
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Raghad AL-Ishaq | -- | 4056 | 2022-05-05 14:57:04 | | | |
| 2 | Amina Yu | -147 word(s) | 3909 | 2022-05-06 04:41:40 | | |
Video Upload Options
Gastrointestinal cancer (GI) is a global health disease with a huge burden on a patient’s physical and psychological aspects of life and on health care providers. It is associated with multiple disease related challenges which can alter the patient’s quality of life and well-being. GI cancer development is influenced by multiple factors such as diet, infection, environment, and genetics. Although activating immune pathways and components during cancer is critical for the host’s survival, cancerous cells can target those pathways to escape and survive. As the gut microbiome influences the development and function of the immune system, research is conducted to investigate the gut microbiome–immune interactions, the underlying mechanisms, and how they reduce the risk of GI cancer.
1. Gastrointestinal Cancer
2. The Immune System in Cancer Pathogenesis
3. Gut Microbiota: Role in GI Cancer Immunity
4. Microbiota–Immune Interactions: Role in GI Cancer Development
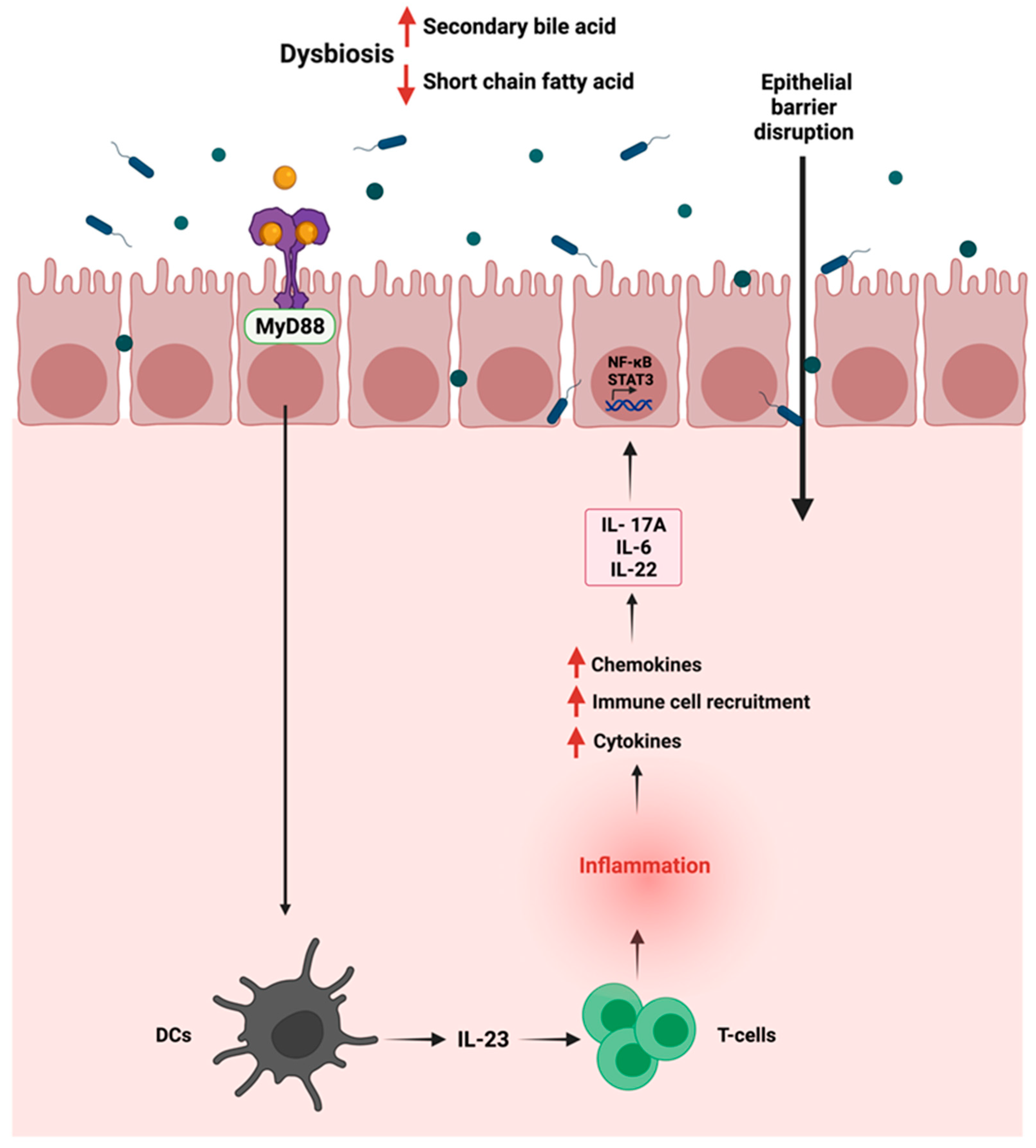
4.1. Inflammation
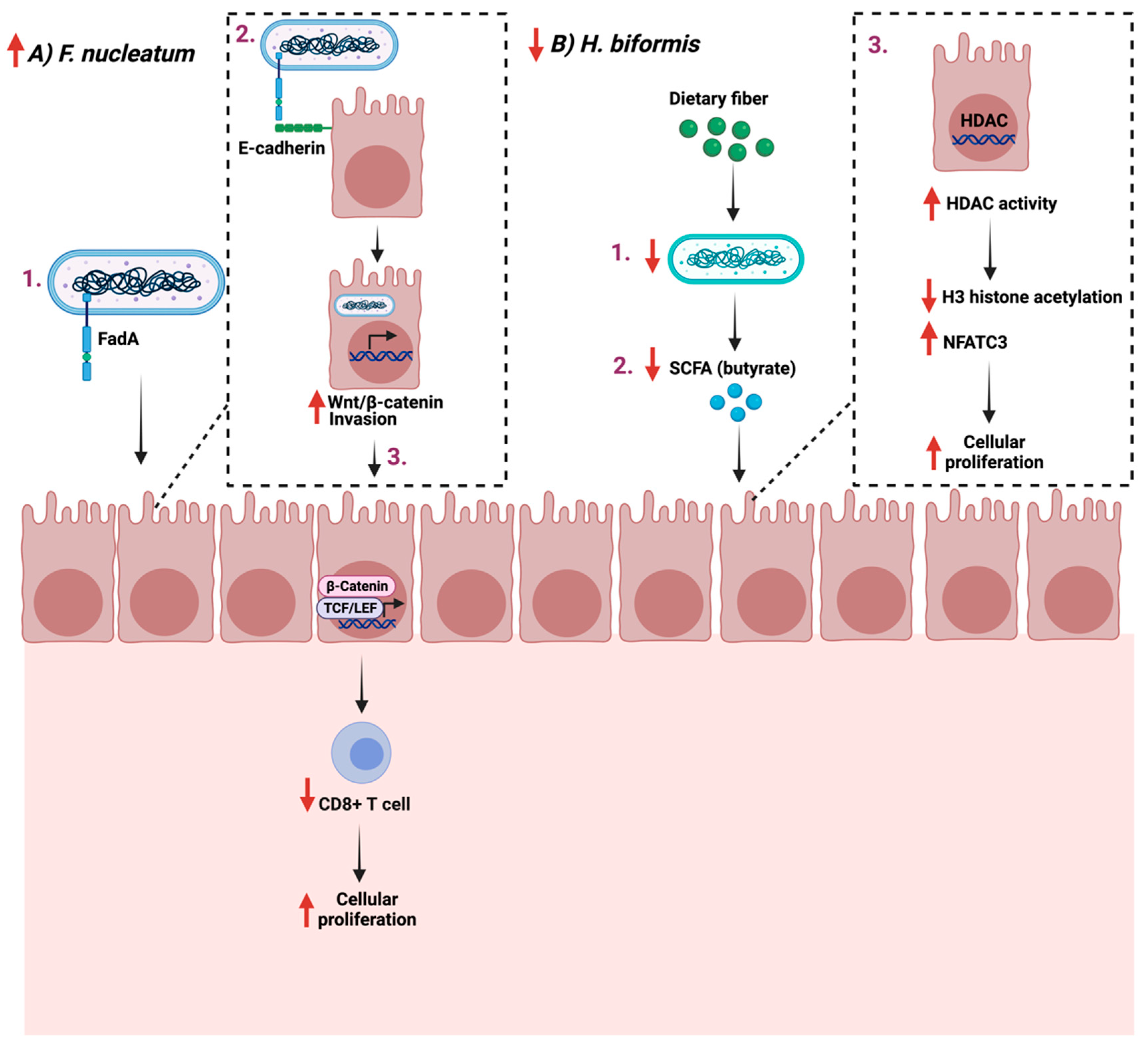
4.2. Cellular Proliferation
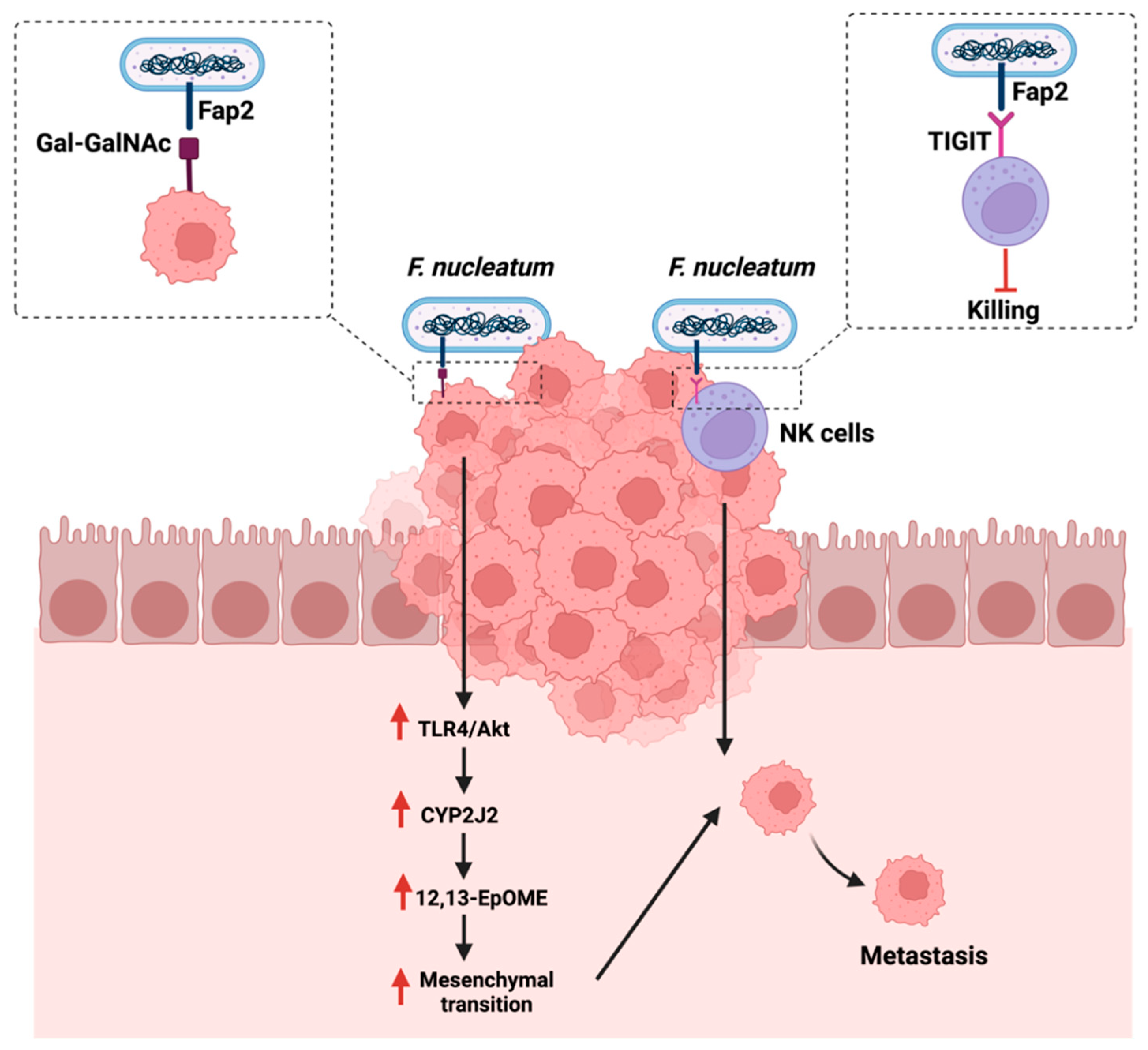
4.3. Metastasis
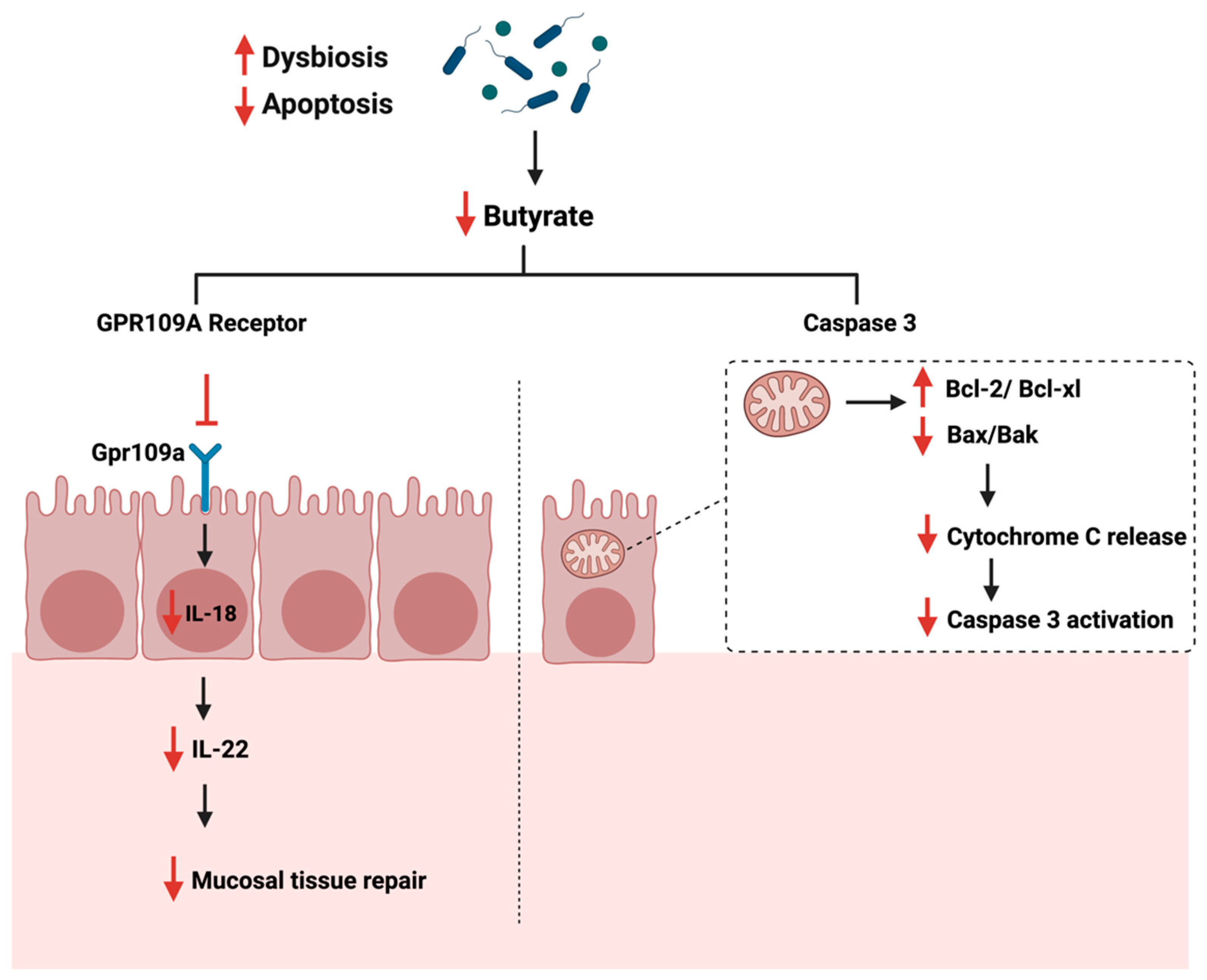
4.4. Apoptosis
5. Influence of Gut Microbiome on Immunotherapy
6. Future of GI Cancer Treatment?
The gut microbiome can interfere directly or indirectly with current treatments such as chemotherapy and immunotherapy, which might impact a treatment’s outcome. Manipulating the gut microbiome composition using fecal microbiota transplantation or phytochemicals might improve therapeutic outcomes [95]. Fecal microbiota transplantation (FMT) is known as the transplantation of microbes from the gut of a healthy donor to a recipient either through the upper or lower gastrointestinal tract [96]. It was first documented in clinical use in 1958 to treat Clostridium difficile infection as it helped treat 80% of the affected patients [97]. The advantages of using FMT include its safety and its ability to restore intestinal microbial diversity [98]. Limited are available that investigates the role and the application of FMT in the context of GI cancer treatment. It was found that reported the effectiveness of FMT in mice receiving intestinal microbiota from wild mice, as the results showed better resistance to CRC [99]. Additionally, and on a different approach, the usage of phytochemicals for GI cancer treatment has recently gained attention. The bioactive plant-derived compounds generally have lower oral bioavailability due to poor aqueous solubility, and therefore, the gut microbiome is essential for the metabolism and absorption of bioactive compounds [100]. Several data support the role of 13 bioactive secondary compounds on GI cancer [101]. For example, lutein, an abundant fat-soluble bioactive compound found primarily in green leaved vegetables, was reported to significantly reduce aberrant crypt foci (ACF) in the colon of mice, thus reducing cellular proliferation [102]. Despite those reports that support potential treatments, research is much needed to investigate the potential synergetic effects between the currently used treatments and FMT or phytochemicals. Additionally, attention should be given to the required concentration and the appropriate delivery mode of FMT and phytochemicals to avoid toxicity and possible side effects. Moreover, looking at the role of gut enzymes in the metabolism and the utilization of those natural bioactive compounds, more is needed to investigate the underlying mechanisms played by those enzymes that might affect the treatment outcome, as shown in [103].
7. Conclusions
The gut microbiome plays an essential role in mediating the immune response, impacting its activities, development, and function. Generally, and during cancer, signature microbes in the gut influence the anti-tumor activities by producing specific metabolites or inducing T-cell responses. On the other hand, some reported bacterial species enhance cellular proliferation and metastasis during cancer and understanding those interactions in the context of cancer may provide potential therapeutic targets. Despite the advances in the field, more research is needed to understand the underlying mechanisms, investigate the impact on current treatments, and identify specific microbes and immune cells that might lead to this interaction. Additionally, clinical trials are essential to assess the influence ofimmune–gut interaction on immunotherapy treatment in clinical settings.
References
- Hassanzade, J.; Molavi, E.V.H.; Farahmand, M.; Rajaiifard, A.R. Incidence and Mortality Rate of Common Gastrointestinal Cancers in South of Iran, a Population Based Study. Iran. J. Cancer Prev. 2011, 4, 163–169.
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012.
- Krieghoff-Henning, E.; Folkerts, J.; Penzkofer, A.; Weg-Remers, S. Cancer-an overview. Med. Mon. Pharm. 2017, 40, 48–54.
- Zali, H.; Rezaei-Tavirani, M.; Azodi, M. Gastric cancer: Prevention, risk factors and treatment. Gastroenterol. Hepatol. Bed Bench 2011, 4, 175–185.
- Rozen, P. Cancer of the gastrointestinal tract: Early detection or early prevention? Eur. J. Cancer Prev. 2020, 13, 71–75.
- Correa, P. Gastric cancer: Overview. Gastroenterol. Clin. N. Am. 2020, 42, 211–217.
- Igney, F.H.; Krammer, P.H. Death and anti-death: Tumour resistance to apoptosis. Nat. Rev. Cancer 2002, 2, 277–288.
- Qiao, L.; Wong, B.C. Targeting apoptosis as an approach for gastrointestinal cancer therapy. Drug Resist. Updates 2009, 12, 55–64.
- Turvey, S.E.; Broide, D.H. Innate immunity. J. Allergy Clin. Immunol. 2010, 125, S24–S32.
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49.
- Adams, J.L.; Smothers, J.; Srinivasan, R.; Hoos, A. Big opportunities for small molecules in immuno-oncology. Nat. Rev. Drug Discov. 2015, 14, 603–622.
- Pandya, P.H.; Murray, M.E.; Pollok, K.E.; Renbarger, J.L. The Immune System in Cancer Pathogenesis: Potential Therapeutic Approaches. J. Immunol. Res. 2016, 2016, 4273943.
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10.
- Harris, T.J.; Drake, C.G. Primer on tumor immunology and cancer immunotherapy. J. Immunother. Cancer 2013, 1, 12.
- Gregory, A.D.; Houghton, A.M. Tumor-associated neutrophils: New targets for cancer therapy. Cancer Res. 2011, 71, 2411–2416.
- Kwak, Y.; Seo, A.N.; Lee, H.E.; Lee, H.S. Tumor immune response and immunotherapy in gastric cancer. J. Pathol. Transl. Med. 2020, 54, 20–33.
- Golshani, G.; Zhang, Y. Advances in immunotherapy for colorectal cancer: A review. Ther. Adv. Gastroenterol. 2020, 13, 1756284820917527.
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86.
- Nasr, R.; Shamseddine, A.; Mukherji, D.; Nassar, F.; Temraz, S. The Crosstalk between Microbiome and Immune Response in Gastric Cancer. Int. J. Mol. Sci. 2020, 21, 6586.
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74.
- Meng, C.; Bai, C.; Brown, T.D.; Hood, L.E.; Tian, Q. Human Gut Microbiota and Gastrointestinal Cancer. Genomics Proteomics Bioinformatics 2018, 16, 33–49.
- Pham, F.; Moinard-Butot, F.; Coutzac, C.; Chaput, N. Cancer and immunotherapy: A role for microbiota composition. Eur. J. Cancer 2021, 155, 145–154.
- Yu, Q.; Jia, A.; Li, Y.; Bi, Y.; Liu, G. Microbiota regulate the development and function of the immune cells. Int. Rev. Immunol. 2018, 37, 79–89.
- Wesemann, D.R.; Portuguese, A.J.; Meyers, R.M.; Gallagher, M.P.; Cluff-Jones, K.; Magee, J.M.; Alt, F.W. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature 2013, 501, 112–115.
- Lathrop, S.K.; Bloom, S.M.; Rao, S.M.; Nutsch, K.; Lio, C.W.; Santacruz, N.; Hsieh, C.S. Peripheral education of the immune system by colonic commensal microbiota. Nature 2011, 478, 250–254.
- Zitvogel, L.; Ma, Y.; Raoult, D.; Kroemer, G.; Gajewski, T.F. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 2018, 359, 1366–1370.
- Cenit, M.C.; Sanz, Y.; Codoner-Franch, P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 2017, 23, 5486–5498.
- Tajik, N.; Frech, M.; Schulz, O.; Schalter, F.; Lucas, S.; Azizov, V.; Durholz, K.; Steffen, F.; Omata, Y.; Rings, A. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 2020, 11, 1995.
- Panebianco, C.; Andriulli, A.; Pazienza, V. Pharmacomicrobiomics: Exploiting the drug-microbiota interactions in anticancer therapies. Microbiome 2018, 6, 92.
- Dzutsev, A.; Badger, J.H.; Perez-Chanona, E.; Roy, S.; Salcedo, R.; Smith, C.K.; Trinchieri, G. Microbes and Cancer. Annu. Rev. Immunol. 2017, 35, 199–228.
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970.
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84.
- Tsuei, J.; Chau, T.; Mills, D.; Wan, Y.J. Bile acid dysregulation, gut dysbiosis, and gastrointestinal cancer. Exp. Biol. Med. 2014, 239, 1489–1504.
- Ge, Y.; Wang, X.; Guo, Y. Gut microbiota influence tumor development and Alter interactions with the human immune system. J. Exp. Clin. Cancer Res. 2021, 40, 42.
- Zhang, J.; Zhang, F.; Zhao, C.; Xu, Q.; Liang, C.; Yang, Y.; Wang, H.; Shang, Y.; Wang, Y.; Mu, X. Dysbiosis of the gut microbiome is associated with thyroid cancer and thyroid nodules and correlated with clinical index of thyroid function. Endocrine 2019, 64, 564–574.
- Aggarwal, B.B. Nuclear factor-kappaB: The enemy within. Cancer Cell 2004, 6, 203–208.
- Mantovani, A. Cancer: Inflammation by remote control. Nature 2005, 435, 752–753.
- Yao, H.; Wan, J.Y.; Zeng, J.; Huang, W.H.; Sava-Segal, C.; Li, L.; Yuan, C.S. Effects of compound K, an enteric microbiome metabolite of ginseng, in the treatment of inflammation associated colon cancer. Oncol. Lett. 2018, 15, 8339–8348.
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126.
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 36–44.
- Lee, T.C.; Huang, Y.C.; Lu, Y.Z.; Yeh, Y.C.; Yu, L.C. Hypoxia-induced intestinal barrier changes in balloon-assisted enteroscopy. J. Physiol. 2018, 596, 3411–3424.
- Tanaka, T.; Kohno, H.; Suzuki, R.; Hata, K.; Sugie, S.; Niho, N. Dextran sodium sulfate strongly promotes colorectal carcinogenesis in Apc (Min/+) mice: Inflammatory stimuli by dextran sodium sulfate results in development of multiple colonic neoplasms. Int. J. Cancer 2006, 118, 25–34.
- Song, X.; Gao, H.; Lin, Y.; Yao, Y.; Zhu, S.; Wang, J. Alterations in the Microbiota Drive Interleukin-17C Production from Intestinal Epithelial Cells to Promote Tumorigenesis. Immunity 2014, 40, 140–152.
- Cheng, Y.; Ling, Z.; Li, L. The Intestinal Microbiota and Colorectal Cancer. Front. Immunol. 2020, 11, 615056.
- Zou, S.; Fang, L.; Lee, M.H. Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol. Rep. 2018, 6, 1–12.
- Chu, F.; Li, Y.; Meng, X.; Li, Y.; Li, T.; Zhai, M.; Ding, X. Gut Microbial Dysbiosis and Changes in Fecal Metabolic Phenotype in Precancerous Lesions of Gastric Cancer Induced With N-Methyl-N’-Nitro-N-Nitrosoguanidine, Sodium Salicylate, Ranitidine, and Irregular Diet. Front. Physiol. 2021, 12, 733979.
- Matson, J.P.; Cook, J.G. Cell cycle proliferation decisions: The impact of single cell analyses. FEBS J. 2017, 284, 362–375.
- Hallstrom, T.C.; Nevins, J.R. Balancing the decision of cell proliferation and cell fate. Cell Cycle 2009, 8, 532–535.
- Duronio, R.J.; Xiong, Y. Signaling pathways that control cell proliferation. Cold Spring Harb. Perspect. Biol. 2013, 5, a008904.
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Nowsheen, S. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35, S25–S54.
- von Frieling, J.; Fink, C.; Hamm, J.; Klischies, K.; Forster, M.; Bosch, T.C.G.; Sommer, F. Grow with the Challenge-Microbial Effects on Epithelial Proliferation, Carcinogenesis, and Cancer Therapy. Front. Microbiol. 2018, 9, 2020.
- McAllister, F.; Housseau, F.; Sears, C.L. Microbiota and immune responses in colon cancer: More to learn. Cancer J. 2014, 20, 232–236.
- Wu, J.; Li, Q.; Fu, X. Fusobacterium nucleatum Contributes to the Carcinogenesis of Colorectal Cancer by Inducing Inflammation and Suppressing Host Immunity. Transl. Oncol. 2019, 12, 846–851.
- Han, Y.W. Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbiol. 2015, 23, 141–147.
- Bullman, S.; Pedamallu, C.S.; Sicinska, E.; Clancy, T.E.; Zhang, X.; Cai, D.; Meyerson, M. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 2017, 358, 1443–1448.
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206.
- Pai, S.G.; Carneiro, B.A.; Mota, J.M.; Costa, R.; Leite, C.A.; Barroso-Sousa, R.; Giles, F.J. Wnt/beta-catenin pathway: Modulating anti-cancer immune response. J. Hematol. Oncol. 2017, 10, 101.
- Zagato, E.; Pozzi, C.; Bertocchi, A.; Schioppa, T.; Saccheri, F.; Guglietta, S.; Rescigno, M. Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nat. Microbiol. 2020, 5, 511–524.
- Hanus, M.; Parada-Venegas, D.; Landskron, G.; Wielandt, A.M.; Hurtado, C.; Alvarez, K.; De la Fuente, M. Immune System, Microbiota, and Microbial Metabolites: The Unresolved Triad in Colorectal Cancer Microenvironment. Front. Immunol. 2021, 12, 612826.
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28.
- Hunter, K.W.; Crawford, N.P.; Alsarraj, J. Mechanisms of metastasis. Breast Cancer Res. 2008, 10, S2.
- McCoy, A.N.; Araujo-Perez, F.; Azcarate-Peril, A.; Yeh, J.J.; Sandler, R.S.; Keku, T.O. Fusobacterium is associated with colorectal adenomas. PLoS ONE 2013, 8, e53653.
- Sakamoto, Y.; Mima, K.; Ishimoto, T.; Ogata, Y.; Imai, K.; Miyamoto, Y.; Baba, H. Relationship between Fusobacterium nucleatum and anti-tumor immunity in colorectal cancer liver metastasis. Cancer Sci. 2021, 112, 4470–4477.
- Wang, S.; Liu, Y.; Li, J.; Zhao, L.; Yan, W.; Lin, B.; Wei, Y. Fusobacterium nucleatum Acts as a Pro-carcinogenic Bacterium in Colorectal Cancer: From Association to Causality. Front. Cell Dev. Biol. 2021, 9, 710165.
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum-symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166.
- Kaczanowski, S. Apoptosis: Its origin, history, maintenance and the medical implications for cancer and aging. Phys. Biol. 2016, 13, 031001.
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592.
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012, 19, 107–120.
- Jan, R.; Chaudhry, G.E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull 2019, 9, 205–218.
- Zhang, L.; Yu, J. Role of apoptosis in colon cancer biology, therapy, and prevention. Curr. Colorectal. Cancer Rep. 2013, 9, 331–340.
- Woznicki, J.A.; Flood, P.; Bustamante-Garrido, M.; Stamou, P.; Moloney, G.; Fanning, A.; Nally, K. Human BCL-G regulates secretion of inflammatory chemokines but is dispensable for induction of apoptosis by IFN-gamma and TNF-alpha in intestinal epithelial cells. Cell Death Dis. 2020, 11, 68.
- Tang, W.; Liu, J.; Ma, Y.; Wei, Y.; Liu, J.; Wang, H. Impairment of Intestinal Barrier Function Induced by Early Weaning via Autophagy and Apoptosis Associated with Gut Microbiome and Metabolites. Front. Immunol. 2021, 12, 804870.
- Waldecker, M.; Kautenburger, T.; Daumann, H.; Busch, C.; Schrenk, D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J. Nutr. Biochem. 2008, 19, 587–593.
- Chen, J.; Zhao, K.N.; Vitetta, L. Effects of Intestinal Microbial(-)Elaborated Butyrate on Oncogenic Signaling Pathways. Nutrients 2019, 11, 1026.
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277.
- Tian, Y.; Xu, Q.; Sun, L.; Ye, Y.; Ji, G. Short-chain fatty acids administration is protective in colitis-associated colorectal cancer development. J. Nutr. Biochem. 2018, 57, 103–109.
- Wang, T.; Cai, G.; Qiu, Y.; Fei, N.; Zhang, M.; Pang, X.; Zhao, L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012, 6, 320–329.
- Salcedo, R.; Worschech, A.; Cardone, M.; Jones, Y.; Gyulai, Z.; Dai, R.-M.; Wang, E.; Ma, W.; Haines, D.; O’Huigin, C. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: Role of interleukin 18. J. Exp. Med. 2010, 207, 1625–1636.
- Tye, H.; Yu, C.H.; Simms, L.A.; de Zoete, M.R.; Kim, M.L.; Zakrzewski, M.; Masters, S.L. NLRP1 restricts butyrate producing commensals to exacerbate inflammatory bowel disease. Nat. Commun. 2018, 9, 3728.
- Pan, L.J.; Ma, S.Y.; Wen, J.; Zhang, X.Q.; Xing, H.J.; Jia, C.S. Direct contact moxibustion promotes apoptosis of gastric cancer cells in rats by regulating intestinal flora. J. Tradit. Chin. Med. 2021, 41, 943–952.
- Zhang, Y.; Zhou, L.; Bao, Y.L.; Wu, Y.; Yu, C.L.; Huang, Y.X.; Sun, Y.; Zheng, L.H.; Li, Y.X. Butyrate induces cell apoptosis through activation of JNK MAP kinase pathway in human colon cancer RKO cells. Chem. Biol. Interact. 2010, 185, 174–181.
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792.
- Urruticoechea, A.; Alemany, R.; Balart, J.; Villanueva, A.; Vinals, F.; Capella, G. Recent advances in cancer therapy: An overview. Curr. Pharm. Des. 2010, 16, 3–10.
- Seager, R.J.; Hajal, C.; Spill, F.; Kamm, R.D.; Zaman, M.H. Dynamic interplay between tumour, stroma and immune system can drive or prevent tumour progression. Converg. Sci. Phys. Oncol. 2017, 3, 034002.
- Inthagard, J.; Edwards, J.; Roseweir, A.K. Immunotherapy: Enhancing the efficacy of this promising therapeutic in multiple cancers. Clin. Sci. 2019, 133, 181–193.
- Farkona, S.; Diamandis, E.P.; Blasutig, I.M. Cancer immunotherapy: The beginning of the end of cancer? BMC Med. 2016, 14, 73.
- Galluzzi, L.; Vacchelli, E.; Bravo-San Pedro, J.M.; Buque, A.; Senovilla, L.; Baracco, E.E.; Kroemer, G. Classification of current anti-cancer immunotherapies. Oncotarget 2014, 5, 12472–12508.
- Dine, J.; Gordon, R.; Shames, Y.; Kasler, M.K.; Barton-Burke, M. Immune Checkpoint Inhibitors: An Innovation in Immunotherapy for the Treatment and Management of Patients with Cancer. Asia Pac. J. Oncol. Nurs. 2017, 4, 127–135.
- Koury, J.; Lucero, M.; Cato, C.; Chang, L.; Geiger, J.; Henry, D.; Tran, A. Immunotherapies: Exploiting the Immune System for Cancer Treatment. J. Immunol. Res. 2018, 2018, 9585614.
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Sznol, M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454.
- Manz, S.M.; Losa, M.; Fritsch, R.; Scharl, M. Efficacy and side effects of immune checkpoint inhibitors in the treatment of colorectal cancer. Ther. Adv. Gastroenterol. 2021, 14, 17562848211002018.
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Libra, M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers 2019, 11, 38.
- Shui, L.; Yang, X.; Li, J.; Yi, C.; Sun, Q.; Zhu, H. Gut Microbiome as a Potential Factor for Modulating Resistance to Cancer Immunotherapy. Front. Immunol. 2019, 10, 2989.
- Vetizou, M.; Pitt, J.M.; Daillere, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Zitvogel, L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084.
- Silva, M.; Brunner, V.; Tschurtschenthaler, M. Microbiota and Colorectal Cancer: From Gut to Bedside. Front. Pharmacol. 2021, 12, 760280.
- Chen, D.; Wu, J.; Jin, D.; Wang, B.; Cao, H. Fecal microbiota transplantation in cancer management: Current status and perspectives. Int. J. Cancer 2019, 145, 2021–2031.
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415.
- Kim, K.O.; Gluck, M. Fecal Microbiota Transplantation: An Update on Clinical Practice. Clin. Endosc. 2019, 52, 137–143.
- Rosshart, S.P.; Vassallo, B.G.; Angeletti, D. Wild mouse gut microbiota promotes host fitness and improves disease resistance. Cell 2017, 171, 1015–1028.e13.
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22.
- Al-Ishaq, R.K.; Overy, A.J.; Busselberg, D. Phytochemicals and Gastrointestinal Cancer: Cellular Mechanisms and Effects to Change Cancer Progression. Biomolecules 2020, 10, 105.
- Gali-Muhtasib, H.U.; Younes, I.H.; Karchesy, J.J.; el-Sabban, M.E. Plant tannins inhibit the induction of aberrant crypt foci and colonic tumors by 1,2-dimethylhydrazine in mice. Nutr. Cancer 2001, 39, 108–116.
- Al-Ishaq, R.K.; Liskova, A.; Kubatka, P.; Busselberg, D. Enzymatic Metabolism of Flavonoids by Gut Microbiota and Its Impact on Gastrointestinal Cancer. Cancers 2021, 13, 3934.




