Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Brian Freeland | -- | 4220 | 2022-05-05 11:58:34 | | | |
| 2 | Amina Yu | -13 word(s) | 4207 | 2022-05-06 04:26:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Freeland, B.; Mccarthy, E.; Balakrishnan, R.; Fahy, S.; , .; Rochfort, K.; Dabros, M.; Marti, R.; Kelleher, S. Polylactic Acid for Single-Use Laboratory Components. Encyclopedia. Available online: https://encyclopedia.pub/entry/22611 (accessed on 07 February 2026).
Freeland B, Mccarthy E, Balakrishnan R, Fahy S, , Rochfort K, et al. Polylactic Acid for Single-Use Laboratory Components. Encyclopedia. Available at: https://encyclopedia.pub/entry/22611. Accessed February 07, 2026.
Freeland, Brian, Eanna Mccarthy, Rengesh Balakrishnan, Samantha Fahy, , Keith Rochfort, Michal Dabros, Roger Marti, Susan Kelleher. "Polylactic Acid for Single-Use Laboratory Components" Encyclopedia, https://encyclopedia.pub/entry/22611 (accessed February 07, 2026).
Freeland, B., Mccarthy, E., Balakrishnan, R., Fahy, S., , ., Rochfort, K., Dabros, M., Marti, R., & Kelleher, S. (2022, May 05). Polylactic Acid for Single-Use Laboratory Components. In Encyclopedia. https://encyclopedia.pub/entry/22611
Freeland, Brian, et al. "Polylactic Acid for Single-Use Laboratory Components." Encyclopedia. Web. 05 May, 2022.
Copy Citation
Sustainability needs have driven interest in polymers which are degradable, recyclable, and/or derived from eco-friendly input materials. Biomass-based polymers are one option with many benefits to sustainability: reducing dependence on fossil fuel extraction; produced from renewable resources; may make use of waste materials; and better biodegradability. Polylactic Acid (PLA) is one of the most commonly used bioplastics, in 2021 it was reported to hold the largest market share worldwidefor biodegradable bioplastics manufacturing capacity.
polylactic acid
3D printing
biodegradable polymers
PLA
1. Polylactic Acid (PLA)
Sustainability needs have driven interest in polymers which are degradable, recyclable, and/or derived from eco-friendly input materials. Biomass-based polymers are one option with many benefits to sustainability: reducing dependence on fossil fuel extraction; produced from renewable resources; may make use of waste materials; and better biodegradability [1]. Polylactic Acid (PLA) is one of the most commonly used bioplastics, in 2021 it was reported to hold the largest market share worldwide [2] for biodegradable bioplastics manufacturing capacity. PLA’s manufacturing capacity accounted for 42% of the total biodegradable, bioplastics production worldwide, with its nearest rival polyhydroxyalkanoates (PHA) [3][4] accounting for only 4% of worldwide production [2].
PLA can be produced from starch using fermentation by microorganism to create the monomer lactic acid, which is polymerised to form PLA [1]. As such, it can be produced from renewable sources. The reduction of CO2 emissions is one of the most advantageous aspects of PLA manufacturing when compared to alternative hydrocarbon-based polymers. Carbon dioxide is thought to be the most significant contributor to global warming and climate change. PLA has the potential to release less greenhouse emissions than rival hydrocarbon-based polymers because CO2 is absorbed from the air when maize is cultivated [5]. On the other hand, agricultural waste material which may otherwise go unutilised can be used to produce the lactic acid, such as dairy waste, cottonseed, tobacco waste, wheat straw, corn cobs, coffee pulp, food waste, stillage, and used brewer’s grain [6].
2. Mechanical Properties of PLA
Typical PLA is a brittle material [1]. Anderson et al. [7] compare PLA’s properties to those of two polymers commonly used in single-use labware, polystyrene (PS) and PET. Brittle PLA’s impact strength is similar to polystyrene, with tensile strength and modulus that are more comparable to PET. However, the mechanical properties of PLA can range from soft, elastic materials to stiff rigid ones, depending on several physical factors such as the molecular weight and crystallinity, and the use of polymer blending or composite additives.
Perego et al. [8] report on the effect of molecular weight and crystallinity on the mechanical properties of several PLA variants; Poly(L-lactide) (PLLA) and Poly(D,L-lactide) (PDLLA). L-lactide and D-lactide are two stereoisomers of lactic acid (see Figure 1), which can lead to differing properties in the PLA produced from them [1]. Annealed PLLA samples were also characterised to investigate the effect of crystallinity. Molecular weight was shown to have a much stronger effect when the crystallinity was higher. Impact resistance was also strongly influenced by crystallinity.
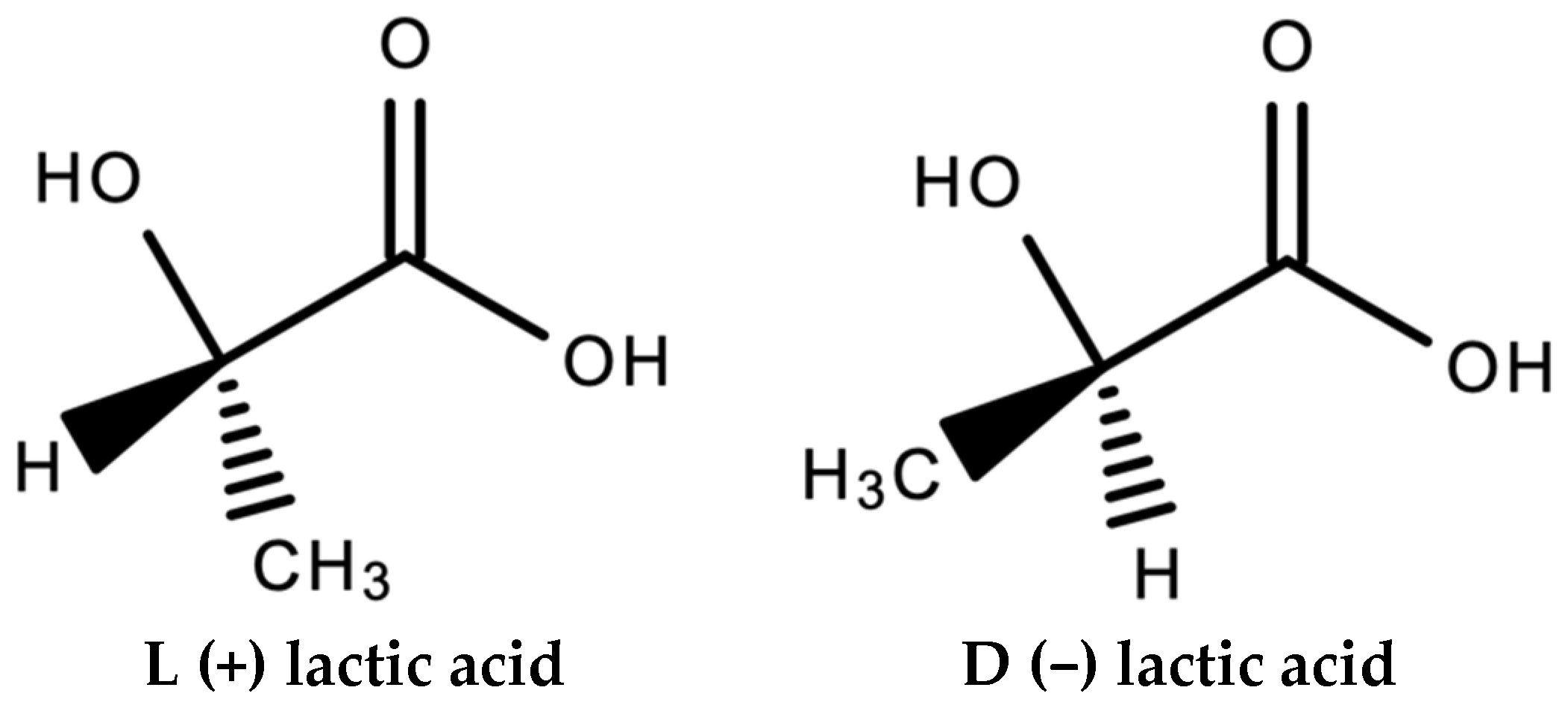
Figure 1. Stereoisomers of lactic acid.
3. Thermal Properties of PLA
The isomer composition can also affect the thermal properties of PLA. Ahmed and Varshney, investigated samples of PLA derived from L-lactide, D-lactide, or both with varied molecular weights [9]. Avinc and Khoddami, [10] illustrate the molecular configurations of PLA with differing isomer compositions (see Figure 2). The results for the varied PLA samples. It was noted that the melt and glass temperatures (Tm and Tg, respectively) tend to increase with the number average molecular weight (Mn) irrespective of isomer. A range of glass temperatures (Tg) can be achieved, with low values giving easier processability and higher values allowing higher operating temperatures for parts produced.
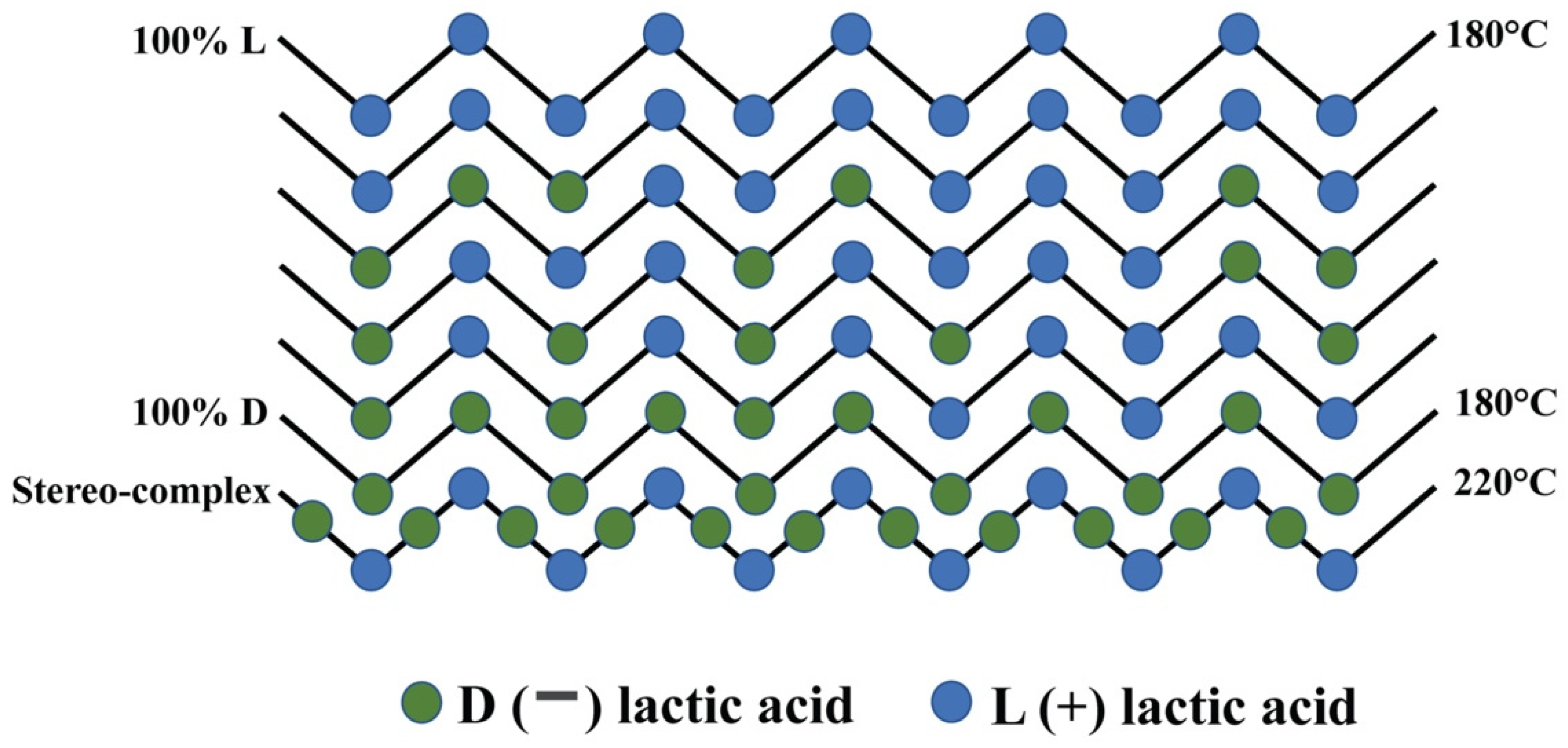
Figure 2. Examples of molecular configurations of PLA obtained through combining the two lactic acids.
4. Mitigating PLA’s Functional Limitations
Standard PLA has some limitations which may make it unsuitable for certain applications. The low glass and melt temperature make it unsuitable for high temperature operations [9]. Sin et al. [1] report that PLA is suitable for room temperatures but seldom used for higher temperature applications as it begins to lose structural integrity at ≥60 °C, making it unsuitable for PCR tests, boiling water, and thermal sterilisation. PLA’s ductile-brittle temperature, the temperature below which polymers are fully brittle, is −47 °C [11].
Janorkar et al. [12] report reductions in the molecular weight of PLA after exposure to a UV sterilisation lamp, which may indicate standard PLA is unsuitable for UV sterilisation. The UV resistance of PLA can be improved by some additives [13][14][15]. Ho et al. [15] found the addition of bamboo charcoal particles to PLA mitigated deterioration of the mechanical properties after UV exposure. Man, et al. [13] report improved UV resistance with the addition of rutile TiO2 in spin coating and extrusion-injection processing. Zhou et al. [16] found that PLA-TiO2 nanocomposite membranes could be safely treated with UV-assisted cleaning. Cao et al. [17] coated TiO2 nanoparticles with SiO2 then D-lactide based PLA to create double shell particles, and used this to reinforce L-lactide based PLA. The reinforced PLA was able to maintain its mechanical properties by >90% after 72 h of UV exposure. The initial mechanical properties were also enhanced by the reinforcement, with the tensile strength being 49% higher.
5. Biological Compatibility Requirements of PLA
When considering a material to interface with a biological system, the biocompatibility of the material is critical in preserving the systems biological integrity. Biocompatibility is a measure of a material’s ability to come into contact with a biological system without eliciting detrimental responses those which elicit little to no effect can be thus considered as inert and biocompatible in nature [18]. This is of particular importance from a standardisation perspective as the choice of alternatives to petroleum-based, non-biodegradable plastics increases steadily [2]. In producing a material, elements such as process contaminants, residues, leachables, and products of degradation may comprise a percentage of the material and in-turn may potentially influence certain biological interactions differently to that intended with the original material. In that way, any and all compounds and additives which may be present and/or intentionally added to improve the properties of these alternatives, must be assessed in terms of their biocompatibility for not just the sample in which it will come into contact with, but also the user [4]. In that way, biocompatibility testing is incredibly important in order to determine whether the alternative material is question is indeed fit for purpose, and functions in exactly the same way as those materials which it is replacing. As such, all potential materials must meet criteria developed by the International Organisation for Standardisation (ISO) in which lab materials made from said alternatives must meet a series of standards which are recognised by regulatory authorities all over the world [19].
Depending on the purpose of the material, the testing methods use to evaluate the biocompatibility can vary widely. These tests, with methodologies spanning in vitro and in vivo, can vary in turnaround time from days to several months depending on the requirement for the specific test data, though some tests may not be required depending on the application [19]. Biological properties such as genotoxicity, hemocompatibility, sensitisation, irritation, implantation and system toxicity are among the indices typically examined with respect to lab materials, though the most common assays used are those which identify the cytotoxicity of the material. Direct contact cell culture assays which evaluate the impact of the material on cell adhesion, cell activation, and/or cell death are used extensively in biocompatibility ones of novel materials [20], as well as extractions in which the leachable materials from the test material are harvested in response to different solvents, and analysed for potentially harmful chemicals or cytotoxic molecules [21]. The aforementioned are mandatory for all lab-based product evaluation programs run by national regulatory bodies, with additional tests such as material properties (chemical, mechanical and thermal) with respect to the potential application also required. Moreover, considerations must also made for instances of misuse of the material/product from both biological and material perspectives [19].
Should a material meet the designated standards, and indeed be deemed biocompatible and fit for its intended purpose, the impact of sterilisation on the material should also be considered. Methods of sterilisation can vary in nature, and as all lab materials must be sterilised before coming into contact with a biological system/host, the degree of stress imparted by a means of sterilisation should be taken into consideration. Single-use plastics only need to tolerate one cycle of sterilization; however, multiple-use products can be subjected to several cycles in their life-time and are required to be able to withstand such without any change in their functional and mechanical properties [22]. As this is deemed an integral process which all lab materials are subjected to prior to use, the impact of sterilisation techniques on prospective replacement materials such as PLA are determined very early in the characterisation process.
6. Solvent Interaction with PLA
Generally, PLA is not dissolved in water, selective alcohols, and alkanes; however, amorphous PLA is highly soluble with organic solvents [1]. Hansen, [23] reports the solubility parameters for several solvents at 25 °C. δd is the dispersion solubility parameter, δp is the polar solubility parameter, δh is the hydrogen solubility parameter, and δt is the total solubility parameter. Close values in the total solubility parameters for two materials (Sin et al. [1] specify <2.5 difference in δt) indicate solubility. Agrawal et al. [24] calculated solubility parameters for standard PLA with a number of methods. It is indicated that standard PLA is expected to dissolve in acetone, benzene, chloroform, 1-4 dioxane, 1-3 dioxolane, ethyl acetate, furan, isoamyl alcohol, methylene dichloride, methyl ethyl ketone, tetrahydrofuran, toluene, and xylene. Nampoothiri et al. [25] note that PLA is only weakly soluble in acetone, ethyl benzene, toluene, and tetrahydrofuran at room temperature but can be readily dissolved with heating. However, high crystallinity PLLA can resist acetone, ethyl acetate, and tetrahydrofuran [1][25].
7. Effect of Temperature on Leachables from PLA
Mutsuga et al. [26] reported on the leaching of lactic acid, lactide, and oligomers from PLA at different temperatures. PLA sheets procured from different manufacturers were placed into glass tubes with 100 mL water, and water with 4% acetic acid or 20% ethanol, and the presence of migrated compounds was measured with liquid chromatography/mass spectrometry after several fixed periods. They note that migration into the mixture was present but low for 20 or 40 °C, but that at 60 °C or higher there are significant migrant levels due to the decomposition of the PLA. Lactide migration levels were raised to 0.24 mg.cm2 at 60 °C, 0.64 mg.cm2 at 80 °C, and 4.12 mg.cm2 at 95 °C and only at that higher temperature oligomers were identified at 1.98 mg.cm2. After the migration test at 95 °C, the sample turned cloudy. It was observed that LA was calculated to be formed at 95 °C from lactide produced by PLA degradation. It was noted that migrant levels were worse for samples with a higher ratio of D-lactide. For the lower temperatures, the amount of lactic acid is lower than those present in some common food ingredients, making PLA suitable for food packaging applications [1][26]. However, the leaching of material may interfere with sensitive lab applications, particularly those involving higher temperature chemical processes.
8. Additives to PLA
Plasticisers may be used to increase the ductility of brittle polymers. For PLA, the glassy brittle polymer can be plasticised using its own monomer (lactic acid/lactide) to increase its flexibility. Sinclair reports the tensile properties for different percentages of lactide plasticiser [27]. The data for different percentage of plasticiser illustrate the broad range of properties that can be obtained, and some similarities to conventional thermoplastics can be noted. This allows PLA to compete as a sustainable packaging material; however, It was noted that degradation is increased with increasing plasticiser [27].
In addition to using plasticisers to improve ductility, other additives may be used with PLA to improve or tailor other properties. Polymer blends are another approach for achieving desired properties. PLA can be blended with several other polymers. For example, PLA can be blended with rubbery polycaprolactone (PCL) to increase elongation at break with reduced tensile strength and stiffness [28][29]. Impact modifiers can be added to improve the impact strength, and nanoscale clay particles can be added to improve stiffness [30]. Additives may be mixed to achieved the desired balance of properties, as illustrated in Figure 3 [30].
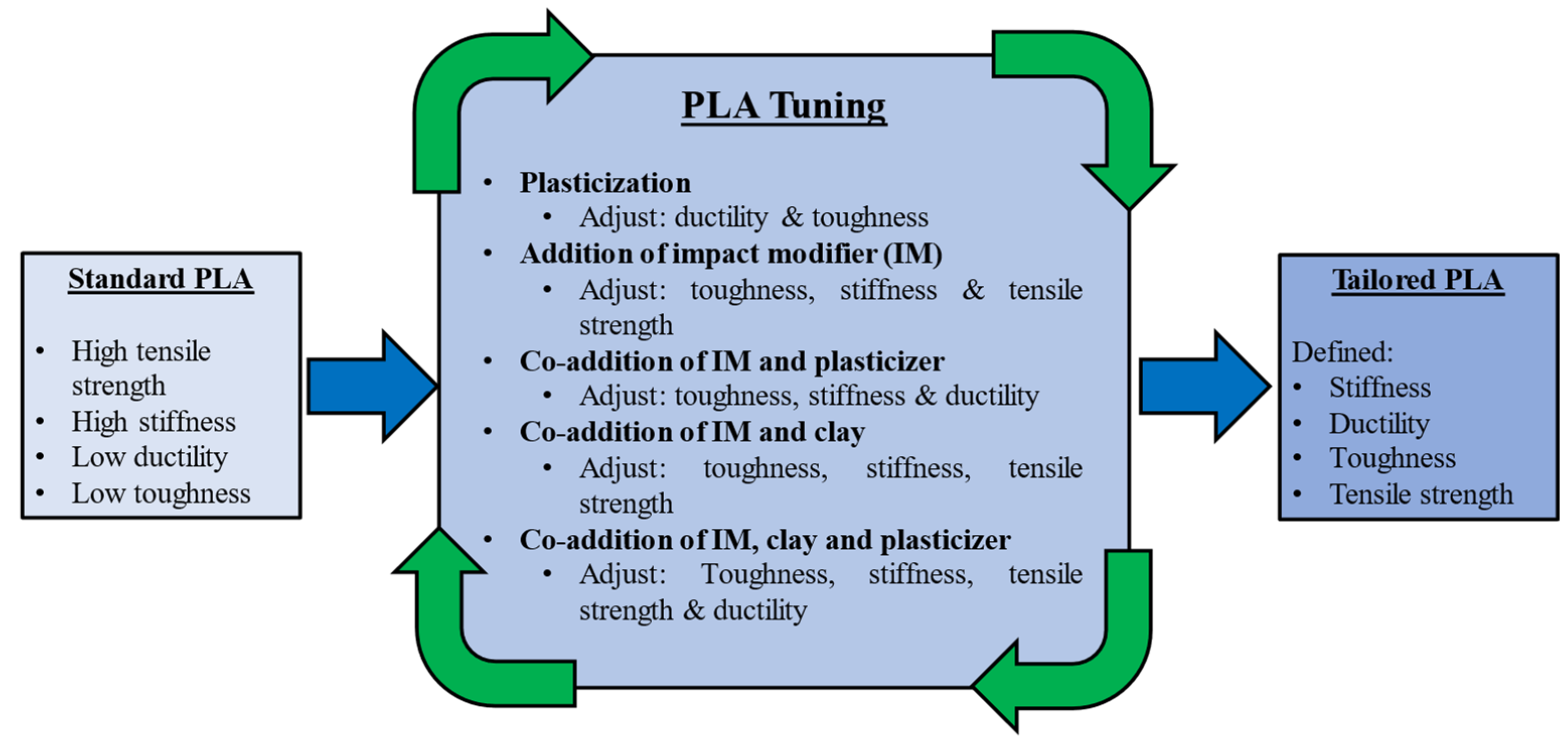
Figure 3. Tuning the properties of PLA with a variety of additives.
Additives can also be used to improve the heat resistance. Heat resistance is evaluated by several methods characterizing how the material’s resistance to deformation changes with temperature. The Heat Deflection Temperature (HDT) for PLA, defined as the temperature at which a defined specimen deflects 250 µm under a specified load and heating rate, is 55 °C, and the Vicat Softening Temperature (VST) for PLA, defined as the temperature at which a specimen is penetrated to 1 mm by a flat ended 1 mm2 area pin under a specified load and heating rate, is 65 °C [31]. Heat resistance can be improved by increasing the crystallinity (using nucleating agents or processing strategy), by polymer blending, or by other reinforcement additives. Using nucleation additive dibenzoylhydrazide with PLLA, Kawamoto et al. [32] were able to achieve a HDT of 124 °C. The stiffness and Izod impact strength were also improved, 4.1 GPa and 7.9 kJ m−2, respectively. In terms of processing, Tábi et al. [33] produced PLA samples using injection molding with different mold materials. Additively manufactured epoxy-based molds were able to deliver higher crystallinity due to their slowing cooling rates compared to steel molds. Using PLA containing nucleation agents (talc and polyethylene glycol (PEG)) with both mold types at room temperature, the VST was improved from 60–65 °C for the steel molds, to 118–124 °C for the epoxy-based molds. The strength of the interfacial bond between adjacent layers dictates the mechanical characteristics of 3D components manufactured using the fused filament fabrication (FFF) process. When PEGs are added to FFF-printed PLA components, the interlayer bond strength is increased, lowering the mechanical anisotropy from 32% for pristine PLA parts to 16% for PLA/PEG parts. Additionally, PEGs with a molecular weight greater than 8000 g/mol have a significant impact on the mechanical characteristics of PLA components [34]. Hriţuc et al. [35] looked into the 3D printing processes that use polylactide (PLA) wire to make parts in a wide range of shapes and sizes. The fused deposition modelling process had some differences between the desired dimensions and the real dimensions that were made. Taguchi models used show that in the case of tubular parts made of PLA, printing speed and plate temperature have the biggest impact on the height and diameter of 3D printing.
PLA can also be used as a matrix in composite materials. Composite materials are made up of two or more different component materials with advantageous properties. For polymers, reinforcement with stiff fibres is a popular approach. Fibre-Reinforced Plastics (FRPs), using fibres such as glass or carbon in the polymer matrix, can provide high specific stiffness, specific strength, impact strength, and damping [36]. Their ability to deliver high strength-to-weight ratios has made FRPs popular in automotive, aerospace, and wind turbine applications [36][37][38][39][40][41][42]. Glass fibres have been used with PLA, providing increases in tensile strength, flexural strength, impact strength, and heat deflection temperature of 183%, 134%, 331%, 313%, respectively, using 30% glass fibre [43]. Carbon fibres have also been used with PLA, giving increases in tensile strength, tensile modulus, flexural strength, and flexural modulus of 73%, 438%, 53%, and 400%, respectively, with 30% carbon fibre [44]. It was reported by Agüero et al. [45] that they made and characterised green composites that used PLA-based fillers and additives that came from the linen processing industry. They showed that the waste from flax (Linum usitatissimum L.) or byproducts can be used to get renewable raw materials that can be used to make green composites for market applications like rigid food packaging and food-contact disposable items in the circular economy and bioeconomy.
Sustainable, Compostable PLA Additives
Sustainable composites can be achieved by combining PLA with eco-friendly additives [46][47]. Cali et al. [48] report on the use of PLA matrices with agricultural waste fillers to create composite filaments which can be used in additive manufacturing (AM). Combining an eco-friendly, biodegradable polymer with biodegradable waste materials that might otherwise go unused to improve the mechanical properties of produced parts is a boon for the sustainability of composite parts, and of additive manufacturing as a technique. It was used the filaments to successfully produce two biomedical prototypes [48]. Similarly, Matsuzaki et al. [49] describe a method where jute fibre is fed into a heated nozzle with pure PLA filaments during the AM process, achieving modulus and strength increases of 157% and 134%, respectively, compared to pure PLA. By combining PLA with poly (ε-caprolactone) (PCL), a biodegradable, water insoluble polyester, López-Rodríguez et al. [50] found that an increase of PCL led to a decrease in the Young’s modulus and the tensile strength of the composite (from 56.8 MPa at 0% PCL to 12.5 MPa at 80% PCL). At a composition ratio of 80% PLA and 20% PCL this blend was found to have similar mechanical properties to PS. Composite blends of PLA and silk fibroin (SF) containing 2–10 wt.% of PLA dispersed in a SF matrix displayed an increase in Young’s modulus (from 2876 MPa at 0% SF to 3480 MPa at 90% SF), tensile strength (from 23.2 MPa at 0% SF to 28.5 MPa at 90% SF), and hydrophobicity in comparison to neat PLA [51]. The mechanical properties of blends containing poly(butylene succinate) (PBS) and PLA were investigated by Chen et al. [52] and Wang et al. [53]. PBS is typically added into a blend with PLA in order to increase the toughness of the material without compromising the biodegradability of the plastic material [54][55][56]. More recently still, Rojas-Martínez et al. [57] have published results showing that PLA blended with keratin and chitosan can be 3D printed into scaffolds. This year, Brounstein et al. [58] reported the blending of PLA with TiO2, ZnO, and ceramics (up to 30 wt.%) to produce antimicrobial composites. Banerjee et al. [59] evaluated the many kinds of nanoparticles employed in the manufacture of PLA nanocomposites, including nanoclay, nanocelluloses, carbon nanotubes, and graphene, covering all key processing, characterisation, and application elements. Dedukh et al. discussed new developments in the usage of composite materials manufactured from PLA in bone surgery and the use of 3D printing to create implants [60][61][62]. The usage of PLLA and PLA blends will rise as learned more about how to adjust the mechanical response of this essential class of materials. These bioresorbable polymers have the capacity to breakdown under biomedically relevant situations. This degradation is regulated by the moleculer weight and orientations, crystallinity, and the chemical and load environment [63]. The impact of acrylated epoxidized soybean oil (AESO) addition on the mechanical, thermal, and thermomechanical characteristics of PLA components formed by injection moulding was learned by Quiles-Carrillo et al. [64] PLA components with 2.5–7.5 wt.% AESO exhibited a significant increase in elongation at break and impact-absorbed energy, but their tensile and flexural strength, as well as thermomechanical characteristics, were preserved or slightly enhanced. It also had a stronger thermal stability and a reduced crystallinity. Thus, using AESO to generate toughened PLA materials of great interest in rigid packaging, automotive, or building and construction applications might be deemed an ecologically acceptable alternative. PLA is gaining a reputation for being a flexible material, from which composites and blend materials can be produced, in order to fine tune the required physical and chemical properties. The key factor for consideration for these developments is not only the improved properties of the PLA, but also the degradation pathways of the materials and end destination of the additives.
9. PLA Industrial Synthesis Processes
The industrial production of PLA today is mainly based on the ring opening polymerization (ROP) of lactide because polycondensation of lactic acid requires rather harsh conditions, for example., high temperatures (180–200 °C), vacuum (at least 5 mbar) and long reaction times to obtain PLA of high molecular weights [65][66]. In contrast, ROP works at rather quite mild conditions (max 130 °C, reaction times of several hours) and yields PLA with narrow PDI and high molecular weights of up to 100 kDa, which are important for reasonable mechanical properties. Metal alkoxides as for example tin(II) octoate are preferred as industrial catalysts as they give high molecular weight and no loss of optical purity [65][66]. The chiral integrity is crucial for the properties of PLA. Gupta et al. [67] reviewed the uses of PLA and its potential value in a variety of emerging technologies, including orthopaedics, drug administration, sutures, and scaffolds, and have piqued researchers’ curiosity in this innovative field. Additionally, they addressed developing PLA using a range of catalysts to meet a variety of performance needs.
Cargill Inc. was the first to industrialize the ROP process from L-lactide in the early 1990s. The required lactide is produced starting from lactic acid in a continuous process; the LA is first condensed to produce a low molecular weight prepolymer PLA from which by controlled depolymerization produces the lactide. The typical operating conditions for the reactor were residence time about 1 h, vacuum pressure 4 mbar, temperature 210 °C, and catalyst amount 0.05 wt.% tin(II) octoate in the feed. The crude lactide is separated and purified by distillation as the specifications for lactide are stringent, especially in terms of free acidity, water content, and stereochemical purity [66]. The industrial PLA synthesis is often referred to as a two-step process because lactide synthesis and ROP are combined [66][68]. As an example, as a typical ROP process, Figure 4 shows that of NatureWorks based on the original Cargill-Dow patented process. NatureWorks produces the USA with a plant with a total capacity of 150,000 t/yr. The second largest plant is the 75,000 t PLA plant in Thailand under the joint venture of Total and Corbion companies [68]. For this later process Sulzer developed together Corbion a continuous process based on the use of static mixers, called SMR™ (Sulzer Mixing Reactor, Sulzer, Winterthur, Switzerland) [69]. This reactor is characterized by a precise control of heat transfer and mixing effects, which allow a high turnover and a consistently high polymer quality. Subsequent devolatization via a static degassing technology allows the elimination of volatiles in the PLA and thus the recycling of unreacted lactide [69].
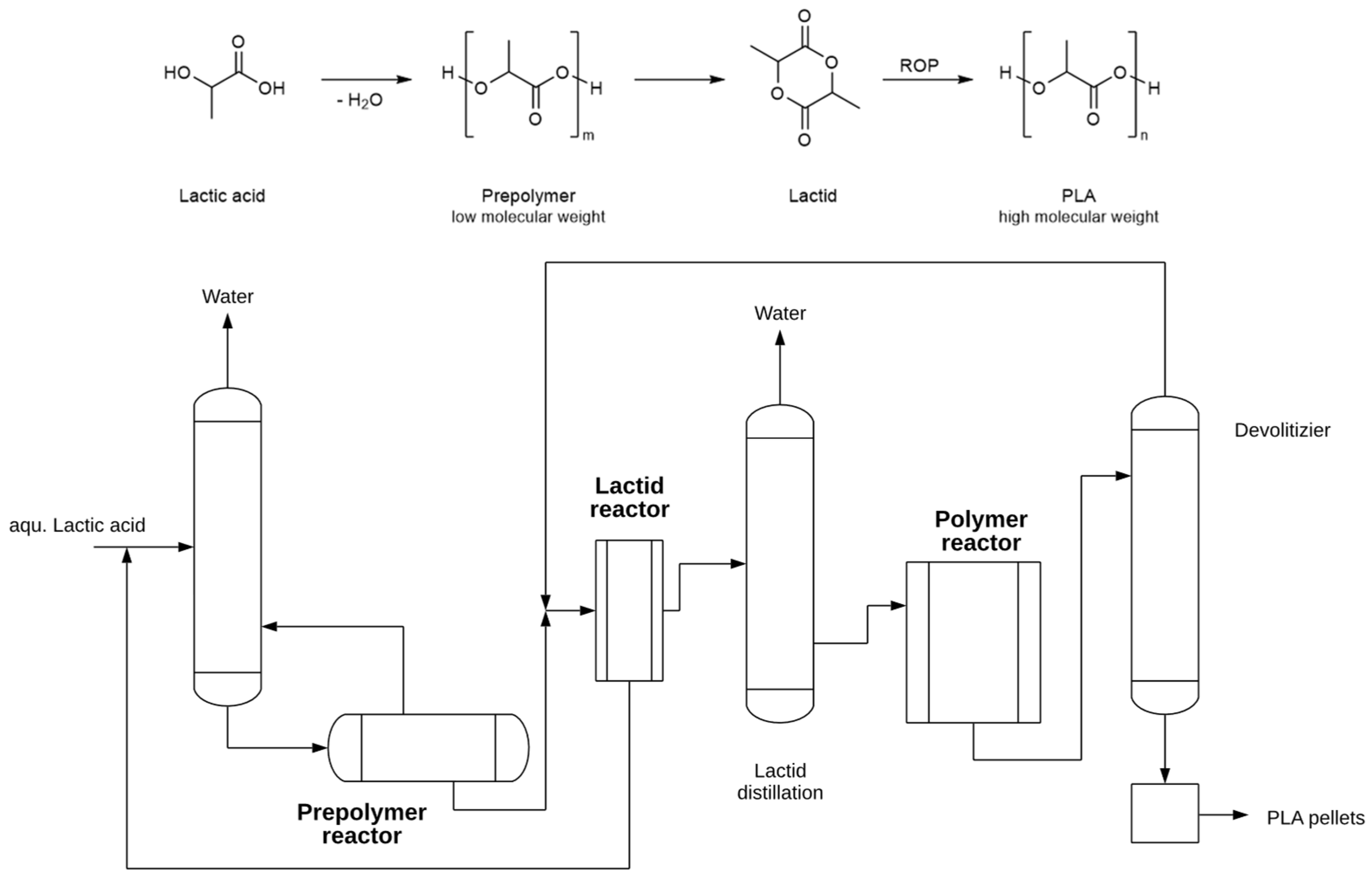
Figure 4. Schematic diagram of a typical combined lactide/ROP process for production of PLA.
10. PLA Current Applications
PLA is used in a wide range of applications ranging domestic, engineering, agricultural, and biomedical sectors [1][70]. PLA fibres can be used in packaging, clothing, furnishings, bedding (pillows, mattresses and so on), and other applications such as hygiene products [10][71]. Lunt and Shafer, [71] noted the advantages of PLA fibres for garments: better wicking and moisture regain; more comfortable; good resilience; unaffected by UV; low flammability; lower stiffness leads to better drape/hang; good crease resistance; dyeability; and sustainability. Superior self-extinguishing behaviour and lower smoke generation compared to other polymer fibres like PET, along with greater resilience and better sustainability, make PLA attractive for furnishings such as drapes and upholstery [71]. The renewable and biodegradable nature of PLA makes it well suited for single-use applications, such as packaging and containers [9]. For example, Swiftpak produce PLA insulation bags [72] and PLA bottles EU produce PLA bottles for milk and water [73]. Common packaging polymers like PET, PVC, polyethylene, polystyrene, and polyamide are petrochemical based with poor degradability. PLA’s properties are adaptable as described above which give scope to tailor the material to a range of packaging products. The Food and Drug Administration (FDA) in America has approved PLA for use in all food packaging applications [74]. PLA has been a popular material for additive manufacturing or 3D printing [47][75][76]. The relatively low glass and melt temperature make PLA easy to process with thermal methods like Fused Deposition Modelling (FDM). For rapid modelling or prototyping, many parts may be produced and discarded, so good degradability and good recyclability as a thermoplastic are other advantages to PLA. As it is non-toxic to the human body, and a bioabsorbable polymer, PLA is attractive for medical applications [74]. It may be used for sutures, dental implants, and drug-delivery devices [77].
PLA for Labware Applications
PLA’s biodegradable attributes makes it attractive for disposable single-use labware items; however, uptake of PLA in labware has been limited. Properties like temperature, UV, and chemical resistance may be limiting factors for this application. SP Scienceware subsidiary Bel-Art produces a range of “Earth-Friendly” spoons and sampling sticks [78][79]. Baden et al. [80] describe the use of additive manufacturing to produce labware in-house using PLA among other materials; however, the focus here was on longer term jigs and fixtures, rather than lab consumables. Gordeev et al. [81] investigated the use of AM to produce chemistry equipment from several engineering polymers including PLA. 3D printed test tubes of PP, PLA, Acrylonitrile Butadiene Styrene (ABS), and PETG were produced and characterised, and the suitability of the materials was assessed. Polypropylene is the most suitable material for chemical experiments due to its high resistance to chemical reagents; PLA labware, on the other hand, has superior properties: it has almost no pores and is very tight, the material does not shrink significantly, and the material is convenient for additional mechanical post-processing. While PETG products are partially transparent, which is an undeniable benefit, the layered structure created during printing precludes the use of PETG printed labware owing to its high porosity. ABS’s limited chemical resistance severely limits its use in chemistry. As a consequence, PP and PLA are much more appropriate for printing labware than ABS or PETG. The following is a general order of the functioning of plastic materials for chemical applications: PP > PLA > ABS > PETG. The mild solvents were Et2O, EtOH, hexane, and H2O, and the aggressive solvents were acetone, MeCN, CH2Cl2, THF, toluene, and DMSO.
References
- Sin, L.T.; Rahmat, A.R.; Rahman, W.A.W.A. Polylactic Acid: PLA Biopolymer Technology and Applications; William Andrew: Oxford, UK, 2012.
- EuropeanBioplastics. Available online: https://www.european-bioplastics.org/market/ (accessed on 16 December 2021).
- Hwang, K.-R.; Jeon, W.; Lee, S.Y.; Kim, M.-S.; Park, Y.-K. Sustainable bioplastics: Recent progress in the production of bio-building blocks for the bio-based next-generation polymer PEF. Chem. Eng. J. 2020, 390, 124636.
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are bioplastics and plant-based materials safer than conventional plastics? In vitro toxicity and chemical composition. Environ. Int. 2020, 145, 106066.
- Vink, E.T.; Rabago, K.R.; Glassner, D.A.; Gruber, P.R. Applications of life cycle assessment to NatureWorks™ polylactide (PLA) production. Polym. Degrad. Stab. 2003, 80, 403–419.
- Djukić-Vuković, A.; Mladenović, D.; Ivanović, J.; Pejin, J.; Mojović, L. Towards sustainability of lactic acid and poly-lactic acid polymers production. Renew. Sustain. Energy Rev. 2019, 108, 238–252.
- Anderson, K.S.; Schreck, K.M.; Hillmyer, M.A. Toughening polylactide. Polym. Rev. 2008, 48, 85–108.
- Perego, G.; Cella, G.D.; Bastioli, C. Effect of molecular weight and crystallinity on poly (lactic acid) mechanical properties. J. Appl. Polym. Sci. 1996, 59, 37–43.
- Ahmed, J.; Varshney, S.K. Polylactides—Chemistry, properties and green packaging technology: A review. Int. J. Food Prop. 2011, 14, 37–58.
- Avinc, O.; Khoddami, A. Overview of poly (lactic acid)(PLA) fibre. Fibre Chem. 2010, 42, 68–78.
- Kaiser, M.; Anuar, H.; Razak, S. Ductile–brittle transition temperature of polylactic acid-based biocomposite. J. Thermoplast. Compos. Mater. 2013, 26, 216–226.
- Janorkar, A.V.; Metters, A.T.; Hirt, D.E. Degradation of poly (L-lactide) films under ultraviolet-induced photografting and sterilization conditions. J. Appl. Polym. Sci. 2007, 106, 1042–1047.
- Man, C.; Zhang, C.; Liu, Y.; Wang, W.; Ren, W.; Jiang, L.; Reisdorffer, F.; Nguyen, T.P.; Dan, Y. Poly (lactic acid)/titanium dioxide composites: Preparation and performance under ultraviolet irradiation. Polym. Degrad. Stab. 2012, 97, 856–862.
- Mohr, L.; Capelezzo, A.; Baretta, C.; Martins, M.; Fiori, M.; Mello, J. Titanium dioxide nanoparticles applied as ultraviolet radiation blocker in the polylactic acid bidegradable polymer. Polym. Test. 2019, 77, 105867.
- Ho, M.-P.; Lau, K.-T.; Wang, H.; Hui, D. Improvement on the properties of polylactic acid (PLA) using bamboo charcoal particles. Compos. Part B Eng. 2015, 81, 14–25.
- Zhou, S.; Zhang, Y.; Ni, L.; Pei, Y.; Zhang, H.; Zhang, H. Applied organic-inorganic nanocomposite of PLA-TiO2 for preparing polysulfone membrane: Structure, performance and UV-assisted cleaning strategy. Water Sci. Technol. 2021, 83, 198–211.
- Cao, Y.; Xu, P.; Lv, P.; Lemstra, P.J.; Cai, X.; Yang, W.; Dong, W.; Chen, M.; Liu, T.; Du, M. Excellent UV resistance of polylactide by interfacial stereocomplexation with double-shell-structured TiO2 nanohybrids. ACS Appl. Mater. Interfaces 2020, 12, 49090–49100.
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953.
- Qin, Y. 14—Biocompatibility testing for medical textile products. In Medical Textile Materials; Woodhead Publishing: Cambridge, UK, 2016.
- Anderson, J. Biocompatibility. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Moller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 9, pp. 363–383.
- Jenke, D. Evaluation of the chemical compatibility of plastic contact materials and pharmaceutical products; safety considerations related to extractables and leachables. J. Pharm. Sci. 2007, 96, 2566–2581.
- Massey, L.K. Introduction to Sterilization Methods. In The Effect of Sterilization Methods on Plastics and Elastomers, 2nd ed.; William Andrew: Norwich, NY, USA, 2005; pp. 5–18.
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC press: Boca Raton, FL, USA, 2007.
- Agrawal, A.; Saran, A.D.; Rath, S.S.; Khanna, A. Constrained nonlinear optimization for solubility parameters of poly (lactic acid) and poly (glycolic acid)—validation and comparison. Polymer 2004, 45, 8603–8612.
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501.
- Mutsuga, M.; Kawamura, Y.; Tanamoto, K. Migration of lactic acid, lactide and oligomers from polylactide food-contact materials. Food Addit. Contam. 2008, 25, 1283–1290.
- Sinclair, R. The case for polylactic acid as a commodity packaging plastic. J. Macromol. Sci. Part A Pure Appl. Chem. 1996, 33, 585–597.
- Hiljanen-Vainio, M.; Varpomaa, P.; Seppälä, J.; Törmälä, P. Modification of poly (L-lactides) by blending: Mechanical and hydrolytic behavior. Macromol. Chem. Phys. 1996, 197, 1503–1523.
- Wang, L.; Ma, W.; Gross, R.; McCarthy, S. Reactive compatibilization of biodegradable blends of poly (lactic acid) and poly (ε-caprolactone). Polym. Degrad. Stab. 1998, 59, 161–168.
- Notta-Cuvier, D.; Odent, J.; Delille, R.; Murariu, M.; Lauro, F.; Raquez, J.; Bennani, B.; Dubois, P. Tailoring polylactide (PLA) properties for automotive applications: Effect of addition of designed additives on main mechanical properties. Polym. Test. 2014, 36, 1–9.
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on polylactic acid (PLA) based sustainable materials for durable applications: Focus on toughness and heat resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916.
- Kawamoto, N.; Sakai, A.; Horikoshi, T.; Urushihara, T.; Tobita, E. Physical and mechanical properties of poly (l-lactic acid) nucleated by dibenzoylhydrazide compound. J. Appl. Polym. Sci. 2007, 103, 244–250.
- Tábi, T.; Kovács, N.; Sajó, I.; Czigány, T.; Hajba, S.; Kovács, J. Comparison of thermal, mechanical and thermomechanical properties of poly (lactic acid) injection-molded into epoxy-based Rapid Prototyped (PolyJet) and conventional steel mold. J. Therm. Anal. Calorim. 2016, 123, 349–361.
- Gao, X.; Qi, S.; Zhang, D.; Su, Y.; Wang, D. The role of poly (ethylene glycol) on crystallization, interlayer bond and mechanical performance of polylactide parts fabricated by fused filament fabrication. Addit. Manuf. 2020, 35, 101414.
- Hriţuc, A.; Slătineanu, L.; Mihalache, A.; Dodun, O.; Coteaţă, M.; Nagîţ, G. Accuracy of polylactide parts made by 3D printing. In Proceedings of the Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 2020; p. 1900064.
- Cheon, S.S.; Jeong, K.S. Composite side-door impact beams for passenger cars. Compos. Struct. 1997, 38, 229–239.
- Joo, S.-J.; Yu, M.-H.; Kim, W.S.; Lee, J.-W.; Kim, H.-S. Design and manufacture of automotive composite front bumper assemble component considering interfacial bond characteristics between over-molded chopped glass fiber polypropylene and continuous glass fiber polypropylene composite. Compos. Struct. 2020, 236, 111849.
- Poulikidou, S.; Jerpdal, L.; Björklund, A.; Åkermo, M. Environmental performance of self-reinforced composites in automotive applications—Case study on a heavy truck component. Mater. Des. 2016, 103, 321–329.
- Yakout, M.; Elbestawi, M. Additive manufacturing of composite materials: An overview. In Proceedings of the 6th International Conference on Virtual Machining Process Technology (VMPT), Montréal, QC, Canada, 29 May–2 June 2017.
- Brøndsted, P.; Lilholt, H.; Lystrup, A. Composite materials for wind power turbine blades. Annu. Rev. Mater. Res. 2005, 35, 505–538.
- Su, H.; Kam, T. Reliability analysis of composite wind turbine blades considering material degradation of blades. Compos. Struct. 2020, 234, 111663.
- Rahimizadeh, A.; Kalman, J.; Henri, R.; Fayazbakhsh, K.; Lessard, L. Recycled glass fiber composites from wind turbine waste for 3D printing feedstock: Effects of fiber content and interface on mechanical performance. Materials 2019, 12, 3929.
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46.
- Chen, R.; Misra, M.; Mohanty, A.K. Injection-moulded biocomposites from polylactic acid (PLA) and recycled carbon fibre: Evaluation of mechanical and thermal properties. J. Thermoplast. Compos. Mater. 2014, 27, 1286–1300.
- Agüero, Á.; Lascano, D.; Garcia-Sanoguera, D.; Fenollar, O.; Torres-Giner, S. Valorization of linen processing by-products for the development of injection-molded green composite pieces of polylactide with improved performance. Sustainability 2020, 12, 652.
- Quiles-Carrillo, L.; Montanes, N.; Garcia-Garcia, D.; Carbonell-Verdu, A.; Balart, R.; Torres-Giner, S. Effect of different compatibilizers on injection-molded green composite pieces based on polylactide filled with almond shell flour. Compos. Part B Eng. 2018, 147, 76–85.
- Quiles-Carrillo, L.; Montanes, N.; Lagaron, J.M.; Balart, R.; Torres-Giner, S. On the use of acrylated epoxidized soybean oil as a reactive compatibilizer in injection-molded compostable pieces consisting of polylactide filled with orange peel flour. Polym. Int. 2018, 67, 1341–1351.
- Calì, M.; Pascoletti, G.; Gaeta, M.; Milazzo, G.; Ambu, R. A new generation of bio-composite thermoplastic filaments for a more sustainable design of parts manufactured by FDM. Appl. Sci. 2020, 10, 5852.
- Matsuzaki, R.; Ueda, M.; Namiki, M.; Jeong, T.-K.; Asahara, H.; Horiguchi, K.; Nakamura, T.; Todoroki, A.; Hirano, Y. Three-dimensional printing of continuous-fiber composites by in-nozzle impregnation. Sci. Rep. 2016, 6, 23058.
- López-Rodríguez, N.; López-Arraiza, A.; Meaurio, E.; Sarasua, J. Crystallization, morphology, and mechanical behavior of polylactide/poly (ε-caprolactone) blends. Polym. Eng. Sci. 2006, 46, 1299–1308.
- Zhu, H.; Feng, X.; Zhang, H.; Guo, Y.; Zhang, J.; Chen, J. Structural characteristics and properties of silk fibroin/poly (lactic acid) blend films. J. Biomater.Sci. Polym. Ed. 2009, 20, 1259–1274.
- Chen, G.-X.; Kim, H.-S.; Kim, E.-S.; Yoon, J.-S. Compatibilization-like effect of reactive organoclay on the poly (l-lactide)/poly (butylene succinate) blends. Polymer 2005, 46, 11829–11836.
- Wang, R.; Wan, C.; Wang, S.; Zhang, Y. Morphology, mechanical properties, and durability of poly (lactic acid) plasticized with Di (isononyl) cyclohexane-1, 2-dicarboxylate. Polym. Eng. Sci. 2009, 49, 2414–2420.
- Park, J.W.; Im, S.S. Phase behavior and morphology in blends of poly (L-lactic acid) and poly (butylene succinate). J. Appl. Polym. Sci. 2002, 86, 647–655.
- Yokohara, T.; Yamaguchi, M. Structure and properties for biomass-based polyester blends of PLA and PBS. Eur. Polym. J. 2008, 44, 677–685.
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and Its Blends with Poly(butylene succinate) (PBS): A Brief Review. Polymers 2019, 11, 1193.
- Rojas-Martínez, L.; Flores-Hernandez, C.; López-Marín, L.; Martinez-Hernandez, A.; Thorat, S.; Vasquez, C.R.; Del Rio-Castillo, A.; Velasco-Santos, C. 3D printing of PLA composites scaffolds reinforced with keratin and chitosan: Effect of geometry and structure. Eur. Polym. J. 2020, 141, 110088.
- Brounstein, Z.; Yeager, C.M.; Labouriau, A. Development of Antimicrobial PLA Composites for Fused Filament Fabrication. Polymers 2021, 13, 580.
- Banerjee, R.; Ray, S.S. An overview of the recent advances in polylactide-based sustainable nanocomposites. Polym. Eng. Sci. 2021, 61, 617–649.
- Dedukh, N.; Makarov, V.; Pavlov, A. Polylactide-based biomaterial and its use as bone implants (analytical literature review). Pain Jt. Spine 2019, 9, 28–35.
- Moetazedian, A.; Gleadall, A.; Han, X.; Silberschmidt, V.V. Effect of environment on mechanical properties of 3D printed polylactide for biomedical applications. J. Mech. Behav. Biomed. Mater. 2020, 102, 103510.
- Bulanda, K.; Oleksy, M.; Oliwa, R.; Budzik, G.; Gontarz, M. Biodegradable polymer composites based on polylactide used in selected 3D technologies. Polimery 2020, 65, 557–562.
- Bergström, J.S.; Hayman, D. An overview of mechanical properties and material modeling of polylactide (PLA) for medical applications. Ann. Biomed. Eng. 2016, 44, 330–340.
- Quiles-Carrillo, L.; Duart, S.; Montanes, N.; Torres-Giner, S.; Balart, R. Enhancement of the mechanical and thermal properties of injection-molded polylactide parts by the addition of acrylated epoxidized soybean oil. Mater. Des. 2018, 140, 54–63.
- Castro-Aguirre, E.; Iniguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly (lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366.
- Masutani, K.; Kimura, Y. PLA Synthesis. From the Monomer to the Polymer. In Poly(Lactic Acid) Science and Technology Processing, Properties, Additives and Applications; Jimenez, A., Peltzer, M., Ruseckaite, R., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2015; pp. 3–36.
- Gupta, A.; Kumar, V. New emerging trends in synthetic biodegradable polymers–Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074.
- Jem, K.J.; Tan, B. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70.
- Wintergerste, T. Great advances in bioplastics. Sulzer Tech. Rev. 2012, 13–14.
- Andrzejewska, A. One Year Evaluation of material properties changes of polylactide parts in various hydrolytic degradation conditions. Polymers 2019, 11, 1496.
- Lunt, J.; Shafer, A.L. Polylactic acid polymers from com. Applications in the textiles industry. J. Ind. Text. 2000, 29, 191–205.
- Swiftpak. Eco-friendly Packaging—PLA Packaging: The Ultimate Guide. Available online: https://www.swiftpak.co.uk/insights/pla-packaging-the-ultimate-guide (accessed on 15 December 2021).
- EU, P.B. LCA of PLA Bottle Solutions for Extended Shelf-Life (ESL) Milk. Available online: https://plabottles.eu/lca-of-pla-bottle-for-milk/ (accessed on 15 December 2021).
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392.
- Donate, R.; Monzón, M.; Alemán-Domínguez, M.E. Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties. e-Polymers 2020, 20, 571–599.
- Liu, Z.; Wang, Y.; Wu, B.; Cui, C.; Guo, Y.; Yan, C. A critical review of fused deposition modeling 3D printing technology in manufacturing polylactic acid parts. Int. J. Adv. Manuf. Technol. 2019, 102, 2877–2889.
- Gad, S.C. Integrated Safety and Risk Assessment for Medical Devices and Combination Products; Springer Nature: Cham, Switzerland, 2020.
- SP Bel-Art. Earth-Friendly 1001 Sticks. Available online: https://www.belart.com/earth-friendly-1001-sticks.html (accessed on 15 December 2021).
- SP Bel-Art. Earth-Friendly Long Handle Sampling Spoon. Available online: https://www.belart.com/earth-friendly-long-handle-sampling-spoons.html (accessed on 15 December 2021).
- Baden, T.; Chagas, A.M.; Gage, G.; Marzullo, T.; Prieto-Godino, L.L.; Euler, T. Open Labware: 3-D printing your own lab equipment. PLoS Biol. 2015, 13, e1002086.
- Gordeev, E.; Degtyareva, E.; Ananikov, V. Analysis of 3D printing possibilities for the development of practical applications in synthetic organic chemistry. Russ. Chem. Bull. 2016, 65, 1637–1643.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.9K
Revisions:
2 times
(View History)
Update Date:
06 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




