Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Li Yao Li | -- | 2321 | 2022-05-05 10:48:33 | | | |
| 2 | Conner Chen | Meta information modification | 2321 | 2022-05-05 11:08:23 | | | | |
| 3 | Conner Chen | -4 word(s) | 2317 | 2022-05-06 02:53:43 | | | | |
| 4 | Conner Chen | -2 word(s) | 2315 | 2022-05-07 10:54:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, L.Y.; , .; Andersson-Engels, S. Ultrafast Laser in Orthopedic Surgery. Encyclopedia. Available online: https://encyclopedia.pub/entry/22601 (accessed on 07 January 2026).
Li LY, , Andersson-Engels S. Ultrafast Laser in Orthopedic Surgery. Encyclopedia. Available at: https://encyclopedia.pub/entry/22601. Accessed January 07, 2026.
Li, Li Yao, , Stefan Andersson-Engels. "Ultrafast Laser in Orthopedic Surgery" Encyclopedia, https://encyclopedia.pub/entry/22601 (accessed January 07, 2026).
Li, L.Y., , ., & Andersson-Engels, S. (2022, May 05). Ultrafast Laser in Orthopedic Surgery. In Encyclopedia. https://encyclopedia.pub/entry/22601
Li, Li Yao, et al. "Ultrafast Laser in Orthopedic Surgery." Encyclopedia. Web. 05 May, 2022.
Copy Citation
The potential of ultrafast lasers (pico- to femtosecond) in orthopedics-related procedures has been studied extensively for clinical adoption. As compared to conventional laser systems with continuous wave or longer wave pulse, ultrafast lasers provide advantages such as higher precision and minimal collateral thermal damages. Translation to surgical applications in the clinic has been restrained by limitations of material removal rate and pulse average power, whereas the use in surface texturing of implants has become more refined to greatly improve bioactivation and osteointegration within bone matrices.
ultrafast lasers
orthopedic surgery
clinical translation
1. Ablation Parameters
Early research of ultrafast laser ablation of bone tissues was predominantly focused on modification and microsurgery of teeth, ear [1] and spine, with very few publications in orthopedic surgery of the peripheral extremities (long bones of arm and leg). Despite all the advantages, the main issue that limits its clinical translation and acceptance, namely the low ablation removal rate, has remained the same even to date [2][3][4][5]. For instance, two studies published in 2007 discussed the use of ultrafast laser for bone ablation. Wieger et al. conducted a comparison study of laser osteotomy on bovine bone tissues using a femtosecond (fs) Yb:glass laser (pulse duration = 330 fs; λ = 1040 nm; pulse repetition rate (PRR) = 1 kHz; max. average pulse energy = 130 μJ) and a conventional Er, Cr:YSGG laser system (pulse duration = 53 µs; λ = 2780 nm; PRR = 20 Hz; max. average pulse energy = 300 mJ) [2]. The ablation rate of the femtosecond laser (~4 × 10−4 mm3/s) was found to be approximately 400-fold slower than the Erbium laser (~0.15 mm3/s) given a power density of ~1000 W/cm2, but could be further optimized by increasing the PRR while maintaining the favorable cut surface morphology. The ablation thresholds were calculated to be 0.82, 0.78 and 0.54 J/cm2 for spongiosa bone, compacta bone and cartilage, respectively, with an average of 72.4 laser pulses overlapped. Moreover, an ultrafast laser system consisting of a diode-pumped Yb:glass laser seed source and a Yb:KYW thin disk laser head (pulse duration = 900 fs; λ = 1030 nm; PRR = 45 KHz; max. average pulse energy = 100 μJ) was used by Liu et al. to achieve a maximum ablation rate of 0.15 mm3/s in porcine femora at the given power output, which was acceptable clinically for knee arthroplasty [4]. Additionally, in precision surgery such as stapedotomy, ultrafast laser has also been proven ideal by offering minimal thermal and acoustic damage [1][6].
Encouragingly, in the past decade, several studies have discovered more novel practices using ultrafast lasers and attempted to tackle the obstacles in the clinic. Notably, Subramanian et al. have developed a lightweight, miniaturized surgical ultrafast laser probe that offers clinically acceptable ablation speed in orthopedic surgery, enabling the potential of robotic integration [7]. Another group also established an optical real-time monitoring of ultrafast laser bone drilling utilizing plasma emission spectroscopy, which allowed for differentiation between bone and bone marrow [8]. The feasibility of drilling large-sized, deep holes on cortical bones has also been demonstrated [8][9]. Furthermore, the fastest ablation rate on cortical bone tissues to date is 0.99 mm3/s in the literature, which was performed on fresh ex vivo sheepshank bone under a cooling condition by Zhang et al. in 2020 [8], followed by 0.66 mm3/s on dried ex vivo defrost and dried porcine femurs under a non-cooling condition reported by Gemini et al. in 2021, using industrially available femtosecond laser sources [10], in comparison to mechanical tools that enable drilling and cutting speeds of up to 5 mm/s [11]. The upscaling of ablation rate is fundamentally constrained by the average powers combined with considerations of repetition rate optimization as well as thermal effect on the tissue [5][12]. The phenomenon arises from the fact that, at the given laser average power and wavelength, an increase in single pulse energy resulting from a decrease in PRR improves the ablation rate until the saturation point where laser energy begins to spread outside the penetration volume [13]. Gemini et al. also compared bone ablation efficiency in different wavelength regimes (IR—1030 nm, visible—515 nm, UV—343 nm), average powers (IR—6.27 W, visible—6.27 W, UV—3.9 W), PRRs (250, 500, 1000 kHz) and scanning speeds (1000, 2500, 400 mm/s) with the conclusion that visible regime, the lowest PRR and the highest scanning speed provided the best ablation rate without thermal tissue disturbance because bone chromophores responded differently to the three wavelength regimes [10]. Moreover, the ablation rate is sometimes affected by bone debris accumulation depending on the amount generated, and thus can be further upscaled by immediate debris removal after each pulse using a cooling system [8][14]. It was demonstrated that compressed air flow and water flow could reduce bone debris by 64% and 76%, respectively; however, significant laser energy loss was observed under water cooling conditions leading to the slowest ablation rate [8]. It has also been noted that sample conditions, specifically ex vivo vs. in vivo, dried vs. fresh and storage conditions, as well as bone surface processing such as sanding, polishing or unaltered can have a significant influence on the ablation performance [15][16][17]; thus, it is crucial to simulate a real clinical situation for accurate and consistent assessments. Table 1 summarizes the ablation rates from ultrafast laser bone ablation studies of different bone samples using different laser parameters.
Table 1. Summary of recent studies on the use of ultrafast lasers for orthopedic applications.
| Author/Year | Bone Type | Ablation Rate (mm3/s) | Laser System Parameters | Potential Application |
|---|---|---|---|---|
| Subramanian et al., 2021 [7] | Bovine rib (fresh) | >1.7 × 10−2 | A CaF2 objective; Er-doped fiber; 1552 nm, 600 fs, 303 kHz |
A miniaturized surgical probe for robotic microsurgery such as spine |
| Gemini et al., 2021 [10] | Porcine femurs (defrost and dried) | 0.66 | A Tangerine industrial femtosecond laser, Amplitude Laser; 517 nm, 350 fs, 250 kHz | Clinical automated high-resolution orthopedic surgery |
| Ashforth et al., 2020 [15] | Bovine and ovine cortical bone (fresh) | 0.90 µm/pulse * | A Ti:Sapphire femtosecond pulsed laser; 800 nm, 140 fs, 1 kHz | Handheld or robotic high-precision orthopedic surgery procedures |
| Zhang et al., 2020 [8] | Sheepshank bone (fresh) | 0.99 | A Yb:KGW femtosecond laser; 1030 nm, 230 fs, 200 kHz | Large-size hole drilling with real-time monitoring |
| Aljekhedab et al., 2019 [14] | Bovine cortical bone (fresh) | 0.60 × 10−3 | A Ti:Sapphire femtosecond laser; 800 nm, 210 fs, 1 kHz |
High-precision bone cutting surgery |
| Tulea et al., 2015 [18] | Cow femur cortical bone (fresh, dried or fixed) | 0.19 | A Nd:YVO4 picosecond laser; 532 nm, 25 ps, 20 kHz | Bone surgery |
| Plötz et al., 2014 [12] | Porcine rib (fresh) | 8.7 × 10−2 | A Nd:YVO4 laser; 1064 nm, 8 ps, 500 kHz | Dental surgery |
| Su et al., 2014 [19] | Bovine femoral condyle (fresh) | 0.80 × 10−4 | A Ti:Sapphire femtosecond laser combined with an optical parametric amplifier; 1700 nm, pulse duration N.R., 5 kHz | Microfracture surgery for articular cartilage injury in the knee |
* Reported as depth removal per pulse.
Characterization of the ablation threshold and the incubation effect likewise plays a major part in optimizing ablation performance on a particular bone type. Ablation threshold, which represents the minimal laser fluence needed to initiate material removal from a surface [5][16], can be measured by exposing the surface to ultrafast laser pulses of decreasing energy or beam radius until no material removal occurs [5][18] or using the D2-technique calculation based on the correlation between the diameters of ablated craters and different pulse energy levels [15][16][18][20][21][22][23]. Bone ablation is usually optimized when the pulse energy is sufficiently higher than the threshold to ensure pulse-to-pulse consistency, but not exceeding a limit that could induce collateral thermal damage [24]. The balance between ablation rate and fluence therefore needs to be well characterized for the bone tissue, otherwise negating the most unique advantage of minimizing thermal effects [25][26][27]. On the other hand, incubation effect refers to the phenomenon where a reducing ablation fluence threshold is accompanied by an increasing number of incident laser pulses in a power law relationship [28], and is typically caused by sufficient energy deposit from the few initial pulses for subsequent pulses permitting lower than single-pulse ablation threshold [7][16][23][29][30]. An incubation coefficient value of 1 indicates no incubation effect. Incubation effect is the most profound at lower pulse numbers where ablation threshold is rapidly reduced as the pulse numbers increase until a saturation point [16][31], and has been shown to modify tissue structures significantly enough, especially at higher PRR, to create beam distortion, shadowing and substantial light scattering due to debris shielding, thus leading to considerable thermal disturbance and decrease in ablation rate [10][26]. With that being said, a recent study by Ashforth et al. concluded that there was none to very little incubation effect found for two types of cortical bones (bovine and ovine) by showing the same incubation coefficient (1.02 ± 0.05) [15]. The possible reason, as the authors explained, could be due to the already high level of microscopic inhomogeneity of native bone tissues; the newly introduced structure defects from laser ablation were consequently negligible. For clinical translation, such behaviors are indeed beneficial in the way that the efficiency of ultrafast laser ablation can remain consistent while drilling into different bone structures. Table 2 outlines the ablation threshold for different tissue types using different laser parameters.
Table 2. Summary of ablation thresholds determined for different bone types. N = Number of pulses.
| Author/Year | Bone Type | Ablation Threshold (J/cm2) | Pulse Duration (fs) | Wavelength (nm) |
|---|---|---|---|---|
| Subramanian et al., 2021 [7] | Bovine cortical bone (fresh; unaltered) |
1.38 ± 0.18 (N = 25.83 *; multi-pulse threshold) |
600 | 1552 |
| Ashforth et al., 2020 [15] | Bovine and ovine cortical bone (fresh; unaltered) |
0.92 (bovine) 0.97 (ovine) (N = 1000) |
140 | 800 |
| Plötz et al., 2014 [12] | Porcine cortical bone (fresh; unaltered) |
1.5 (N = Not Reported) |
8 × 103 | 1064 |
| Cangueiro et al., 2012 [23] | Bovine cortical bone (fresh; polished) |
0.32 ± 0.04 (N = 100) |
500 | 1030 |
| Nicolodelli et al., 2012 [26] | Bovine cortical bone (fresh; polished) |
0.23 (N = 1000) |
70 | 801 |
| Emigh et al., 2012 [16] | Porcine cortical bone (fresh; unaltered) |
1.75 ± 0.55 (N = 1000) |
170 | 800 |
| Lim et al., 2009 [29] | Bovine cortical bone (fresh; polished) |
1.22 ± 0.29 (strong) 0.79 ± 0.18 (gentle) (N = 1000) |
150 | 775 |
| Girard et al., 2007 [5] | Porcine cortical bone (fresh; polished) |
0.69 ± 0.08 (N = 1000) |
200 | 775 |
* Estimated averaged N value predicted by simulation.
2. Thermal Effect
As discussed before, ultrafast laser ablation is overall characterized by minimal thermal damage and limited heat diffusion outside of the focal volume, because the dominant mechanism, namely multiphoton absorption of light and avalanche ionization or hydrodynamic plasma expansion [32], is not thermally mediated with only little heat deposition, making it an auspicious technique in the clinic [33][34][35][36]. Still early studies demonstrated some carbonization, cracking and melting [19][23][26], which could be mitigated by employing cooling systems [8][37]. Nevertheless, recent studies have shown more optimized performance [38][39][40]. In particular, Ashforth et al. reported no observations of a heat-affected zone at the maximum laser fluence and pulse numbers by assessing any forms of carbonization, discoloration and microcracking around the craters using light microscopy [15]. Canteli et al. evaluated thermal effects on fresh bovine femur using a nanosecond laser source (20 ns, 355 nm, 2–100 kHz) as compared to a picosecond laser source (12 ps, 1064 nm, 100–600 kHz), and found that the picosecond laser, although not as ideal as femtosecond lasers, resulted in significantly reduced heating compared to the nanosecond laser [39]. The study by Gemini et al. provided optimization strategies and described the observation of increasing thermal accumulation while decreasing scanning speed and increasing PRR individually in the IR and visible wavelength regimes [10]. When both the scanning speed and PRR were increased, not only thermal loads increased but expanding plasma plume was also produced, leading to reduced ablation efficiency and precision. However, the observation did not apply to the UV regime, where collagen and hemoglobin are the main absorbers. No specific behavior was detected with changing parameters; the thermal load was comparatively high enough at the lowest PRR to generate laser-induced bone calcination. In Figure 1, laser-irradiated damages such as the typical thermal-induced particle-like roughness and micro-cracks can be seen under SEM images at a higher PRR (Figure 1a), whereas native bone structures containing blood vessels and osteocytes were preserved at a lower PRR (Figure 1b,c) [10]. The interrelationship between scanning speed, PRR and wavelength choice therefore requires thorough investigation for an optimized laser ablation performance without collateral thermal damages on bone tissues. Similarly, Gill et al. studied temperature distributions of dried bovine bone irradiated also by a Tangerine laser (Amplitude, 320 fs, 1030 nm) using different PRRs [40]. It was found that carbonization occurred at high enough PRRs where thermal dissipation was exceeded by accumulation causing irreversible tissue damage, and therefore the importance of rigorous laser parameter selection was again emphasized in order to minimize thermal effects while maximizing ablation rates. Furthermore, the pattern of scanning path can also influence heat accumulation and thus reduce the ablation rate. Circular scanning motion was found to generate significant thermal damage (charring) as compared to scanning in line paths because of poor heat dissipation [8]. Additionally, with large-size and deep holes, ultrafast laser can still produce slight charring around the edge.
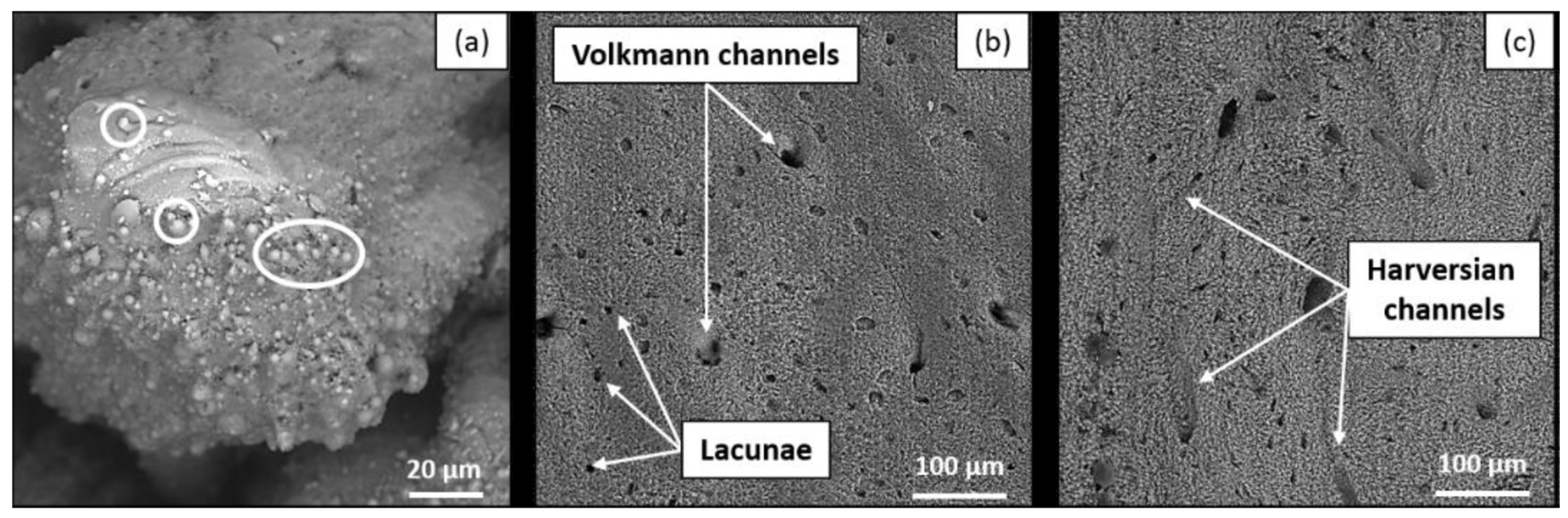
Figure 1. Bone tissues after ultrafast laser ablation under SEM imaging in the visible regime showing (a) laser-induced thermal damages (spherical structures indicated by the white circle) at a higher PRR (515 nm, 1000 kHz, 4000 mm/s, 6.27 W), while (b) shows native bone structures such as Volkmann channels and lacunae at a lower PRR (515 nm, 250 kHz, 4000 mm/s, 6.27 W), as well as the Harversian channels shown in (c) after ablation in the IR regime (1030 nm, 250 kHz, 1000 mm/s, 6.27 W).
3. Surface Morphology
Surface morphology is usually assessed using scanning electron microscopy (SEM), confocal microscopy, X-ray computed microtomography (µCT) and histology to evaluate thermal effects on the tissue [8][9][10]. By visually examining the condition of craters or holes on bone tissue after laser ablation on the microscopic level, physical features such as charring, roughness and micro-cracks can be identified to determine and compare the degree of laser-induced damage. As previously mentioned, a picosecond or femtosecond pulsed laser system generates little to no thermal effect during bone ablation compared to longer pulse or mechanical tools [25]. Several authors have described, under SEM or histological observations, that the bottom and side walls of the laser-ablated cavity are smooth and homogeneous with a well-defined geometry [6][14][15][39]. No significant signs of charring, melting or major debris accumulation were found [23][25][29]. Figure 2, shows an image comparison of ultrafast laser ablation under different cooling conditions, namely no cooling (a, a1, a2), gas (b, b1, b2) or water (c, c1, c2) cooling, using white light imaging, SEM and histology, respectively, which demonstrated great uniformity and precise cutting with, overall, no observation of cracks, especially for cooling-assisted drillings. Some microcracks were, however, observed in the inner wall without cooling, which could potentially delay bone healing [8].
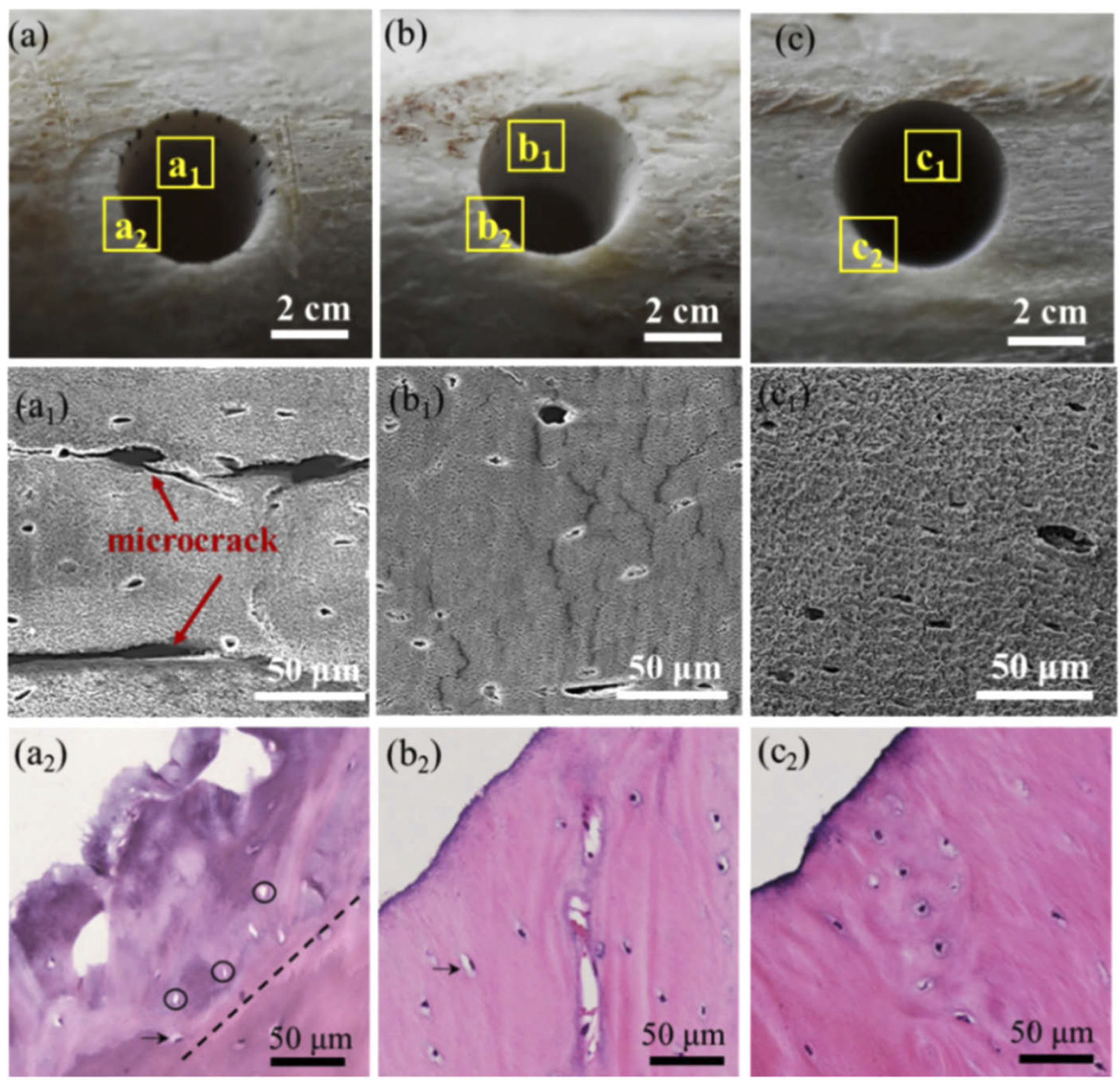
Figure 2. Morphology of laser-drilled bone tissues under different environmental cooling conditions. Vertical panels (a–c) correspond to without cooling, gas cooling and water cooling; horizontal panels (subscript: none, 1, 2) correspond to white light, SEM and histology images.
References
- Armstrong, W.B.; Neev, J.A.; Da Silva, L.B.; Rubenchik, A.M.; Stuart, B.C. Ultrashort pulse laser ossicular ablation and stapedotomy in cadaveric bone. Lasers Surg. Med. 2002, 30, 216–220.
- Wieger, V.; Zoppel, S.; Wintner, E. Ultrashort pulse laser osteotomy. Laser Phys. 2007, 17, 438–442.
- Strassl, M.; Wieger, V.; Brodoceanu, D.; Beer, F.; Moritz, A.; Wintner, E. Ultra-Short Pulse Laser Ablation of Biological Hard Tissue and Biocompatibles. J. Laser Micro/Nanoeng. 2008, 3, 30–40.
- Liu, Y.; Niemz, M. Ablation of femural bone with femtosecond laser pulses—A feasibility study. Lasers Med. Sci. 2007, 22, 171–174.
- Girard, B.; Yu, D.; Armstrong, M.R.; Wilson, B.C.; Clokie, C.M.L.; Miller, R.J.D. Effects of femtosecond laser irradiation on osseous tissues. Lasers Surg. Med. 2007, 39, 273–285.
- McCaughey, R.G.; Sun, H.; Rothholtz, V.S.; Juhasz, T.; Wong, B.J.-F. Femtosecond laser ablation of the stapes. J. Biomed. Opt. 2009, 14, 024040.
- Subramanian, K.; Andrus, L.; Pawlowski, M.; Wang, Y.; Tkaczyk, T.; Ben-Yakar, A. Ultrafast laser surgery probe with a calciumfluoride miniaturized objective for bone ablation. Biomed. Opt. Express 2021, 12, 4779.
- Zhang, J.; Guan, K.; Zhang, Z.; Guan, Y. In vitro evaluation of ultrafast laser drilling large-size holes on sheepshank bone. Opt. Express 2020, 28, 25528.
- An, R.; Khadar, G.W.; Wilk, E.I.; Emigh, B.; Haugen, H.K.; Wohl, G.R.; Dunlop, B.; Anvari, M.; Hayward, J.E.; Fang, Q. Ultrafast laser ablation and machining large-size structures on porcine bone. J. Biomed. Opt. 2013, 18, 070504.
- Gemini, L.; Al-Bourgol, S.; Machinet, G.; Bakkali, A.; Faucon, M.; Kling, R. Ablation of Bone Tissue by Femtosecond Laser: A Path to High-Resolution Bone Surgery. Materials 2021, 14, 2429.
- Fisher, C.; Harty, J.; Yee, A.; Li, C.L.; Komolibus, K.; Grygoryev, K.; Lu, H.; Burke, R.; Wilson, B.C.; Andersson-Engels, S. Perspective on the integration of optical sensing into orthopedic surgical devices. J. Biomed. Opt. 2022, 27, 010601.
- Plötz, C.; Schelle, F.; Bourauel, C.; Frentzen, M.; Meister, J. Ablation of porcine bone tissue with an ultrashort pulsed laser (USPL) system. Lasers Med. Sci. 2014, 30, 977–983.
- Kramer, T.; Remund, S.; Jäggi, B.; Schmid, M.; Neuenschwander, B. Ablation dynamics—From absorption to heat accumulation/ultra-fast laser matter interaction. Adv. Opt. Technol. 2018, 7, 129–144.
- Aljekhedab, F.; Zhang, W.; Haugen, H.K.; Wohl, G.R.; El-Desouki, M.M.; Fang, Q. Influence of environmental conditions in bovine bone ablation by ultrafast laser. J. Biophotonics 2019, 12, e201800293.
- Ashforth, S.A.; Oosterbeek, R.N.; Bodley, O.L.C.; Mohr, C.; Aguergaray, C.; Simpson, M.C. Femtosecond lasers for high-precision orthopedic surgery. Lasers Med. Sci. 2020, 35, 1263–1270.
- Emigh, B.; An, R.; Hsu, E.M.; Crawford, T.H.R.; Haugen, H.K.; Wohl, G.R.; Hayward, J.E.; Fang, Q. Porcine cortical bone ablation by ultrashort pulsed laser irradiation. J. Biomed. Opt. 2012, 17, 0280011–0280016.
- Kim, B.; Feit, M.D.; Rubenchik, A.M.; Joslin, E.J.; Eichler, J.; Stoller, P.C.; Da Silva, L.B. Effects of high repetition rate and beam size on hard tissue damage due to subpicosecond laser pulses. Appl. Phys. Lett. 2000, 76, 4001–4003.
- Tulea, C.; Caron, J.; Gehlich, N.; Lenenbach, A.; Noll, R.; Loosen, P. Laser cutting of bone tissue under bulk water with a pulsed ps-laser at 532 nm. J. Biomed. Opt. 2015, 20, 105004.
- Su, E.; Sun, H.; Juhasz, T.; Wong, B.J.F. Preclinical investigations of articular cartilage ablation with femtosecond and pulsed infrared lasers as an alternative to microfracture surgery. J. Biomed. Opt. 2014, 19, 098001.
- Mannion, P.T.; Magee, J.; Coyne, E.; O’Connor, G.M.; Glynn, T.J. The effect of damage accumulation behaviour on ablation thresholds and damage morphology in ultrafast laser micro-machining of common metals in air. Appl. Surf. Sci. 2004, 233, 275–287.
- Ben-Yakar, A.; Byer, R.L. Femtosecond laser ablation properties of borosilicate glass. J. Appl. Phys. 2004, 96, 5316–5323.
- Liu, J.M. Simple technique for measurements of pulsed Gaussian-beam spot sizes. Opt. Lett. 1982, 7, 196–198.
- Cangueiro, L.T.; Vilar, R.; do Rego, A.M.B.; Muralha, V.S.F. Femtosecond laser ablation of bovine cortical bone. J. Biomed. Opt. 2012, 17, 125005.
- Mortensen, L.J.; Alt, C.; Turcotte, R.; Masek, M.; Liu, T.-M.; Côté, D.C.; Xu, C.; Intini, G.; Lin, C.P. Femtosecond laser bone ablation with a high repetition rate fiber laser source. Biomed. Opt. Express 2015, 6, 32–42.
- Lo, D.D.; Mackanos, M.A.; Chung, M.T.; Hyun, J.S.; Montoro, D.T.; Grova, M.; Liu, C.; Wang, J.; Palanker, D.; Connolly, A.J.; et al. Femtosecond plasma mediated laser ablation has advantages over mechanical osteotomy of cranial bone. Lasers Surg. Med. 2012, 44, 805–814.
- Nicolodelli, G.; Lizarelli, R.D.F.Z.; Bagnato, V.S. Influence of effective number of pulses on the morphological structure of teeth and bovine femur after femtosecond laser ablation. J. Biomed. Opt. 2012, 17, 048001.
- Nguyen, J.; Ferdman, J.; Zhao, M.; Huland, D.; Saqqa, S.; Ma, J.; Nishimura, N.; Schwartz, T.H.; Schaffer, C.B. Sub-surface, micrometer-scale incisions produced in rodent cortex using tightly-focused femtosecond laser pulses. Lasers Surg. Med. 2011, 43, 382–391.
- Jee, Y.; Becker, M.F.; Walser, R.M. Laser-induced damage on single-crystal metal surfaces. J. Opt. Soc. Am. B 1988, 5, 648–659.
- Lim, Y.C.; Altman, K.J.; Farson, D.F.; Flores, K.M. Micropillar fabrication on bovine cortical bone by direct-write femtosecond laser ablation. J. Biomed. Opt. 2009, 14, 064021.
- Marjoribanks, R.S.; Dille, C.; Schoenly, J.E.; McKinney, L.; Mordovanakis, A.; Kaifosh, P.; Forrester, P.; Qian, Z.; Covarrubias, A.; Feng, Y.; et al. Ablation and thermal effects in treatment of hard and soft materials and biotissues using ultrafast-laser pulse-train bursts. Photon. Lasers Med. 2012, 1, 155–169.
- Rosenfeld, A.; Lorenz, M.; Stoian, R.; Ashkenasi, D. Ultrashort-laser-pulse damage threshold of transparent materials and the role of incubation. Appl. Phys. A 1999, 69, S373–S376.
- Stuart, B.C.; Feit, M.D.; Rubenchik, A.M.; Shore, B.W.; Perry, M.D. Laser-Induced Damage in Dielectrics with Nanosecond to Subpicosecond Pulses. Phys. Rev. Lett. 1995, 74, 2248–2251.
- Hoy, C.L.; Ferhanoglu, O.; Yildirim, M.; Kim, K.H.; Karajanagi, S.S.; Chan, K.M.C.; Kobler, J.B.; Zeitels, S.M.; Ben-Yakar, A. Clinical Ultrafast Laser Surgery: Recent Advances and Future Directions. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 710081.
- Lee, Y.M.; Tu, R.Y.; Chiang, A.C.; Huang, Y.C. Average-power mediated ultrafast laser osteotomy using a mode-locked Nd:YVO laser oscillator. J. Biomed. Opt. 2007, 12, 060505.
- Daskalova, A.; Bashir, S.; Husinsky, W. Morphology of ablation craters generated by ultra-short laser pulses in dentin surfaces: AFM and ESEM evaluation. Appl. Surf. Sci. 2010, 257, 1119–1124.
- Kim, B.; Feit, M.D.; Rubenchik, A.M.; Joslin, E.J.; Celliers, P.; Eichler, J.; Da Silva, L.B. Influence of pulse duration on ultrashort laser pulse ablation of biological tissues. J. Biomed. Opt. 2001, 6, 332–338.
- Friedrich, R.E.; Quade, M.; Jowett, N.; Kroetz, P.; Amling, M.; Kohlrusch, F.K.; Zustin, J.; Gosau, M.; Schlüter, H.; Miller, R.J.D. Ablation Precision and Thermal Effects of a Picosecond Infrared Laser (PIRL) on Roots of Human Teeth: A Pilot Study Ex Vivo. In Vivo 2020, 34, 2325–2336.
- Domke, M.; Wick, S.; Laible, M.; Rapp, S.; Huber, H.P.; Sroka, R. Ultrafast dynamics of hard tissue ablation using femtosecond-lasers. J. Biophotonics 2018, 11, e201700373.
- Canteli, D.; Muñoz-García, C.; Morales, M.; Márquez, A.; Lauzurica, S.; Arregui, J.; Lazkoz, A.; Molpeceres, C. Thermal Effects in the Ablation of Bovine Cortical Bone with Pulsed Laser Sources. Materials 2019, 12, 2916.
- Gill, R.K.; Smith, Z.J.; Lee, C.; Wachsmann-Hogiu, S. The effects of laser repetition rate on femtosecond laser ablation of dry bone: A thermal and LIBS study. J. Biophotonics 2016, 9, 171–180.
More
Information
Subjects:
Orthopedics; Optics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
4 times
(View History)
Update Date:
07 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




