammatory Response in Human Polymorphonuclear Neutrophils through Toll-Like Receptor 2 Induction but Display Relative Resistance to Oxidative Damage. Antimicrob. Agents Chemother. 2008, 52, 722–724.
- .Ibrahim, A.S.; Voelz, K. The mucormycete–host interface. Curr. Opin. Microbiol. 2017, 40, 40–45.
- Andrianaki, A.M.; Kyrmizi, I.; Thanopoulou, K.; Baldin, C.; Drakos, E.; Soliman, S.S.M.; Shetty, A.C.; McCracken, C.; Akoumianaki, T.; Stylianou, K.; et al. Iron restriction inside macrophages regulates pulmonary host defense against Rhizopus species. Nat. Commun. 2018, 9, 1–17.
- Kraibooj, K.; Park, H.-R.; Dahse, H.-M.; Skerka, C.; Voigt, K.; Figge, M.T. Virulent strain ofLichtheimia corymbiferashows increased phagocytosis by macrophages as revealed by automated microscopy image analysis. Mycoses 2014, 57, 56–66.
- Ghuman, H.; Voelz, K. Innate and Adaptive Immunity to Mucorales. J. Fungi 2017, 3, 48.
- Perkhofer, S.; Kainzner, B.; Kehrel, B.E.; Dierich, M.P.; Nussbaumer, W.; Lass-Flörl, C. Potential Antifungal Effects of Human Platelets against Zygomycetes In Vitro. J. Infect. Dis. 2009, 200, 1176–1179.
- Simitsopoulou, M.; Roilides, E.; Maloukou, A.; Gil-Lamaignere, C.; Walsh, T.J. Interaction of amphotericin B lipid formulations and triazoles with human polymorphonuclear leucocytes for antifungal activity against Zygomycetes. Mycoses 2008, 51, 147–154.
- Speth, C.; Lass-Flörl, C.; Rambach, G. Platelet immunology in fungal infections. Thromb. Haemost. 2014, 112, 632–639.
- Schmidt, S.; Tramsen, L.; Perkhofer, S.; Lass-Flörl, C.; Hanisch, M.; Röger, F.; Klingebiel, T.; Koehl, U.; Lehrnbecher, T. Rhizopus oryzae hyphae are damaged by human natural killer (NK) cells, but suppress NK cell mediated immunity. Immunobiology 2013, 218, 939–944.
- Schmidt, S.; Tramsen, L.; Lehrnbecher, T. Natural Killer Cells in Antifungal Immunity. Front. Immunol. 2017, 8, 1623.
- Schmidt, S.; Schneider, A.; Demir, A.; Lass-Flörl, C.; Lehrnbecher, T. Natural killer cell-mediated damage of clinical isolates of mucormycetes. Mycoses 2015, 59, 34–38.
- Chamilos, G.; Lewis, R.E.; Hu, J.; Xiao, L.; Zal, T.; Gilliet, M.; Halder, G.; Kontoyiannis, D.P. Drosophila melanogaster as a model host to dissect the immunopathogenesis of zygomycosis. Proc. Natl. Acad. Sci. USA 2008, 105, 9367–9372.
- dos Santos, A.R.; Fraga-Silva, T.F.; Almeida-Donanzam, D.D.F.; dos Santos, R.F.; Finato, A.C.; Soares, C.T.; Lara, V.S.; Almeida, N.L.M.; Andrade, M.I.; de Arruda, O.S.; et al. IFN-γ Mediated Signaling Improves Fungal Clearance in Experimental Pulmonary Mucormycosis. Mycopathologia 2022, 187, 15–30.
- Chinn, R.Y.; Diamond, R.D. Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: Relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect. Immun. 1982, 38, 1123–1129.
- Paltauf, A. Mycosis mucorina. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 2005, 102, 543–564.
- Singh, A.K.; Singh, R.; Joshi, S.R.; Misra, A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102146.
- Al-Tawfiq, J.A.; Alhumaid, S.; Alshukairi, A.N.; Temsah, M.-H.; Barry, M.; Al Mutair, A.; Rabaan, A.A.; Al-Omari, A.; Tirupathi, R.; AlQahtani, M.; et al. COVID-19 and mucormycosis superinfection: The perfect storm. Infection 2021, 49, 833–853.
- Chakrabarti, A. The recent mucormycosis storm over Indian sky. Indian J. Med Microbiol. 2021, 39, 269–270.
- Suvvari, T.K.; Arigapudi, N.; Kandi, V.R.; Kutikuppala, L.S. Mucormycosis: A killer in the shadow of COVID-19. J. De Mycol. Med. 2021, 31, 101161.
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170.
- Arakeri, G.; Rao Us, V.; Mendes, R.A.; Oeppen, R.S.; Brennan, P.A. COVID-associated mucormycosis (CAM): Is the Delta variant a cause? Br. J. Oral Maxillofac. Surg. 2021, 59, 1095–1098.
- Pal, R.; Singh, B.; Bhadada, S.K.; Banerjee, M.; Bhogal, R.S.; Hage, N.; Kumar, A. COVID-19-associated mucormycosis: An updated systematic review of literature. Mycoses 2021, 64, 1452–1459.
- Patel, A.; Agarwal, R.; Rudramurthy, S.M.; Shevkani, M.; Xess, I.; Sharma, R.; Savio, J.; Sethuraman, N.; Madan, S.; Shastri, P.; et al. Multicenter Epidemiologic Study of Coronavirus Disease–Associated Mucormycosis, India. Emerg. Infect. Dis. 2021, 27, 2349–2359.
- Arora, S.; Hemmige, V.S.; Mandke, C.; Chansoria, M.; Rawat, S.K.; Dravid, A.; Sethi, Y.; Medikeri, G.; Jariwala, S.P.; Puius, Y.A. Online Registry of COVID-19–Associated Mucormycosis Cases, India, 2021. Emerg. Infect. Dis. 2021, 27, 2963–2965.
- Sharma, S.; Grover, M.; Bhargava, S.; Samdani, S.; Kataria, T. Post coronavirus disease mucormycosis: A deadly addition to the pandemic spectrum. J. Laryngol. Otol. 2021, 135, 442–447.
- Afzal, S.; Nasir, M. Aspergillosis and mucormycosis in COVID-19 patients; a systematic review and meta-analysis. Medrxiv 2021.
- Ramaswami, A.; Sahu, A.K.; Kumar, A.; Suresh, S.; Nair, A.; Gupta, D.; Chouhan, R.; Bhat, R.; Mathew, R.; Majeed, J.A.; et al. COVID-19-associated mucormycosis presenting to the Emergency Department—an observational study of 70 patients. QJM: Int. J. Med. 2021, 114, 464–470.
- Selarka, L.; Sharma, S.; Saini, D.; Sharma, S.; Batra, A.; Waghmare, V.T.; Dileep, P.; Patel, S.; Shah, M.; Parikh, T.; et al. Mucormycosis and COVID-19: An epidemic within a pandemic in India. Mycoses 2021, 64, 1253–1260.
- Bhanuprasad, K.; Manesh, A.; Devasagayam, E.; Varghese, L.; Cherian, L.M.; Kurien, R.; Karthik, R.; Deodhar, D.; Vanjare, H.; Peter, J.; et al. Risk factors associated with the mucormycosis epidemic during the COVID-19 pandemic. Int. J. Infect. Dis. 2021, 111, 267–270.
- Aranjani, J.M.; Manuel, A.; Razack, H.I.A.; Mathew, S.T. COVID-19–associated mucormycosis: Evidence-based critical review of an emerging infection burden during the pandemic’s second wave in India. PLOS Neglected Trop. Dis. 2021, 15, e0009921.
- Khatri, A.; Chang, K.-M.; Berlinrut, I.; Wallach, F. Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient–case report and review of literature. J. Mycol. Med. 2021, 31, 101125.
- Honavar, S.G.; Sen, M.; Lahane, S.; Lahane, T.P.; Parekh, R. Mucor in a Viral Land: A Tale of Two Pathogens. Indian J. Ophthalmol. 2021, 69, 244–252.
- Freeland, B.; Funnell, M. Corticosteroid-induced hyperglycemia. Nursing 2012, 42, 68–69.
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8.
- Agnihotri, A.K.; Vij, M.; Aruoma, O.I.; Yagnik, V.D.; Bahorun, T.; Villamil, M.E.; Menezes, G.A.; Gupta, V. The double trouble: COVID-19 associated mucormycosis a focused review and future perspectives. Glob. J. Medical, Pharm. Biomed. Updat. 2021, 16, 4.
- Lim, S.; Bae, J.H.; Kwon, H.-S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2020, 17, 11–30.
- Hulter, H.N.; Licht, J.H.; Bonner, E.L.; Glynn, R.D.; Sebastián, A. Effects of glucocorticoid steroids on renal and systemic acid-base metabolism. Am. J. Physiol. Physiol. 1980, 239, F30–F43.
- Damgaci, S.; Ibrahim-Hashim, A.; Enríquez-Navas, P.M.; Pilon-Thomas, S.; Guvenis, A.; Gillies, R.J. Hypoxia and acidosis: Immune suppressors and therapeutic targets. Immunology 2018, 154, 354–362.
- Bhogireddy, R.; Krishnamurthy, V.; Jabaris S, S.L.; Pullaiah, C.P.; Manohar, S. Is Mucormycosis an inevitable complication of COVID-19 in India? Braz. J. Infect. Dis. 2021, 25, 101597.
- Ruhl, L.; Pink, I.; Kühne, J.F.; Beushausen, K.; Keil, J.; Christoph, S.; Sauer, A.; Boblitz, L.; Schmidt, J.; David, S.; et al. Endothelial dysfunction contributes to severe COVID-19 in combination with dysregulated lymphocyte responses and cytokine networks. Signal Transduct. Target. Ther. 2021, 6, 418.
- Elyaspour, Z.; Zibaeenezhad, M.J.; Razmkhah, M.; Razeghian-Jahromi, I. Is It All About Endothelial Dysfunction and Thrombosis Formation? The Secret of COVID-19. Clin. Appl. Thromb. 2021, 27, 10760296211042940.
- Lugito, N.P.H.; Cucunawangsih, C. How Does Mucorales Benefit from the Dysregulated Iron Homeostasis During SARS-CoV-2 Infection? Mycopathologia 2021, 186, 877–882.
- Kawasumi, H.; Gono, T.; Kawaguchi, Y.; Kaneko, H.; Katsumata, Y.; Hanaoka, M.; Kataoka, S.; Yamanaka, H. IL-6, IL-8, and IL-10 Are Associated with Hyperferritinemia in Rapidly Progressive Interstitial Lung Disease with Polymyositis/Dermatomyositis. BioMed Res. Int. 2014, 2014, 815245.
- Perricone, C.; Bartoloni, E.; Bursi, R.; Cafaro, G.; Guidelli, G.M.; Shoenfeld, Y.; Gerli, R. COVID-19 as part of the hyperferritinemic syndromes: The role of iron depletion therapy. Immunol. Res. 2020, 68, 213–224.
- John, T.; Jacob, C.; Kontoyiannis, D. When Uncontrolled Diabetes Mellitus and Severe COVID-19 Converge: The Perfect Storm for Mucormycosis. J. Fungi 2021, 7, 298.
- Edeas, M.; Saleh, J.; Peyssonnaux, C. Iron: Innocent bystander or vicious culprit in COVID-19 pathogenesis? Int. J. Infect. Dis. 2020, 97, 303–305.
- Campione, E.; Cosio, T.; Rosa, L.; Lanna, C.; Di Girolamo, S.; Gaziano, R.; Valenti, P.; Bianchi, L. Lactoferrin as Protective Natural Barrier of Respiratory and Intestinal Mucosa against Coronavirus Infection and Inflammation. Int. J. Mol. Sci. 2020, 21, 4903.
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin as Antiviral Treatment in COVID-19 Management: Preliminary Evidence. Int. J. Environ. Res. Public Heal. 2021, 18, 10985.
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin Against SARS-CoV-2: In Vitro and In Silico Evidences. Front. Pharmacol. 2021, 12, 666600.
- Mahalaxmi, I.; Jayaramayya, K.; Venkatesan, D.; Subramaniam, M.D.; Renu, K.; Vijayakumar, P.; Narayanasamy, A.; Gopalakrishnan, A.V.; Kumar, N.S.; Sivaprakash, P.; et al. Mucormycosis: An opportunistic pathogen during COVID-19. Environ. Res. 2021, 201, 111643.
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128.
- Glowacka, I.; Bertram, S.; Herzog, P.; Pfefferle, S.; Steffen, I.; Muench, M.O.; Simmons, G.; Hofmann, H.; Kuri, T.; Weber, F.; et al. Differential Downregulation of ACE2 by the Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus and Human Coronavirus NL63. J. Virol. 2010, 84, 1198–1205.
- Ong, J.J.; Chan, A.C.; Sharma, A.K.; Sharma, S.; Sharma, V.K. The mucormycosis epidemic within COVID-19 pandemic- lessons from India. Brain, Behav. Immun. 2021, 97, 4–5.
More
Information
Subjects:
Mycology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
COVID-19
Revisions:
2 times
(View History)
Update Date:
06 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




