
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stefania D'Angelo | -- | 4032 | 2022-05-05 14:08:00 | | | |
| 2 | Vivi Li | Meta information modification | 4032 | 2022-05-06 03:36:59 | | |
Video Upload Options
Aging and, particularly, the onset of age-related diseases are associated with tissue dysfunction and macromolecular damage, some of which can be attributed to accumulation of oxidative damage. Recently, growing interest has emerged on the beneficial effects of plant-based diets for the prevention of chronic diseases including obesity, diabetes, and cardiovascular disease. Several studies collectively suggests that the intake of polyphenols and their major food sources may exert beneficial effects on improving insulin resistance and related diabetes risk factors, such as inflammation and oxidative stress. They are the most abundant antioxidants in the diet, and their intake has been associated with a reduced aging in humans. Polyphenolic intake has been shown to be effective at ameliorating several age-related phenotypes, including oxidative stress, inflammation, impaired proteostasis, and cellular senescence, both in vitro and in vivo.
1. Introduction
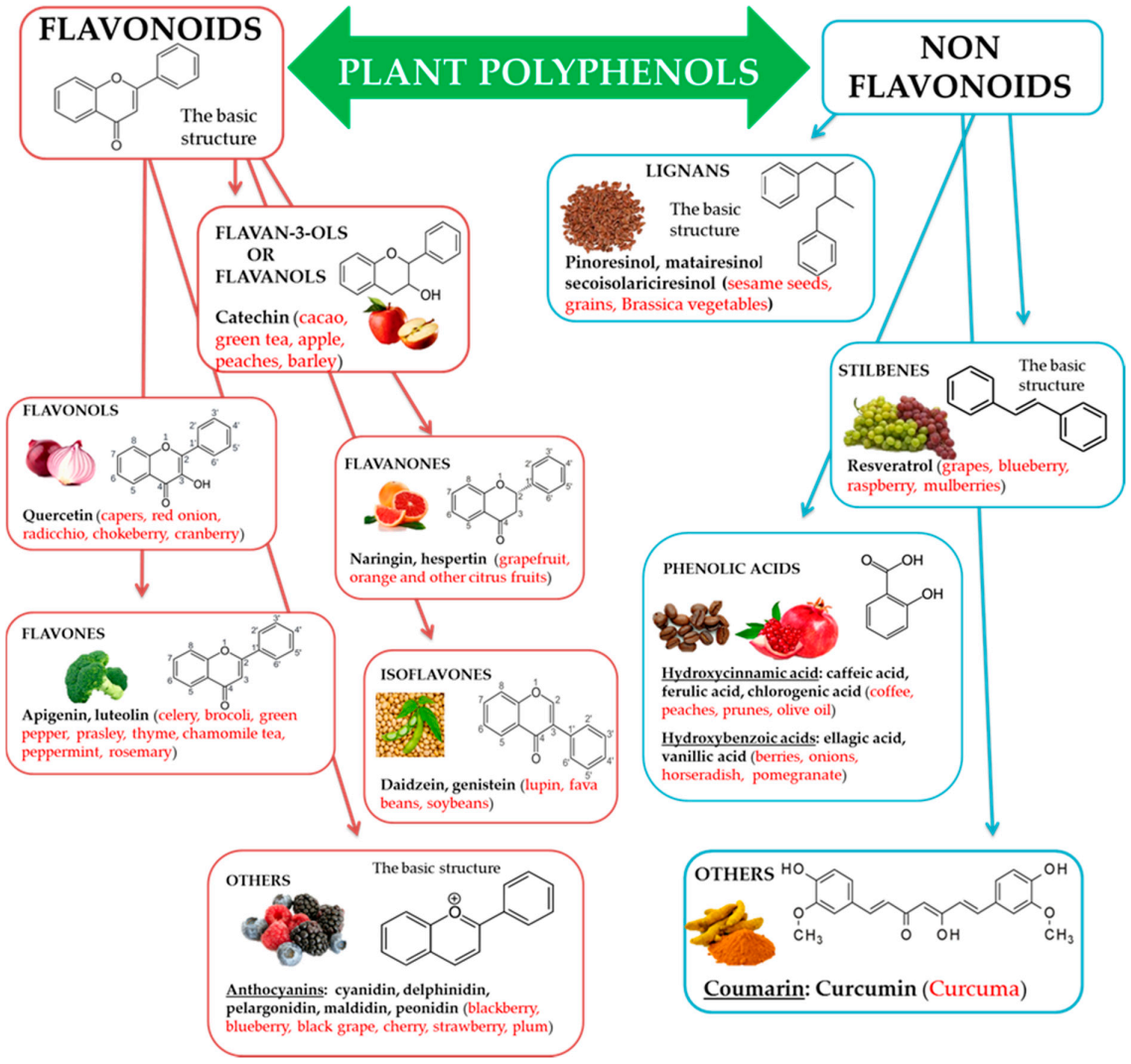
2. Polyphenols: Anti-Senescence Nutraceuticals
3. Action of Polyphenols on Some Hallmarks of Aging

3.1. Polyphenols and Mitochondria
3.2. Polyphenols and Telomeres
3.3. Polyphenols and Sirt-1
3.4. Polyphenols and Male Fertility
References
- Jin, K.; Rose, M.R. Modern Biological Theories of Aging. Aging Dis. 1988, 1, 220–221.
- Queen, B.L.; Tollefsbol, T.O. Polyphenols and aging. Curr. Aging Sci. 2010, 3, 34–42.
- Aunan, J.R.; Watson, M.M.; Hagland, H.R.; Søreide, K. Molecular and biological hallmarks of aging. Br. J. Surg. 2016, 103, e29–e46.
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. Neuroendocrine aspects of skin aging. Int. J. Mol. Sci. 2019, 20, 2798.
- Dhanjal, D.S.; Bhardwaj, S.; Sharma, R.; Bhardwaj, K.; Kumar, D.; Chopra, C.; Nepovimova, E.; Singh, R.; Kuca, K. Plant Fortification of the Diet for Anti-Aging Effects: A Review. Nutrients 2020, 12, 3008.
- Da Costa, J.P.; Vitorino, R.; Silva, G.M.; Vogel, C.; Duarte, A.C.; Rocha-Santos, T. Asynopsis on aging—Theories, mechanisms and future prospects. Aging Res. Rev. 2016, 29, 90–112.
- Gladyshev, V.N. The free radical theory of aging is dead. Long live the damage theory! Antioxid. Redox Signal. 2014, 20, 727–731.
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4.
- Di Meo, S.; Venditti, P. Evolution of the Knowledge of Free Radicals and Other Oxidants. Oxid. Med. Cell. Longev. 2020, 2020, 9829176.
- Viña, J. The free radical theory of frailty: Mechanisms and opportunities for interventions to promote successful aging. Free Radic. Biol. Med. 2019, 134, 690–694.
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772.
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183.
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77.
- Gutteridge, J.M.; Halliwell, B. Free radicals and antioxidants in the year 2000. A historical look to the future. Ann. N. Y. Acad. Sci. 2000, 899, 136–147.
- Reczek, C.R.; Chandel, N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015, 33, 8–13.
- Ingrosso, D.; D’Angelo, S.; Perna, A.F.; Iolascon, A.; Miraglia del Giudice, E.; Perrotta, S.; Zappia, V.; Galletti, P. Increased membrane-protein methylation in hereditary spherocytosis. A marker of cytoskeletal disarray. Eur. J. Biochem. 1995, 228, 894–898.
- Ingrosso, D.; D’Angelo, S.; Perrotta, S.; d’Urzo, G.; Iolascon, A.; Perna, A.F.; Galletti, P.; Zappia, V.; Miraglia del Giudice, E. Cytoskeletal behaviour in Spectrin and Band 3 deficient spherocytic red cells: Evidence for a differentiated splenic conditioning role. Br. J. Haematol. 1996, 93, 38–41.
- D’Angelo, S.; Lembo, S.; Flora, F.; De Bonis, M.L.; Balato, A.; Ayala, F.; Balato, N.; Galletti, P.; Zappia, V. Abnormal isoaspartyl residues in erythrocyte membranes from psoriatic patients. Arch. Dermatol. Res. 2012, 304, 475–479.
- D’Angelo, S.; Trojsi, F.; Salvatore, A.; Daniele, L.; Raimo, M.; Galletti, P.; Monsurrò, M.R. Accumulation of altered aspartyl residues in erythrocyte membrane proteins from patients with sporadic amyotrophic lateral sclerosis. Neurochem. Int. 2013, 63, 626–634.
- D’Angelo, S.; Rosa, R. Oxidative stress and sport performance. Sport Sci. 2020, 13 (Suppl. S1), 18–22.
- D’Angelo, S. Polyphenols and athletic performance: A review on human data. In Plant Physiological Aspects of Phenolic Compounds; Soto-Hernández, M., García-Mateos, R., Tenango, M.P., Eds.; IntechOpen: London, UK, 2019; pp. 1–24. ISBN 978-1-78984-033-9.
- Jarrett, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Asp. Med. 2012, 33, 399–417.
- Kirkwood, T.B.L. Why and how are we living longer? Exp. Physiol. 2017, 102, 1067–1074.
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217.
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 477.
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2015, 7, 17–44.
- Minuz, P.; Velo, G.; Violi, F.; Ferro, A. Are nutraceuticals the modern panacea? From myth to science. Br. J. Clin. Pharmacol. 2017, 83, 5–7.
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020, 21, 884–896.
- Da Costa, J.P. A current look at nutraceuticals–Key concepts and future prospects. Trends Food Sci. Technol. 2017, 62, 68–78.
- D’Angelo, S.; Tafuri, D. Nutraceutical: Their role in improving sports performance. Sport Sci. 2020, 13 (Suppl. S1), 7–12.
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64 (Suppl. S3), S112–S120.
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87.
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892.
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528.
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90.
- Gutiérrez-Grijalva, E.P.; Ambriz-Pére, D.L.; Leyva-López, N.; Castillo-López, R.I.; Heredia, J.B. Review: Dietary phenolic compounds, health benefits and bioaccessibility. Arch. Latinoam. Nutr. 2016, 66, 87–100.
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333.
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246.
- Quero, J.; Mármol, I.; Cerrada, E.; Rodríguez-Yoldi, M.J. Insight into the potential application of polyphenol-rich dietary intervention in degenerative disease management. Food Funct. 2020, 11, 2805–2825.
- Devi, S.A.; Chamoli, A. Polyphenols as an Effective Therapeutic Intervention against Cognitive Decline during Normal and Pathological Brain Aging. Adv. Exp. Med. Biol. 2020, 1260, 159–174.
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278.
- Menaa, F.; Menaa, A.; Tréton, J. Polyphenols against Skin Aging, Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 819–830.
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835.
- Tešic, Ž.; Gašic, U.; Milojkovic-Opsenica, D. Polyphenolic Profile of the Fruits Grown in Serbia. In Advances in Plant. Phenolics: From Chemistry to Human Health, 1st ed.; ACS Symposium Series; Jayaprakasha, G.K., Patil, B.S., Gattuso, G., Eds.; American Chemical Society: Washington, DC, USA, 2018; Chapter 3; Volume 1286, pp. 47–66.
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044.
- Gašić, U.; Ćirić, I.; Pejčić, T.; Radenković, D.; Djordjević, V.; Radulović, S.; Tešić, Ž. Polyphenols as Possible Agents for Pancreatic Diseases. Antioxidants 2020, 9, 547.
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250.
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83.
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural antioxidants: Multiple mechanisms to protect skin from solar radiation. Front. Pharmacol. 2018, 9, 392.
- Pangestuti, R.; Siahaan, E.A.; Kim, S.K. Photoprotective Substances Derived from Marine Algae. Mar. Drugs 2018, 16, 399.
- Barbosa, M.; Valentão, P.; Andrade, P.B. Polyphenols from Brown Seaweeds (Ochrophyta, Phaeophyceae): Phlorotannins in the Pursuit of Natural Alternatives to Tackle Neurodegeneration. Mar. Drugs. 2020, 18, 654.
- Thring, T.; Hili, P.; Naughton, D. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27.
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464.
- Zappia, V.; Galletti, P.; Manna, C.; D’Angelo, S.; Napoli, D.; De Bonis, M.L.; Capasso, G. Effects of Hydroxytyrosol on Cyclosporine Nephrotoxicity. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Oxford, UK, 2010; pp. 1245–1252.
- D’Angelo, S.; Sammartino, D. Protective Effect of Annurca Apple Extract against Oxidative Damage in Human Erythrocytes. Curr. Nutr. Food Sci. 2015, 11, 248–256.
- D’Angelo, S.; Martino, E.; Ilisso, C.P.; Bagarolo, M.L.; Porcelli, M.; Cacciapuoti, G. Pro-oxidant and pro-apoptotic activity of polyphenol extract from Annurca apple and its underlying mechanisms in human breast cancer cells. Int. J. Oncol. 2017, 51, 939–948.
- D’Angelo, S.; Martino, E.; Cacciapuoti, G. Effects of Annurca Apple (Malus pumila cv Annurca) Polyphenols on Breast Cancer Cells. Curr. Nutr. Food Sci. 2019, 15, 745–751.
- Vuoso, D.C.; D’Angelo, S.; Ferraro, R.; Caserta, S.; Guido, S.; Cammarota, M.; Porcelli, M.; Cacciapuoti, G. Annurca apple polyphenol extract promotes mesenchymal-to-epithelial transition and inhibits migration in triple-negative breast cancer cells through ROS/JNK signaling. Sci. Rep. 2020, 10, 15921.
- Boccellino, M.; Quagliuolo, L.; D’Angelo, S. Annurca Apple Biophenols’ Effects in Combination with Cisplatin on A549 Cells. Curr. Nutr. Food Sci. 2021, 17, 111–120.
- Martino, E.; Vuoso, D.C.; D’Angelo, S.; Mele, L.; D’Onofrio, N.; Porcelli, M.; Cacciapuoti, G. Annurca apple polyphenol extract selectively kills MDA-MB-231 cells through ROS generation, sustained JNK activation and cell growth and survival inhibition. Sci. Rep. 2019, 10, 13045.
- D’Angelo, S.; Morana, A.; Salvatore, A.; Zappia, V.; Galletti, P. Protective effect of polyphenols from Glycyrrhiza glabra against oxidative stress in Caco-2 cells. J. Med. Food 2009, 12, 1326–1333.
- D’Angelo, S.; Cimmino, A.; Raimo, M.; Salvatore, A.; Zappia, V.; Galletti, P. Effect of reddening-ripening on the antioxidant activity of polyphenol extracts from cv. ‘Annurca’ apple fruits. J. Agric. Food Chem. 2007, 55, 9977–9985.
- Silva, R.F.M.; Pogacnik, L. Polyphenols fromfood and natural products: Neuroprotection and safety. Antioxidants 2020, 9, 61.
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235.
- D’Angelo, S. Polyphenols: Potential beneficial effects of these phytochemicals in athletes. Curr. Sports Med. Rep. 2020, 19, 260–265.
- D’Angelo, S.; Rosa, R. The impact of supplementation with Pomegranate fruit (Punica Granatum L.) on sport performance. Sport Sci. 2020, 13 (Suppl. S1), 29–37.
- D’Angelo, S.; Ascione, A. Guaranà and physical performance: A myth or reality? J. Hum. Sport Exerc. 2020, 15, S539–S551.
- D’Angelo, S. Current Evidence on the Effect of Dietary Polyphenols Intake on Brain Health. Curr. Nutr. Food Sci. 2020, 16, 1170–1182.
- Gulcin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391.
- Bhat, M.I.; Kapila, R. Dietary metabolites derived from gut microbiota: Critical modulators of epigenetic changes in mammals. Nutr. Rev. 2017, 75, 374–389.
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Giustina, A.; Gazzaruso, C. Microbiota and metabolic diseases. Endocrine 2018, 61, 357–371.
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422.
- D’Angelo, S.; Donini, L. The relationship between microbiota and exercise. Sport Sci. 2020, 14 (Suppl. S1), 24–29.
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452.
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 8, 73.
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem 2015, 26, 651–660.
- Sung, M.M.; Kim, T.T.; Denou, E.; Soltys, C.M.; Hamza, S.M.; Byrne, N.J.; Masson, G.; Park, H.; Wishart, D.S.; Madsen, K.L.; et al. Improved glucose homeostasis in obese mice treated with resveratrol is associated with alterations in the gut microbiome. Diabetes 2017, 66, 418–425.
- Espley, R.V.; Butts, C.A.; Laing, W.A.; Martell, S.; Smith, H.; McGhie, T.K.; Zhang, J.; Paturi, G.; Hedderley, D.; Bovy, A.; et al. Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. J. Nutr. 2014, 144, 146–154.
- Wilson, M.A.; Shukitt-Hale, B.; Kalt, W.; Ingram, D.K.; Joseph, J.A.; Wolkow, C.A. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 2006, 5, 59–68.
- Peng, C.; Chan, H.Y.; Li, Y.M.; Huang, Y.; Chen, Z.Y. Black tea theaflavins extend the lifespan of fruit flies. Exp. Gerontol. 2009, 44, 773–783.
- Sunagawa, T.; Shimizu, T.; Kanda, T.; Tagashira, M.; Sami, M.; Shirasawa, T. Procyanidins from apples (Malus pumila Mill.) extend the lifespan of Caenorhabditis elegans. Planta Med. 2011, 77, 122–127.
- Bass, T.M.; Weinkove, D.; Houthoofd, K.; Gems, D.; Partridge, L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech. Aging Dev. 2007, 128, 546–552.
- Liao, V.H.; Yu, C.W.; Chu, Y.J.; Li, W.H.; Hsieh, Y.C.; Wang, T.T. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech. Aging Dev. 2011, 132, 480–487.
- Abbas, S.; Wink, M. Epigallocatechin gallate inhibits beta amyloid oligomerization in Caenorhabditis elegans and affects the daf-2/insulin-like signaling pathway. Phytomedicine 2010, 17, 902–909.
- Gomes, E.C.; Silva, A.N.; Oliveira, M.R.D. Oxidants, antioxidants, and the beneficial roles of exercise-induced production of reactive species. Oxidative Med. Cell. Longev. 2012, 2012, 756132.
- Salehi, A.; Emami, S.; Keighobadi, M.; Mirzaei, H. An overview of the effects of polyphenols on cardiac mitochondrial function. J. Maz. Univ. Med. Sci. 2019, 28, 211–224.
- Maleki, M.; Khelghati, N.; Alemi, F.; Bazdar, M.; Asemi, Z.; Majidinia, M.; Sadeghpoor, A.; Mahmoodpoor, A.; Jadidi-Niaragh, F.; Targhazeh, N.; et al. Stabilization of telomere by the antioxidant property of polyphenols: Anti-aging potential. Life Sci. 2020, 259, 118341.
- Barbosa, M.C.; Grosso, R.A.; Fader, C.M. Hallmarks of aging: An autophagic perspective. Front. Endocrinol. 2019, 9, 790.
- Höhn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; König, J.; Grune, T.; Castro, J.P. Happily (n) ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017, 11, 482–501.
- Rolt, A.; Cox, L.S. Structural basis of the anti-aging effects of polyphenolics: Mitigation of oxidative stress. BMC Chem. 2020, 14, 50.
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of cellular senescence. Trends Cell Biol. 2018, 28, 436–453.
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115.
- Jeppesen, D.K.; Bohr, V.A.; Stevnsner, T. DNA repair deficiency in neurodegeneration. Prog. Neurobiol. 2011, 94, 166–200.
- Kennedy, S.R.; Salk, J.J.; Schmitt, M.W.; Loeb, L.A. Ultra-sensitive sequencing reveals an age-related increase in somatic mitochondrial mutations that are inconsistent with oxidative damage. PLoS Genet. 2013, 9, e1003794.
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905.
- Santo, A.; Zhu, H.; Li, Y.R. Free radicals: From health to disease. React. Oxyg. Species 2016, 2, 245–263.
- Nissanka, N.; Moraes, C.T. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 2018, 592, 728–742.
- Quijano, C.; Cao, L.; Fergusson, M.M.; Romero, H.; Liu, J.; Gutkind, S.; Rovira, I.I.; Mohney, R.P.; Karoly, E.D.; Finkel, T. Oncogene-induced senescence results in marked metabolic and bioenergetic alterations. Cell Cycle 2012, 11, 1383–1392.
- Bakula, D.; Scheibye-Knudsen, M. MitophAging: Mitophagy in Aging and Disease. Front. Cell Dev. Biol. 2020, 8, 239.
- Evans, C.S.; Holzbaur, E.L.F. Autophagy and mitophagy in ALS. Neurobiol. Dis. 2019, 122, 35–40.
- Zole, E.; Ranka, R. Mitochondria, its DNA and telomeres in aging and human population. Biogerontology 2018, 19, 189–208.
- Peng, K.; Tao, Y.; Zhang, J.; Wang, J.; Ye, F.; Dan, G.; Zhao, Y.; Cai, Y.; Zhao, J.; Wu, Q.; et al. Resveratrol Regulates Mitochondrial Biogenesis and Fission/Fusion to Attenuate Rotenone-Induced Neurotoxicity. Oxid. Med. Cell. Longev. 2016, 2016, 6705621.
- Ferrara, L.; Joksimovic, M.; D’Angelo, S. Modulation of mitochondrial biogenesis: Action of physical activity and phytochemicals. J. Phys. Educ. Sport 2021, 21, 425–433.
- Fivenson, E.M.; Lautrup, S.; Sun, N.; Scheibye-Knudsen, M.; Stevnsner, T.; Nilsen, H.; Bohr, V.A.; Fang, E.F. Mitophagy in neurodegeneration and aging. Neurochem. Int. 2017, 109, 202–209.
- Ames, B.N. Endogenous oxidative DNA damage, aging, and cancer. Free Radic. Res. Commun. 1989, 7, 121–128.
- Pourahmad, J.; Salimi, A.; Seydi, E. Role of Oxygen Free Radicals in Cancer Development and Treatment. In Free Radicals and Diseases; InTech: London, UK, 2016.
- Hornsby, P.J. Telomerase and the aging process. Exp. Gerontol. 2007, 42, 575–581.
- Schmidt, J.C.; Cech, T.R. Human telomerase: Biogenesis, trafficking, recruitment, and activation. Genes Dev. 2015, 29, 1095–1105.
- Shay, J.W.; Wright, W.E. Hallmarks of telomeres in aging research. J. Pathol. 2007, 211, 114–123.
- Boccardi, V.; Paolisso, G.; Mecocci, P. Nutrition and lifestyle in healthy aging: The telomerase challenge. Aging 2016, 8, 12–15.
- Gomez-Delgado, F.; Delgado-Lista, J.; Lopez-Moreno, J.; Rangel-Zuñiga, O.A.; Alcala-Diaz, J.F.; Leon-Acuña, A.; Corina, A.; Yubero-Serrano, E.; Torres-Peña, J.D.; Camargo, A.; et al. Telomerase RNA Component Genetic Variants Interact With the Mediterranean Diet Modifying the Inflammatory Status and its Relationship With Aging: CORDIOPREV Study. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 327–332.
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in grape seeds-biochemistry and functionality. J. Med. Food 2003, 6, 291–299.
- Sheng, R.; Gu, Z.L.; Xie, M.L. Epigallocatechin gallate, the major component of polyphenols in green tea, inhibits telomere attrition mediated cardiomyocyte apoptosis in cardiac hypertrophy. Int. J. Cardiol. 2013, 162, 199–209.
- Gardner, E.; Ruxton, C.; Leeds, A. Black tea–helpful or harmful? A review of the evidence. Eur. J. Clin. Nutr. 2007, 61, 3–18.
- Hsu, S.C.; Huang, S.M.; Chen, A.; Sun, C.Y.; Lin, S.H.; Chen, J.S.; Liu, S.T.; Hsu, Y.J. Resveratrol increases anti-aging Klotho gene expression via the activating transcription factor 3/c-Jun complex-mediated signaling pathway. Int. J. Biochem. Cell Biol. 2014, 53, 361–371.
- Ong, A.L.C.; Ramasamy, T.S. Role of Sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming. Aging Res. Rev. 2018, 43, 64–80.
- Imai, S.; Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014, 24, 464–471.
- Gurău, F.; Baldoni, S.; Prattichizzo, F.; Espinosa, E.; Amenta, F.; Procopio, A.D.; Albertini, M.C.; Bonafè, M.; Olivieri, F. Anti-senescence compounds: A potential nutraceutical approach to healthy aging. Aging Res. Rev. 2018, 46, 14–31.
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127.
- Sarubbo, F.; Esteban, S.; Miralles, A.; Moranta, D. Effects of Resveratrol and other Polyphenols on Sirt1: Relevance to Brain Function during Aging. Curr. Neuropharmacol. 2018, 16, 126–136.
- Lee, S.H.; Lee, J.H.; Lee, H.Y.; Min, K.J. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019, 52, 24–34.
- D’Angelo, S.; Mele, E.; Di Filippo, F.; Viggiano, A.; Meccariello, R. Sirt1 activity in the brain: Simultaneous effects on energy homeostasis and reproduction. Int. J. Environ. Res. Public Health 2021, 18, 1243.
- Zou, P.; Liu, X.; Li, G.; Wang, Y. Resveratrol pretreatment attenuates traumatic brain injury in rats by suppressing NLRP3 inflammasome activation via SIRT1. Mol. Med. Rep. 2018, 17, 3212–3217.
- Sarubbo, F.; Ramis, M.R.; Kienzer, C.; Aparicio, S.; Esteban, S.; Miralles, A.; Moranta, D. Chronic Silymarin, Quercetin and Naringenin Treatments Increase Monoamines Synthesis and Hippocampal Sirt1 Levels Improving Cognition in Aged Rats. J. Neuroimmune Pharmacol. 2018, 13, 24–38.
- Yao, H.; Rahman, I. Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem. Pharmacol. 2012, 84, 1332–1339.
- Pierantoni, R.; Cobellis, G.; Meccariello, R.; Fasano, S. Evolutionary aspects of cellular communication in the vertebrate hypothalamo-hypophysio-gonadal axis. Int. Rev. Cytol. 2002, 218, 69–141.
- Chianese, R.; Cobellis, G.; Chioccarelli, T.; Ciaramella, V.; Migliaccio, M.; Fasano, S.; Pierantoni, R.; Meccariello, R. Kisspeptins, Estrogens and Male Fertility. Curr. Med. Chem. 2016, 23, 4070–4091.
- Almeida, S.; Rato, L.; Sousa, M.; Alves, M.-G.; Oliveira, P.F. Fertility and Sperm Quality in the Aging Male. Curr. Pharm. Des. 2017, 23, 442–4437.
- Jenkins, T.G.; Aston, K.I.; Meyer, T.; Carrell, D.T. The Sperm Epigenome, Male Aging, and Potential Effects on the Embryo. Adv. Exp. Med. Biol. 2015, 868, 81–93.
- Paoli, D.; Pecora, G.; Pallotti, F.; Faja, F.; Pelloni, M.; Lenzi, A.; Lombardo, F. Cytological and molecular aspects of the aging sperm. Hum. Reprod. 2019, 34, 218–227.
- Chianese, R.; Troisi, J.; Richards, S.; Scafuro, M.; Fasano, S.; Guida, M.; Pierantoni, R.; Meccariello, R. Bisphenol A in Reproduction: Epigenetic Effects. Curr. Med. Chem. 2018, 25, 748–770.
- Skoracka, K.; Eder, P.; Łykowska-Szuber, L.; Dobrowolska, A.; Krela-Kaźmierczak, I. Diet and Nutritional Factors in Male (In)fertility-Underestimated Factors. J. Clin. Med. 2020, 9, 1400.
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant supplements and semen parameters: An evidence based review. Int. J. Reprod. Biomed. 2016, 14, 729–736.
- Truong, T.; Gardner, D.K. Antioxidants improve IVF outcome and subsequent embryo development in the mouse. Hum. Reprod. 2017, 32, 2404–2413.
- Wu, Z.H.; Ke, X.W.; Feng, S.Y.; Zhang, L.; Wu, J.F.; Cheng, W.; Cheng, J.J.; Zhang, J.D.; Zhang, Y.G. Tea polyphenols reduces the apoptosis of spermatogenic cells in rats with experimental varicocele. Zhonghua Nan Ke Xue 2015, 21, 702–707.
- Opuwari, C.; Monsees, T. Green tea consumption increases sperm concentration and viability in male rats and is safe for reproductive, liver and kidney health. Sci. Rep. 2020, 10, 15269.
- Opuwari, C.S.; Monsees, T.K. In vivo effects of black tea on the male rat reproductive system and functions of the kidney and liver. Andrologia 2020, 52, e13552.
- Zhu, Y.; Yin, Q.; Yang, Y. Comprehensive Investigation of Moringa oleifera from Different Regions by Simultaneous Determination of 11 Polyphenols Using UPLC-ESI-MS/MS. Molecules 2020, 25, 676.
- Moichela, F.T.; Adefolaju, G.A.; Henkel, R.R.; Opuwari, C.S. Aqueous leaf extract of Moringa oleifera reduced intracellular ROS production, DNA fragmentation and acrosome reaction in Human spermatozoa in vitro. Andrologia 2021, 53, e13903.
- Azadi, L.; Tavalaee, M.; Deemeh, M.R.; Arbabian, M.; Nasr-Esfahani, M.H. Effects of Tempol and Quercetin on Human Sperm Function after Cryopreservation. Cryo Lett. 2017, 38, 29–36.
- Kawasaki, Y.; Sakurai, D.; Yoshihara, T.; Tsuchida, M.; Harakawa, S.; Suzuki, H. Effect of quercetin on the motility of cryopreserved canine spermatozoa. Cryobiology 2020, 96, 50–54.
- Ahmed, H.; Jahan, S.; Salman, M.M.; Ullah, F. Stimulating effects of Quercetin (QUE) in tris citric acid extender on post thaw quality and in vivo fertility of buffalo (Bubalus bubalis) bull spermatozoa. Theriogenology 2019, 134, 18–23.
- Mao, T.; Han, C.; Wei, B.; Zhao, L.; Zhang, Q.; Deng, R.; Liu, J.; Luo, Y.; Zhang, Y. Protective Effects of Quercetin against Cadmium Chloride-Induced Oxidative Injury in Goat Sperm and Zygotes. Biol. Trace Elem. Res. 2018, 185, 344–355.
- Cui, X.; Jing, X.; Wu, X.; Yan, M. Protective effect of resveratrol on spermatozoa function in male infertility induced by excess weight and obesity. Mol. Med. Rep. 2016, 14, 4659–4665.
- Alamo, A.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Giacone, F.; Calabrese, V.; La Vignera, S.; Calogero, A.E. Environment and Male Fertility: Effects of Benzo-α-Pyrene and Resveratrol on Human Sperm Function In Vitro. J. Clin. Med. 2019, 8, 561.
- Gadani, B.; Bucci, D.; Spinaci, M.; Tamanini, C.; Galeati, G. Resveratrol and Epigallocatechin-3-gallate addition to thawed boar sperm improves in vitro fertilization. Theriogenology 2017, 90, 88–93.
- Shabani Nashtaei, M.; Nekoonam, S.; Naji, M.; Bakhshalizadeh, S.; Amidi, F. Cryoprotective effect of resveratrol on DNA damage and crucial human sperm messenger RNAs, possibly through 5′ AMP-activated protein kinase activation. Cell Tissue Bank. 2018, 19, 87–95.
- Bucci, D.; Spinaci, M.; Yeste, M.; Mislei, B.; Gadani, B.; Prieto Martinez, N.; Love, C.; Mari, G.; Tamanini, C.; Galeati, G. Combined effects of resveratrol and epigallocatechin-3-gallate on post thaw boar sperm and IVF parameters. Theriogenology 2018, 117, 16–25.





