Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Esmaiel Jabbari | -- | 3224 | 2022-05-04 18:43:47 | | | |
| 2 | Camila Xu | Meta information modification | 3224 | 2022-05-05 09:00:43 | | | | |
| 3 | Camila Xu | Meta information modification | 3224 | 2022-05-05 09:07:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jabbari, E.; Akbari, A.; Bigham, A.; Rahimkhoei, V.; , . Natural Antiviral Polymers. Encyclopedia. Available online: https://encyclopedia.pub/entry/22593 (accessed on 07 February 2026).
Jabbari E, Akbari A, Bigham A, Rahimkhoei V, . Natural Antiviral Polymers. Encyclopedia. Available at: https://encyclopedia.pub/entry/22593. Accessed February 07, 2026.
Jabbari, Esmaiel, Ali Akbari, Ashkan Bigham, Vahid Rahimkhoei, . "Natural Antiviral Polymers" Encyclopedia, https://encyclopedia.pub/entry/22593 (accessed February 07, 2026).
Jabbari, E., Akbari, A., Bigham, A., Rahimkhoei, V., & , . (2022, May 04). Natural Antiviral Polymers. In Encyclopedia. https://encyclopedia.pub/entry/22593
Jabbari, Esmaiel, et al. "Natural Antiviral Polymers." Encyclopedia. Web. 04 May, 2022.
Copy Citation
Natural polymers or biopolymers are classified into polysaccharides, polypeptides (proteins), and nucleic acid polymers (polynucleotides). Natural polymers as components of living systems are derived from plants, animals, and microorganisms.
natural polymers
biopolymers
polysaccharides
1. Introduction
Natural polymers or biopolymers are classified into polysaccharides, polypeptides (proteins), and nucleic acid polymers (polynucleotides) [1]. Natural polymers as components of living systems are derived from plants, animals, and microorganisms [1]. Advantages of natural polymers over synthetic polymers include biocompatibility, non-toxicity, biodegradability, and intrinsic antiviral properties as shown in Figure 1 [2]. Among different classes of biopolymers, polysaccharides and nucleic acid polymers are capable of interfering with the virus-host cell interaction to block fusion, entry, and replication of the virus in the host cell [3].
 Figure 1. Key benefits of natural polymers as antiviral materials.
Figure 1. Key benefits of natural polymers as antiviral materials.2. Polysaccharides
Polysaccharides are an abundant source of renewable and biodegradable polymers. Polysaccharides are made up of more than 10 monosaccharide repeat units connected by glycosilic linkages in branched or linear chains with molecular weights ranging from tens of thousands to a few millions [4]. Like other natural polymers, polysaccharides play vital functions in living systems from cell adhesion to intracellular signaling and maintenance of biological and mechanical properties of tissues [5]. Natural plant polysaccharides are composed of long sequences of monosaccharides with different biological activities including anti-inflammatory, immunomodulatory, antioxidant, and antiviral activities [6][7]. Table 1 presents the general properties of carrageenan, chitin, chitosan, and fucoidan as widely studied polysaccharides with antiviral activities.
Table 1. Polysaccharides, their origin, and molecular characteristics.
| Polysaccharide | Origin | Occurrence/Function | Molecular Characteristics | Ref. |
|---|---|---|---|---|
| Carrageenan | Algae | Structural polysaccharides of marine red algae | Heteropolysaccharide, linear, anionic | [8] |
| Chitin/chitosan | Animal | Chitin: structural polysaccharide from exoskeleton of insects and shells of crustaceans Chitosan: derivative of chitin prepared by deacetylation |
Chitin: homopolysaccharide, linear, neutral Chitosan: heteropolysaccharide, linear, cationic |
[9] |
| Fucoidan | Algae | Structure and composition dependent on species with diverse structures | Heteropolysaccharide, anionic, linear. | [10] |
Polysaccharides possess different types of functional groups including hydroxyl, amine, and carboxylic acid groups which can be used for polymer modification [6][7]. These functional groups are used to tailor the properties of polysaccharides to specific biological applications [11][12]. The inhibitory effect of negatively charged polysaccharides on viral infections is well documented [13][14][15]. Polysaccharides interact with Trans-Activator of Transcription (Tat) regulatory protein of viruses to inhibit their interaction with cell surface receptors like heparan sulfates [16]. As an example, carrageenan which is a naturally sulfated polysaccharide of red algae, inhibits many viral infections including human papillomavirus (HPV), herpes simplex virus type-1 (HSV-1), and influenza A virus (IAV) by interacting with the viral Tat protein [17][18]. Numerous studies have reported the inhibitory effect of plant polysaccharides on replication of human and animal viruses including SARS associated coronaviruses (SARS-CoV) [19][20][21][22]. These include brown algae, Auricularia auricula, Pinus massoniana, and Acanthopanax polysaccharides [23]. . According to these results, highly sulfated long-chain glycomimetic polymers prevented human papillomavirus (HPV16) infection in vitro and in vivo by interaction with the virus prior to cell attachment whereas highly sulfated short-chain glycomimetic oligomers inhibited viral infection post cell attachment [24]. The polyanionic nature of sulfated polysaccharides facilitates interaction with the positively charged domains of the virus envelope glycoproteins to form a non-reversible complex, thus making the viral glycoprotein sites inaccessible to interact with surface receptors of the host cell [25]. This non-reversible polysaccharide-viral interaction blocks binding and fusion of the virus with the host cell which inhibits viral entry in the cell [25]. Seaweed polysaccharides with high anionic sulfate content are attractive as antiviral agents to inhibit infections [26][27][28]. The structural diversity and complexity of marine polysaccharides and their derivatives contribute to their antiviral activity at different stages of viral infections [29]. The antiviral activity of polysaccharides is affected by their molecular weight. As the antiviral activity arises from the interaction of polysaccharides with viral envelope proteins through reversible secondary interactions like electrostatic, nonpolar, and hydrogen bonding, long chains that can form irreversible complexes substantially increase antiviral activity.
2.1. Carrageenan
The cell wall of marine red seaweed contains the polysaccharides agaran and carrageenan that form approximately 50% of the dry mass of seaweed. As a high molecular weight sulfated polysaccharide, carrageenan has long been used as a gelling agent and stabilizer in food [30]. However, carrageenan has many biological activities including anticoagulation, antioxidation, antiviral, and immunostimulatory [31]. Based on sulfate content, there are more than 10 types of carrageenan, but only the types lambda (λ), kappa (κ), and iota (ι) are of particular interest in viral and medical applications [32]. The chemical structure of the three carrageenan types is shown in Figure 2.
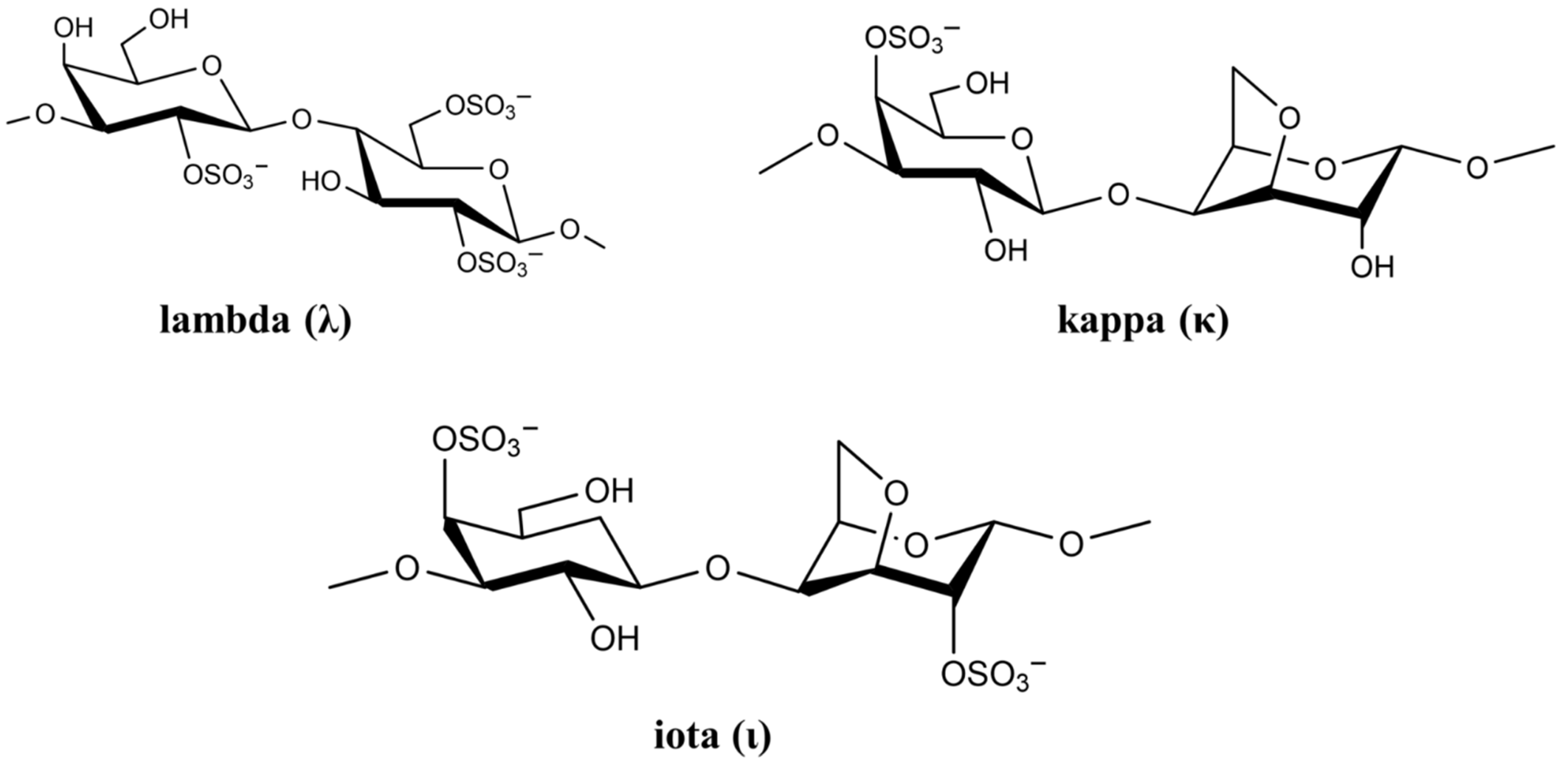 Figure 2. Chemical structure of lambda (λ), kappa (κ), and iota (ι) carrageenan.
Figure 2. Chemical structure of lambda (λ), kappa (κ), and iota (ι) carrageenan.The negatively-charged sulfate groups of carrageenan neutralize the positive charge of the cell surface, leading to inhibition of viral attachment, penetration, and viral uncoating [33]. The biological activity of carrageenan strongly depends on its degree of sulfation, which depends on carrageenan (λ, κ and ι) as well as virus type [34]. List of studies on antiviral activity of λ, κ, and ι carrageenan are presented in Table 2.
Table 2. Antiviral activity of λ, κ, and ι carrageenan against different viruses.
| Type | Virus | Genome | Cell Line | IC50/EC50 (µg/mL) | Ref. |
|---|---|---|---|---|---|
| κ | DENV-1 | RNA | Vero | >50 (EC50) | [35] |
| κ | DENV-2 | RNA | Vero | 1.8 (EC50) | [35] |
| κ | HSV-1 | DNA | Vero | 1.9 (EC50) | [36] |
| κ | HSV-2 | DNA | Vero | 1.6 (EC50) | [36] |
| κ | HIV-1 | RNA | MT-4 | 12 (IC50) | [37] |
| ι | DENV-1 | RNA | Vero | 41 (EC50) | [35] |
| ι | DENV-2 | RNA | Vero | 0.40 (EC50) | [35] |
| ι | H1N1 | RNA | MDCK | 0.39 (IC50) | [38] |
| ι | SARS-CoV-2 | RNA | Vero | 0.05 (IC50) | [39] |
| λ | HIV-1 | RNA | MT-4 | 1.9 (IC50) | [37] |
| λ | H1N1 | RNA | MDCK | 0.04 (IC50) | [17] |
| λ | VSV | RNA | HeLa | 4.0 (IC50) | [37] |
In general, a higher degree of sulfation leads to a higher antiviral activity [29]. It is reported that λ-carrageenan having 35% sulfation exhibits approximately ten times higher inhibition against HIV infection as compared to κ-carrageenan with 25% sulfation [40]. Aside from sulfation, the source of polysaccharide affects its antiviral activity [41]. Carrageenan is capable of affecting virus replication directly through the sulfate groups leading to a decrease in viral activity [42]. For example, carrageenan binding changes the structure of HSV virus, namely the structure of glycoproteins B and C, which results in virus deactivation. Vissani et al. demonstrated that the antiviral activity of ι-carrageenan against herpesvirus-3 is linked to envelope glycoprotein binding [43]. Functional characterization showed that ι-carrageenan and N-sulfonated derivatives of poly (allylamine hydrochloride) blocked the release of human metapneumovirus (hMPV) from infected cells, which reduced viral spreading [44]. A nasal spray for the delivery of carrageenan has been developed for treating patients infected with rhinovirus (HRV), coronavirus (HCoV), and influenza A (IAV) virus. The nasal spray decreased viral symptoms in infected patients and it was most effective against the human coronavirus [45]. It should be pointed out that ι-carrageenan is an approved antiviral drug for use against not only HRV, IAV-H1N1 and HCoV-OC43 but also against viral throat infection [46].
Carrageenan has also been used against human papillomavirus (HPV) which is a non-enveloped small DNA virus capable of infecting mucosal membrane or skin [47]. In one study, carrageenan showed antiviral activity against HPV pseudovirus in a luciferase mouse model without exhibiting a cytotoxic effect [48]. In another study, the antiviral activity of carrageenan was compared with heparin sulfate and highly sulfated synthetic glycomimetic polymers in vitro and in vivo [24]. Both short chain oligomeric sulfated saccharides with 2–10 sugar units and long polymeric saccharides with 40–80 sugar units were synthesized to mimic different properties of glycopolymers, as shown in Figure 3A,B. The in vitro evaluation of the antiviral agents were done with HPV16-eGFP pseudovirus incubated with HeLa cervical cancer cells whereas the in vivo evaluations were done with HPV16-luciferase inoculated in the mouse vagina [24]. The addition of carrageenan, heparin sulfate, and polymeric sulfated saccharides inhibited the attachment of HPV16 pseudovirus to the surface of HeLa cells to block viral infection (Figure 3C) [24]. The effectiveness of polymeric sulfated saccharides with 40 units in inhibiting viral attachment was slightly less than that with 80 units. The in vivo imaging results showed that both carrageenan and heparin sulfate were effective in inhibiting HPV16 infection after 2 days of inoculation [24].
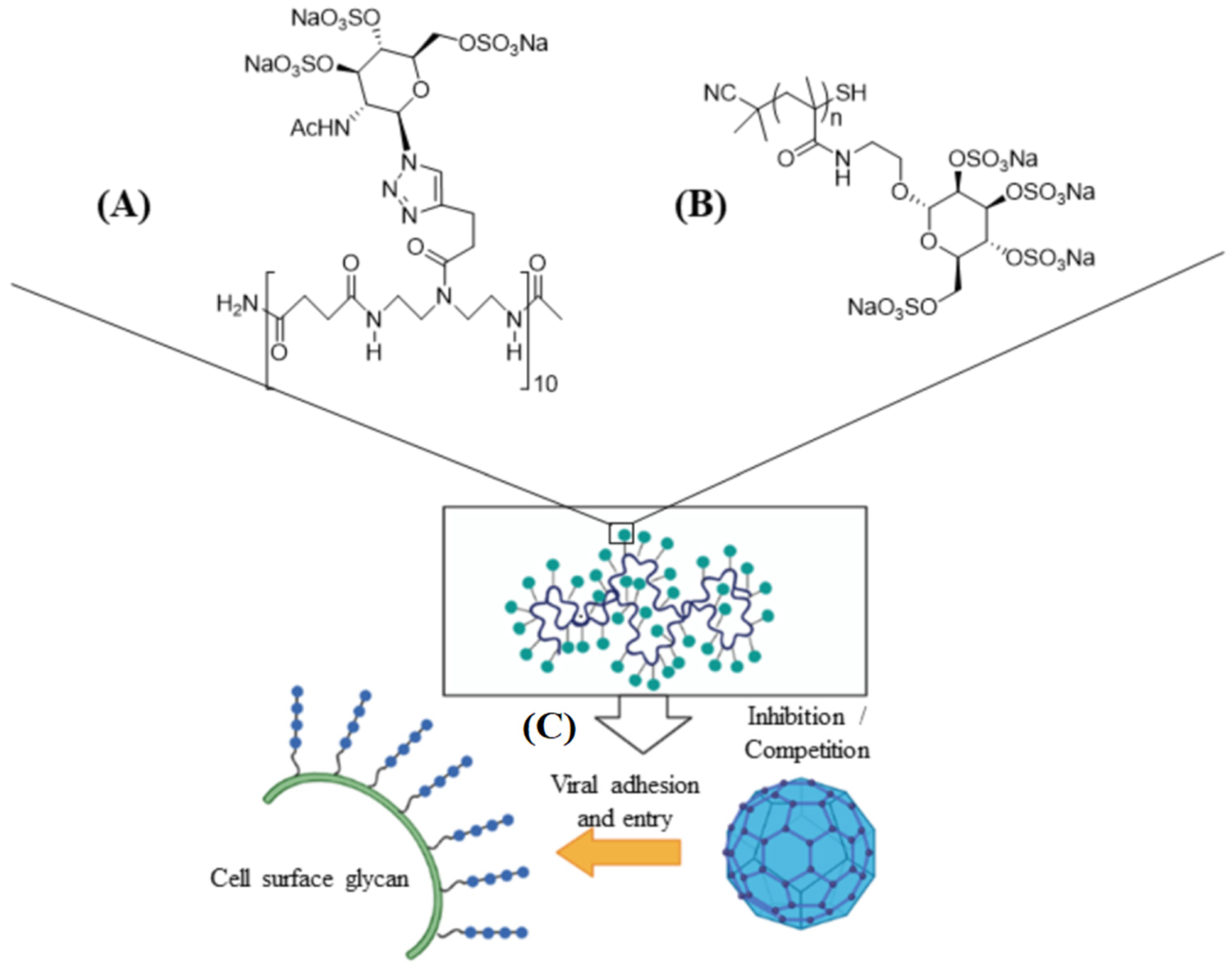 Figure 3. Schematic representation of short ((A), 10 sugar units) and long ((B), n units) chain oligomeric sulfated saccharides to mimic different properties of glycopolymers; (C) the sulfated glycopolymers block viral infection by interfering with virus attachment to the cell surface. Reprinted with permission from Ref. [24]. Copyright 2020, American Chemical Society.
Figure 3. Schematic representation of short ((A), 10 sugar units) and long ((B), n units) chain oligomeric sulfated saccharides to mimic different properties of glycopolymers; (C) the sulfated glycopolymers block viral infection by interfering with virus attachment to the cell surface. Reprinted with permission from Ref. [24]. Copyright 2020, American Chemical Society.2.2. Chitosan and Its Derivatives
Chitin and chitosan due to their biocompatibility, degradability, and non-immunogenicity are used in the development of antimicrobial wound dressing as well as other medical applications [49][50]. Chitin, comprised of N-acetyl-D-glucosamine and D-glucosamine units, is isolated from the shell of arthropods like shrimp, lobster, crab, and insects or produced by fermentation [51]. Chitosan is produced from chitin by partial deacetylation or by enzymatic hydrolysis [52]. Although the antimicrobial properties of chitosan is well-documented, less is known about the antiviral activity of this biopolymer [53]. The degree of acetylation and amination, chitosan concentration, molecular weight, overall charge as well as charge distribution, and functional group modification affects antiviral activity of chitosan [54][55]. The positively charged derivatives of chitosan possess antiviral activity whereas the negatively charged derivatives are not antiviral [50]. Chitosan can inhibit viral infection directly and indirectly or it can be used as a carrier in the delivery of antiviral agents, as shown in Figure 4 [56].
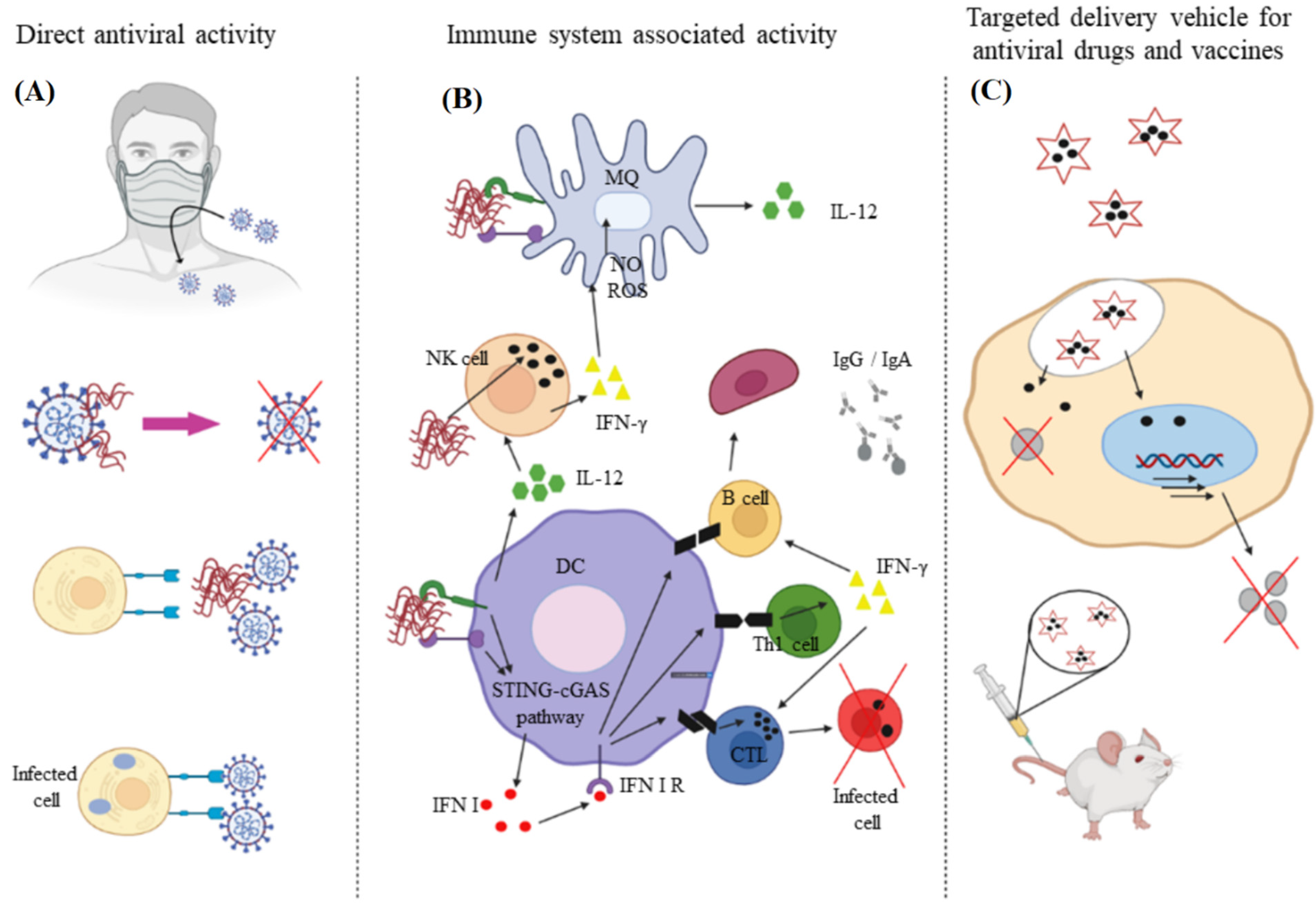 Figure 4. The direct (A) and indirect (B) approaches to using chitosan to inhibit viral infection; (C) chitosan is also used as a carrier for delivery of antiviral agents. Reprinted with permission from Ref. [52]. Copyright 2021, Elsevier.
Figure 4. The direct (A) and indirect (B) approaches to using chitosan to inhibit viral infection; (C) chitosan is also used as a carrier for delivery of antiviral agents. Reprinted with permission from Ref. [52]. Copyright 2021, Elsevier.A sulfated derivative of chitin, namely N-carboxymethyl chitosan-N-O-sulfate (NCMCS), was shown to inhibit uptake and replication of human immunodeficiency virus-1 (HIV-1) in CD4+ human T-lymphoma cells, which was attributed to the interference of NCMCS with the interaction of viral GP120 envelope glycoproteins with CD4+ cell surface receptors [57]. In another study, a sulfated derivative of carboxymethyl chitin (SCM-chitin) was shown to inhibit penetration and subsequent pathogenesis of herpes simplex type-1 virus (HSV-1) in vitro in monkey kidney epithelial cells (Vero) as compared to carboxymethyl chitin which showed no activity [58]. The sulfated derivative of chitosan was also found to protect Vero cells from cytopathic infection by Coxsackie and Rift Valley fever RNA viruses [59]. Chitosan and its derivatives are used as a carrier in delivery of antiviral drugs to reduce the minimum required dose and increase bioavailability, thus improving the drugs’ therapeutic effectiveness (Figure 4C) [60]. In one study, curcumin encapsulated in chitosan nanoparticles (NPs) was shown to have higher antiviral activity against hepatitis C virus genotype 4a (HCV-4a) in human hepatoma cells as compared to unencapsulated curcumin [61]. A delivery system based on zinc-stabilized nanocomplex of chitosan and chondroitin sulfate for delivery of tenofovir was developed for inhibition of HIV-1 viral infection, as shown in Figure 5 [62]. The tenofovir-loaded nanocomplex showed strong antiviral activity against HIV-1 in human peripheral blood mononuclear cells (PBMCs) without a cytotoxic effect. The delivery system showed considerable antiviral activity in the absence of tenofovir [62].
 Figure 5. A nanocomplex for delivery of tenofovir based on zinc-stabilized chitosan and chondroitin sulfate. Abbreviations are TF for tenofovir, CS for chitosan, Chons for chondroitin sulfate, IgA for antibody, PECs for polyelectrolyte complexes, and PBMCs for human peripheral blood mononuclear cells. Reprinted with permission from Ref. [62]. Copyright 2016, American Chemical Society.
Figure 5. A nanocomplex for delivery of tenofovir based on zinc-stabilized chitosan and chondroitin sulfate. Abbreviations are TF for tenofovir, CS for chitosan, Chons for chondroitin sulfate, IgA for antibody, PECs for polyelectrolyte complexes, and PBMCs for human peripheral blood mononuclear cells. Reprinted with permission from Ref. [62]. Copyright 2016, American Chemical Society.Recently, the antiviral activity of quaternary ammonium modified chitosan such as N-(2-hydroxypropyl)-3-trimethylammonium chitosan (HTCC) and N-palmitoyl-N-monomethyl-N,N-dimethyl-N,N,N-trimethyl-6-O-glycolchitosan (GCPQ) is reported against SARS-CoV-2, as shown in Figure 6 [63][64]. Due to the quaternary ammonium functional groups in the structure of HTCC and GCPQ, these positively charged molecules bind electrostatically to coronavirus S proteins to block not only viral entry to host cells but also to prevent viral replication. List of studies on antiviral activity of chitosan and its derivatives are presented in Table 3.
 Figure 6. Chemical structures of N-(2-hydroxypropyl)-3-trimethylammonium chitosan (HTCC) and N-palmitoyl-N-monomethyl-N,N-dimethyl-N,N,N-trimethyl-6-O-glycolchitosan (GCPQ) with quaternary ammonium functional groups.
Figure 6. Chemical structures of N-(2-hydroxypropyl)-3-trimethylammonium chitosan (HTCC) and N-palmitoyl-N-monomethyl-N,N-dimethyl-N,N,N-trimethyl-6-O-glycolchitosan (GCPQ) with quaternary ammonium functional groups.Table 3. Antiviral activity of chitosan and its derivatives.
| Chitosan | Virus | Cell Line | Concentration | Ref. |
|---|---|---|---|---|
| Zinc stabilized chitosan | HIV-1 | Human peripheral blood mononuclear | 0.1–0.2% w/v chitosan in acetic acid | [62] |
| Curcumin-loaded chitosan nanocomposite | Hepatitis C | human hepatoma | 20 µg/mL curcumin in 4% chitosan composite | [61] |
| Chitosan with medium molecular weight | AD169 strain of HCMV | Human embryonic lung fibroblasts | 9–14 mg Fostcanet antiviral drug in 4.5 mg chitosan |
[65] |
| Silver-loaded chitosan | H1N1 Influenza A | Madin-Darby canine kidney | Ag NPs in 10 mg/mL chitosan | [66] |
| Nanosilver-loaded polyquaternary phosphonium oligochitosan | Hepatitis A | Vero | 100 µL/mL silver NPs | [67] |
| 3,6-O-sulfated chitosan | HPV | 293FT, Hela, HaCaT | 2.4, 4.7, 4.2 µg/mL chitosan | [68] |
| HTCC | SARS-CoV2 | human airway epithelium (HAE) | 12.5 µg/mL HTCC | [63] |
| GCPQ | SARS-CoV2 | Vero E6 and A549 | 10 µg/mL GCPQ | [64] |
2.3. Fucoidan
Fucoidan is a sulfated, acidic polysaccharide composed of L-fucose and galactose monosaccharides with smaller proportions of xylose and mannose [69]. It is isolated from the cell wall of marine brown seaweeds like Ascopbyllum nodosum, Fucus vesiculosus, Saccharina japonica, and Sargassum thunbergia. Based on molecular structure, there are two types of fucoidan, as shown in Figure 7, namely type I having (1→3)-l-fucopyranose repeats and type II having (1→3)- and (1→4)-l-fucopyranose repeat units [70]. Depending on degree and pattern of sulfation, molecular weight and composition, fucoidans possess different biological properties including antiviral [71][72][73][74][75][76], anti-inflammatory [77], immunologic [78] and antitumor [79][80] properties.
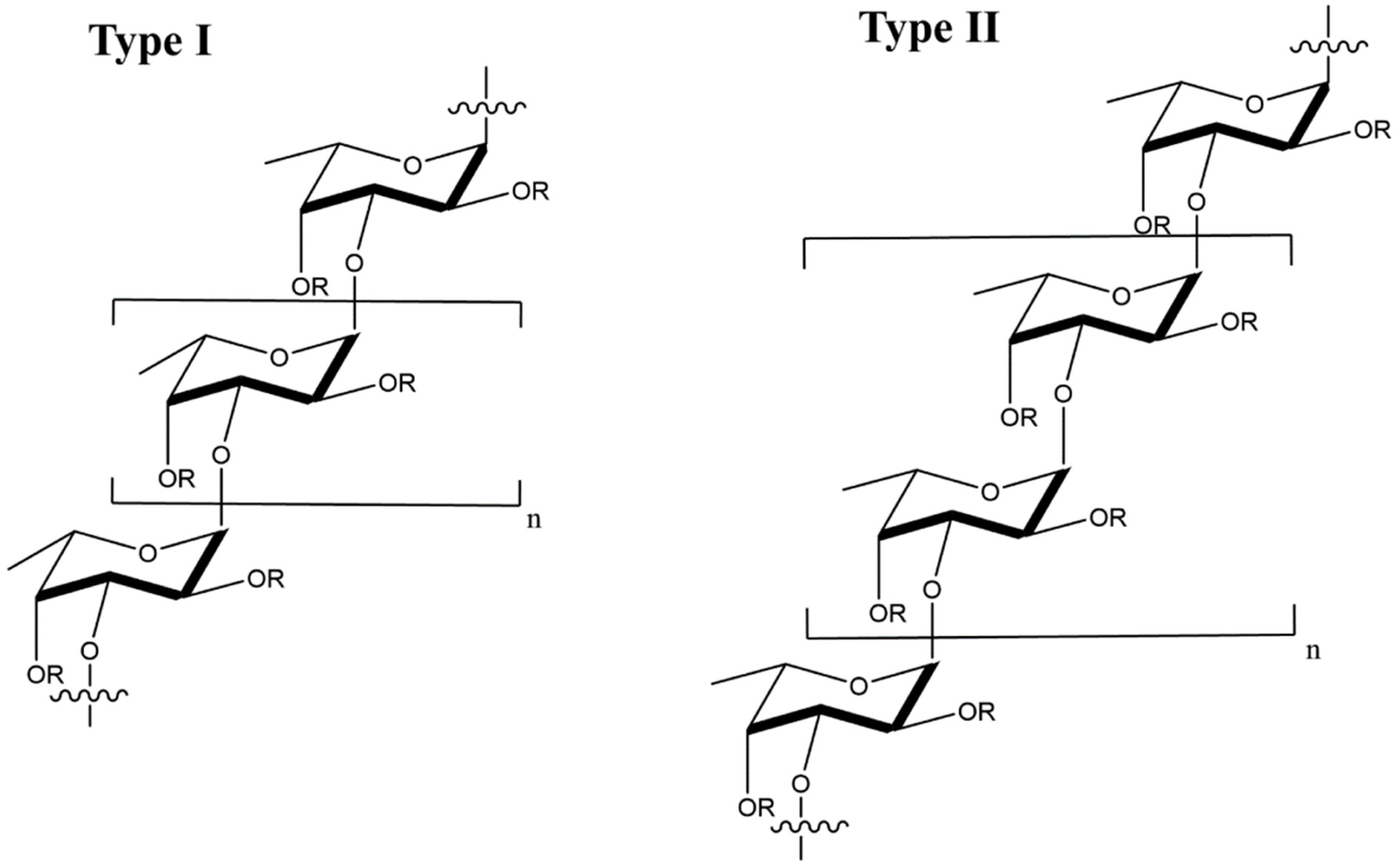 Figure 7. Molecular structure of type I and type II fucoidans from brown seaweed; the R groups represent sulfate, glucuronic acid, or fucopyranose groups.
Figure 7. Molecular structure of type I and type II fucoidans from brown seaweed; the R groups represent sulfate, glucuronic acid, or fucopyranose groups.The mechanism of action for antiviral activity of fucoidans from different sources has been recently studied [71][81]. Fucoidan inactivates the virus before or after cell internalization [82]. After internalization, fucoidan prevents viral transcription as well as other intracellular targets, thus blocking viral replication [82]. List of studies on antiviral activity of fucoidans from different sources are presented in Table 4. The LMW fucoidans showed antiviral activity in HeLa, Hep-2, and MDCK cells infected with influenza A virus at medium and high fucoidan doses in the range of 0.15–2.4 mg/mL whereas low doses were ineffective against influenza A [72]. Further, the survival time of the virus-infected mice increased and their lung index improved with intravenous administration of fucoidan [72]. In this research, fucoidan acted as an adjuvant to suppress HBV DNA, HBV surface antigen (HBsAG) and active HBV proteins implicated in viral replication, namely HBeAg and HBcAg [83].
Table 4. Antiviral activity of fucoidans from different species of seaweed.
| Fucoidan | Virus | Cell Line | IC50 (μg/mL) | Ref. |
|---|---|---|---|---|
| Cladosiphon okamuranus | Newcastle Disease (NDV) | Vero | 0.75 | [84] |
| Scytosiphon lomentaria | HSV-1, HSV-2 | Vero | 1.12–1.22 | [81] |
| Sargassum henslowianum | HSV-1, HSV-2 | Vero | 0.48–0.89 | [82] |
| Sporophyll of Undaria pinnatifida (Mekabu) | Influenza A | MDCK | 15 | [85] |
| Brown Algae: Fucus evanescens | HSV-1, HSV-2, HIV-1 | MT-4 | 25–80 | [86] |
3. Nucleic Acid Polymers
Nucleic acid polymers (NAPs), as phosphorothioate oligonucleotides, possess an-tiviral activity [87]. Oligonucleotides and NAPs can be stabilized against degradation by nucleases made amphipathic by phosphorothioation of non-bridging oxygen atoms through phosphodiester linkages [87]. The antiviral activity of phosphorothioated NAPs was first reported in a study on mechanism of action and the effect of NAPs’ structure on inhibition of HIV-1 infection [83]. It was found that NAPs inhibited viral entry to the host cell and the extent of inhibition was controlled by the size of NAPs, not their sequence distribution. According to this research, NAPs with >20 nucleotides showed antiviral activity [83]. The antiviral activity of NAPs against HIV-1 was at-tributed to the interaction of NAPs with the amphipathic alpha helix triplets found in the core of metastable envelope glycoprotein gp41 of HIV to inhibit viral entry to the host cell. The first step in the viral inhibition is decomplexation of the amphipathic al-pha helices of gp41 by NAPs followed by interaction with hydrophobic moieties of the alpha helices, which explains the dependence of NAP inhibition on size and phos-phorothioation [88]. The interaction of NAPs with amphipathic alpha helix triplets of gp41 was used for targeting and inactivating other viruses including cytomegalovirus, herpesvirus, and lymphocytic choriomeningitis virus [89][90][91][92]. A similar mechanism is proposed for the role of NAPs in blocking the entry of hepatitis C virus (HCV) into host cells [91]. However, one study reported that NAPs did not prevent HCV attach-ment to the host cells but blocked the post-binding step required for viral entry [92]. The post-binding step is posited to involve the interaction of NAPs with other viral proteins including the hypervariable region of glycoprotein E2 or apolipoprotein E [93][94]. The antiviral activity of NAPs assessed against ductal hepatitis B virus (DHBV) with primary duck hepatocytes (PDH) as the host was shown to be dependent on NAP size and amphipathicity [95]. Specifically, NAPs with >40 nucleotides showed higher antiviral activity against DHBV. The therapeutic potential of two NAPs, namely REP 2055 and REP 2139-Ca, were evaluated in patients with HBeAg positive chronic HBV infection in two clinical studies [96][97]. The patients treated with NAP monotherapy showed 2–7 log reduction in serum HBsAg, 3–9 log reduction in serum HBV DNA, and the presence of serum anti-HBsAg antibodies in the patients’ blood [96][97]. These results indicated that NAPs could potentially be used as a component in combination therapies in treating patients with HBV. In another study, the safety and therapeutic efficacy of REP 2139 and REP 2165 NAPs combined with commonly used medication to treat patients with chronic hepatitis B, namely tenofovir disoproxil fumarate (TDF) and pegylated interferon α-2a (PegIFN), were assessed in a randomized phase 2 clinical trial. The REP 2139 NAP and its bioequivalent REP 2165 block the assembly of subviral particles (SVPs) in hepatocytes resulting in clearance of HBsAg surface antigens. The experimental NAP groups were TDF+PegIFN+REP 2139 and TDF+PegIFN+REP 2165 [98]. According to the results, there was no difference in the levels of HBsAg, indicative of HBV infection, anti-HBs, indicative of recovery from HBV infection, and HBV DNA between the two NAP groups [98]. The undesired side effects induced by PegIFN, namely thrombocytopenia and neutropenia, were unaf-fected by the addition of NAPs to TD and PegIFN. However, the levels of HBV-induced transaminases were initially higher in the NAP groups, but the levels returned to nor-mal during therapy and follow-up [98]. Treatment with TDF+PegIFN without NAP resulted in low HBsAg loss and HBsAg seroconversion as well as low functional cure. Conversely, treatment with TDF+PegIFN+NAP resulted in high HBsAg loss and HBsAg seroconversion as well as high functional cure with normal liver function [98]. No differences in HBsAg loss, seroconversion, or functional cure were observed between the two NAP groups. An in vitro model based on HepG2.2.15 hepatocyte cells that constitutively express HBV demonstrated that the NAPs’ endosomal release selectively impaired the secretion of HBsAg without accumulation of HBsAg in the cells [99]. NAPs due to their biocompatibility, degradability, and their ability to penetrate cell membranes have been used as a carrier for delivery of antiviral drugs against classical swine fever virus (CSFV) in infected pigs [100][101]. The enveloped virus with a sin-gle-stranded RNA causing CSFV belongs to the genus Pestivirus in the family Fla-viviridae [102]. The small interfering RNA (siRNA) was delivered to the host cells in a porcine CSFV model using tetrahedral framework nucleic acid (tFNA), as a three-dimensional DNA nanomaterial that self-assembles by complementary base pairing [103]. Concurrent delivery of C3 and C6 siRNAs in self-assembled tFNA to host cells prevented viral replication and exocytosis, as shown in Figure 8 [103].
 Figure 8. Schematic illustration of the mechanism of siRNA delivery by tFNA nanomaterial. Reprinted with permission from Ref. [103]. Copyright 2021, American Chemical Society.
Figure 8. Schematic illustration of the mechanism of siRNA delivery by tFNA nanomaterial. Reprinted with permission from Ref. [103]. Copyright 2021, American Chemical Society.Following penetration through the cell membrane, CSFV is transported to the endoplasmic reticulum where its single-stranded RNA is released for protein translation. Following RNA translation, CSFV is replicated on the cytoplasmic membrane leading to the formation of viral particles in the Golgi apparatus. The siRNAs carried by tFNA degrade the released viral RNA to prevent its translation in the endoplasmic reticulum. List of studies on antiviral activity of NAPs are presented in Table 5.
Table 5. Antiviral activity of NAPs against different viruses with their mechanism of actions.
| NAP | Virus | Cell Line | Mechanism of Action | Ref. |
|---|---|---|---|---|
| Phosphorothioate Oligonucleotides | HIV-1 | H9 | Inhibiting viral entry to the host | [88] |
| amphipathic DNA polymer (40-nucleotide polycytidine) | HSV | Human cervical epithelial (CaSki) | Reduced viral and protein expression | [89] |
| Amphipathic DNA polymers (REP 9, REP 2015 and REP 9C) | Animal | NIH 3T3 | Reduced viral replication | [92] |
| REP 2006 | HBV | DHBV | Reduced viral entry to the host | [95] |
| REP 2055 | HBV | DHBV | blocking release of DHBsAg from the infected hepatocytes | [97] |
References
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D bioprinting. Polymers 2018, 10, 1278.
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021, 2, 1–19.
- Randazzo, W.; Fabra, M.J.; Falcó, I.; López-Rubio, A.; Sánche, G. Polymers and biopolymers with antiviral activity: Potential applications for improving food safety. Comp. Rev. Food Sci. Food Saf. 2018, 17, 754–768.
- Xie, J.-H.; Jin, M.-L.; Morris, G.A.; Zha, X.-Q.; Chen, H.-Q.; Yi, Y.; Li, J.-E.; Wang, Z.-J.; Gao, J.; Nie, S.-P. Advances on bioactive polysaccharides from medicinal plants. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. S1), S60–S84.
- Dwek, R.A. Glycobiology: Toward understanding the function of sugars. Chem. Rev. 1996, 96, 683–720.
- Xie, L.; Shen, M.; Hong, Y.; Ye, H.; Huang, L.; Xie, J. Chemical modifications of polysaccharides and their anti-tumor activities. Carbohydr. Polym. 2020, 229, 115436.
- Pawar, H.A.; Kamat, S.R.; Choudhary, P.D. An overview of natural polysaccharides as biological macromolecules: Their chemical modifications and pharmaceutical applications. Biol. Med. 2015, 7, 1.
- Jiang, J.-L.; Zhang, W.-Z.; Ni, W.-X.; Shao, J.-W. Insight on structure-property relationships of carrageenan from marine red algal: A review. Carbohydr. Polym. 2021, 257, 117642.
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining chitin, chitosan and their melanin complexes from insects. Int. J. Biol. Macromol. 2021, 167, 1319–1328.
- Oliveira, C.; Neves, N.M.; Reis, R.L.; Martins, A.; Silva, T.H. A review on fucoidan antitumor strategies: From a biological active agent to a structural component of fucoidan-based systems. Carbohydr. Polym. 2020, 239, 116131.
- Darge, H.F.; Andrgie, A.T.; Tsai, H.-C.; Lai, J.-Y. Polysaccharide and polypeptide based injectable thermo-sensitive hydrogels for local biomedical applications. Int. J. Biol. Macromol. 2019, 133, 545–563.
- Gupta, N.; Jangid, A.K.; Pooja, D.; Kulhari, H. Inulin: A novel and stretchy polysaccharide tool for biomedical and nutritional applications. Int. J. Biol. Macromol. 2019, 132, 852–863.
- Shi, Q.M.; Wang, A.J.; Lu, Z.H.; Qin, C.J.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453–454, 1–9.
- Lu, W.J.; Yang, Z.F.; Chen, J.; Wang, D.; Zhang, Y. Recent advances in antiviral activities and potential mechanisms of sulfated polysaccharides. Carbohydr. Polym. 2021, 272, 118526.
- Li, Z.H.; Cui, B.; Liu, X.W.; Wang, L.C.; Xian, Q.J.; Lu, Z.X.; Liu, S.T.; Cao, Y.G.; Zhao, Y.R. Virucidal activity and the antiviral mechanism of acidic polysaccharides against Enterovirus 71 infection in vitro. Microbiol. Immunol. 2020, 64, 189–201.
- Zhao, C.; Gao, L.; Wang, C.; Liu, B.; Jin, Y.; Xing, Z. Structural characterization and antiviral activity of a novel heteropolysaccharide isolated from Grifola frondosa against enterovirus 71. Carbohydr. Polym. 2016, 144, 382–389.
- Leibbrandt, A.; Meier, C.; König-Schuster, M.; Weinmüllner, R.; Kalthoff, D.; Pflugfelder, B.; Graf, P.; Frank-Gehrke, B.; Beer, M.; Fazekas, T. Iota-carrageenan is a potent inhibitor of influenza A virus infection. PLoS ONE 2010, 5, e14320.
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Müller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006, 2, e69.
- Chen, Y.; Luo, Q.; Li, S.; Li, C.; Liao, S.; Yang, X.; Zhou, R.; Zhu, Y.; Teng, L.; Chen, H. Antiviral activity against porcine epidemic diarrhea virus of Pogostemon cablin polysaccharide. J. Ethnopharmacol. 2020, 259, 113009.
- Liang, Y.-Y.; Li, K.-W.; Niu, F.-J.; Li, Y.; Wei, H.-C.; Dai, Y.-L.; Wang, Y.-Y.; Zhou, C.-Z.; Wan, X.-H. Salvia plebeia R. Br. polysaccharides (SPP) against RSV (respiratory syncytial virus) infection: Antiviral effect and mechanisms of action. Biomed. Pharmacother. 2021, 141, 111843.
- Wang, T.; Wang, X.; Zhuo, Y.; Si, C.; Yang, L.; Meng, L.; Zhu, B. Antiviral activity of a polysaccharide from Radix Isatidis (Isatis indigotica Fortune) against hepatitis B virus (HBV) in vitro via activation of JAK/STAT signal pathway. J. Ethnopharmacol. 2020, 257, 112782.
- Chen, X.Y.; Han, W.W.; Wang, G.X.; Zhao, X. Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines. Int. J. Biol. Macromol. 2020, 164, 331–343.
- Yu, C.; Wei, K.; Liu, L.; Yang, S.; Hu, L.; Zhao, P.; Meng, X.; Shao, M.; Wang, C.; Zhu, L. Taishan Pinus massoniana pollen polysaccharide inhibits subgroup J avian leucosis virus infection by directly blocking virus infection and improving immunity. Sci. Rep. 2017, 7, 1–14.
- Soria-Martinez, L.; Bauer, S.; Giesler, M.; Schelhaas, S.; Materlik, J.; Janus, K.; Pierzyna, P.; Becker, M.; Snyder, N.L.; Hartmann, L. Prophylactic antiviral activity of sulfated glycomimetic oligomers and polymers. J. Am. Chem. Soc. 2020, 142, 5252–5265.
- Carbone, D.A.; Pellone, P.; Lubritto, C.; Ciniglia, C. Evaluation of microalgae antiviral activity and their bioactive compounds. Antibiotics 2021, 10, 746.
- Sanniyasi, E.; Venkatasubramanian, G.; Anbalagan, M.M.; Raj, P.P.; Gopal, R.K. In vitro anti-HIV-1 activity of the bioactive compound extracted and purified from two different marine macroalgae (seaweeds) (Dictyota bartayesiana J.V.Lamouroux and Turbinaria decurrens Bory). Sci. Rep. 2019, 9, 12185.
- Pereira, L.; Critchley, A.T. The COVID 19 novel coronavirus pandemic 2020: Seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? J. Appl. Phycol. 2020, 32, 1875–1877.
- Alam, M.A.; Parra-Saldivar, R.; Bilal, M.; Afroze, C.A.; Ahmed, M.N.; Iqbal, H.M.N.; Xu, J.L. Algae-derived bioactive molecules for the potential treatment of SARS-CoV-2. Molecules 2021, 26, 2134.
- Andrew, M.; Jayaraman, G. Marine sulfated polysaccharides as potential antiviral drug candidates to treat corona virus disease (COVID-19). Carbohydr. Res. 2021, 505, 108326.
- Aga, M.B.; Dar, A.H.; Nayik, G.A.; Panesar, P.S.; Allai, F.; Khan, S.A.; Shams, R.; Kennedy, J.F.; Altaf, A. Recent insights into carrageenan-based bio-nanocomposite polymers in food applications: A review. Int. J. Biol. Macromol. 2021, 192, 197–209.
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301.
- He, X.; Fang, J.; Guo, Q.; Wang, M.; Li, Y.; Meng, Y.; Huang, L. Advances in antiviral polysaccharides derived from edible and medicinal plants and mushrooms. Carbohydr. Polym. 2020, 229, 115548.
- Hans, N.; Malik, A.; Naik, S. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: Mini review. Bioresour. Technol. Rep. 2020, 13, 100623.
- Ana, P.; Nathalie, B.; Gilles, B.; Daniel, R.; Tomás, M.-S.; Yolanda, F.-P. Anti-Herpes simplex virus (HSV-1) activity and antioxidant capacity of carrageenan-rich enzymatic extracts from Solieria filiformis (Gigartinales, Rhodophyta). Int. J. Biol. Macromol. 2021, 168, 322–330.
- Talarico, L.B.; Damonte, E.B. Interference in dengue virus adsorption and uncoating by carrageenans. Virology 2007, 363, 473–485.
- Kolender, A.A.; Pujol, C.A.; Damonte, E.B.; Cerezo, A.S.; Matulewicz, M. Sulfation of kappa-carrageenan and antiviral activity. An. Asoc. Quim. Argent. 1998, 86, 304–311.
- Baba, M.; Snoeck, R.; Pauwels, R.; De Clercq, E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 1988, 32, 1742–1745.
- Morokutti-Kurz, M.; König-Schuster, M.; Koller, C.; Graf, C.; Graf, P.; Kirchoff, N.; Reutterer, B.; Seifert, J.-M.; Unger, H.; Grassauer, A. The intranasal application of Zanamivir and carrageenan is synergistically active against influenza A virus in the murine model. PLoS ONE 2015, 10, e0128794.
- Morokutti-Kurz, M.; Fröba, M.; Graf, P.; Große, M.; Grassauer, A.; Auth, J.; Schubert, U.; Prieschl-Grassauer, E. Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro. PLoS ONE 2021, 16, e0237480.
- Witvrouw, M.; De Clercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol. Vasc. Sys. 1997, 29, 497–511.
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure–activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15.
- Piccini, L.E.; Carro, A.C.; Quintana, V.M.; Damonte, E.B. Antibody-independent and dependent infection of human myeloid cells with dengue virus is inhibited by carrageenan. Virus Res. 2020, 290, 198150.
- Vissani, A.; Galdo Novo, S.; Ciancia, M.; Zabal, O.A.; Thiry, E.; Bratanich, A.C.; Barrandeguy, M.E. Effects of lambda-carrageenan on equid herpesvirus 3 in vitro. J. Equine Vet. Sci. 2016, 39, S61–S62.
- Ciejka, J.; Botwina, P.; Nowakowska, M.; Szczubiałka, K.; Pyrc, K. Synthetic sulfonated derivatives of poly (allylamine hydrochloride) as inhibitors of human metapneumovirus. PLoS ONE 2019, 14, e0214646.
- Koenighofer, M.; Lion, T.; Bodenteich, A.; Prieschl-Grassauer, E.; Grassauer, A.; Unger, H.; Mueller, C.A.; Fazekas, T. Carrageenan nasal spray in virus confirmed common cold: Individual patient data analysis of two randomized controlled trials. Multidiscip. Respir. Med. 2014, 9, 1–12.
- Morokutti-Kurz, M.; Graf, C.; Prieschl-Grassauer, E. Amylmetacresol/2, 4-dichlorobenzyl alcohol, hexylresorcinol, or carrageenan lozenges as active treatments for sore throat. Int. J. Gen. Med. 2017, 10, 53.
- Buck, C.B.; Cheng, N.; Thompson, C.D.; Lowy, D.R.; Steven, A.C.; Schiller, J.T.; Trus, B.L. Arrangement of L2 within the papillomavirus capsid. J. Virol. 2008, 82, 5190–5197.
- Rodríguez, A.; Kleinbeck, K.; Mizenina, O.; Kizima, L.; Levendosky, K.; Jean-Pierre, N.; Villegas, G.; Ford, B.E.; Cooney, M.L.; Teleshova, N. In vitro and in vivo evaluation of two carrageenan-based formulations to prevent HPV acquisition. Antivir. Res. 2014, 108, 88–93.
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594.
- Riaz Rajoka, M.S.; Mehwish, H.M.; Wu, Y.; Zhao, L.; Arfat, Y.; Majeed, K.; Anwaar, S. Chitin/chitosan derivatives and their interactions with microorganisms: A comprehensive review and future perspectives. Crit. Rev. Biotechnol. 2020, 40, 365–379.
- Shamshina, J.L.; Berton, P.; Rogers, R.D. Advances in functional chitin materials: A review. ACS Sustain. Chem. Eng. 2019, 7, 6444–6457.
- Safarzadeh, M.; Sadeghi, S.; Azizi, M.; Rastegari-Pouyani, M.; Pouriran, R.; Hoseini, M.H.M. Chitin and chitosan as tools to combat COVID-19: A triple approach. Int. J. Biol. Macromol. 2021, 183, 235–244.
- Jana, B.; Chatterjee, A.; Roy, D.; Ghorai, S.; Pan, D.; Pramanik, S.K.; Chakraborty, N.; Ganguly, J. Chitosan/benzyloxy-benzaldehyde modified ZnO nano template having optimized and distinct antiviral potency to human cytomegalovirus. Carbohydr. Polym. 2022, 278, 118965.
- Davydova, V.; Nagorskaya, V.; Gorbach, V.; Kalitnik, A.; Reunov, A.; Solov’Eva, T.; Ermak, I. Chitosan antiviral activity: Dependence on structure and depolymerization method. Appl. Biochem. Microbiol. 2011, 47, 103–108.
- Chirkov, S. The antiviral activity of chitosan. Appl. Biochem. Microbiol. 2002, 38, 1–8.
- Sharma, N.; Modak, C.; Singh, P.K.; Kumar, R.; Khatri, D.; Singh, S.B. Underscoring the immense potential of chitosan in fighting a wide spectrum of viruses: A plausible molecule against SARS-CoV-2? Int. J. Biol. Macromol. 2021, 179, 33–44.
- Sosa, M.A.G.; Fazely, F.; Koch, J.A.; Vercellotti, S.V.; Ruprecht, R.M. N-Carboxymethylchitosan-N, O-sulfate as an anti-HIV-1 agent. Biochem. Biophys. Res. Commun. 1991, 174, 489–496.
- Ishihara, C.; Yoshimatsu, K.; Tsuji, M.; Arikawa, J.; Saiki, I.; Tokura, S.; Azuma, I. Anti-viral activity of sulfated chitin derivatives against Friend murine leukaemia and herpes simplex type-1 viruses. Vaccine 1993, 11, 670–674.
- Hassan, M.I.; Mohamed, A.F.; Taher, F.A.; Kamel, M.R. Antimicrobial activities of chitosan nanoparticles prepared from Lucilia cuprina maggots (Diptera: Calliphoridae). J. Egypt. Soc. Parasitol. 2016, 46, 563–570.
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Chitosan, alginate, hyaluronic acid, gums, and β-glucan as potent adjuvants and vaccine delivery systems for viral threats including SARS-CoV-2: A review. Int. J. Biol. Macromol. 2021, 182, 1931–1940.
- Loutfy, S.A.; Elberry, M.H.; Farroh, K.Y.; Mohamed, H.T.; Mohamed, A.A.; Mohamed, E.B.; Faraag, A.H.I.; Mousa, S.A. Antiviral activity of chitosan nanoparticles encapsulating curcumin against hepatitis C virus genotype 4a in human hepatoma cell lines. Int. J. Nanomed. 2020, 15, 2699.
- Wu, D.; Ensinas, A.; Verrier, B.; Primard, C.; Cuvillier, A.; Champier, G.; Paul, S.; Delair, T. Zinc-stabilized chitosan-chondroitin sulfate nanocomplexes for HIV-1 infection inhibition application. Mol. Pharmaceut. 2016, 13, 3279–3291.
- Milewska, A.; Chi, Y.; Szczepanski, A.; Barreto-Duran, E.; Dabrowska, A.; Botwina, P.; Obloza, M.; Liu, K.; Liu, D.; Guo, X. HTCC as a polymeric inhibitor of SARS-CoV-2 and MERS-CoV. J. Virol. 2021, 95, e01622-20.
- Pyrć, K.; Milewska, A.; Duran, E.B.; Botwina, P.; Lopes, R.; Arenas-Pinto, A.; Badr, M.; Mellor, R.; Kalber, T.L.; Fernandes-Reyes, D. SARS-CoV-2 inhibition in human airway epithelial cells using a mucoadhesive, amphiphilic chitosan that may serve as an anti-viral nasal spray. BioRxiv 2021, 11, 20012.
- Russo, E.; Gaglianone, N.; Baldassari, S.; Parodi, B.; Cafaggi, S.; Zibana, C.; Donalisio, M.; Cagno, V.; Lembo, D.; Caviglioli, G. Preparation, characterization and in vitro antiviral activity evaluation of foscarnet-chitosan nanoparticles. Colloids Surf. B Biointerf. 2014, 118, 117–125.
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8, 1–6.
- Sofy, A.R.; Hmed, A.A.; Abd El Haliem, N.F.; Zein, M.A.-E.; Elshaarawy, R.F. Polyphosphonium-oligochitosans decorated with nanosilver as new prospective inhibitors for common human enteric viruses. Carbohydr. Polym. 2019, 226, 115261.
- Gao, Y.; Liu, W.; Wang, W.; Zhang, X.; Zhao, X. The inhibitory effects and mechanisms of 3, 6-O-sulfated chitosan against human papillomavirus infection. Carbohydr. Polym. 2018, 198, 329–338.
- Zayed, A.; Ulber, R. Fucoidans: Downstream processes and recent applications. Mar. Drugs 2020, 18, 170.
- Wu, L.; Sun, J.; Su, X.; Yu, Q.; Yu, Q.; Zhang, P. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr. Polym. 2016, 154, 96–111.
- Wang, W.; Wu, J.; Zhang, X.; Hao, C.; Zhao, X.; Jiao, G.; Shan, X.; Tai, W.; Yu, G. Inhibition of influenza A virus infection by fucoidan targeting viral neuraminidase and cellular EGFR pathway. Sci. Rep. 2017, 7, 1–14.
- Sun, T.; Zhang, X.; Miao, Y.; Zhou, Y.; Shi, J.; Yan, M.; Chen, A. Studies on antiviral and immuno-regulation activity of low molecular weight fucoidan from Laminaria japonica. J. Ocean Univ. China 2018, 17, 705–711.
- Thuy, T.T.T.; Ly, B.M.; Van, T.T.T.; Van Quang, N.; Tu, H.C.; Zheng, Y.; Seguin-Devaux, C.; Mi, B.; Ai, U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015, 115, 122–128.
- Cao, Y.-G.; Hao, Y.; Li, Z.-H.; Liu, S.-T.; Wang, L.-X. Antiviral activity of polysaccharide extract from Laminaria japonica against respiratory syncytial virus. Biomed. Pharmacother. 2016, 84, 1705–1710.
- Trejo-Avila, L.M.; Elizondo-Gonzalez, R.; Rodriguez-Santillan, P.; Aguilar-Briseño, J.A.; Ricque-Marie, D.; Rodriguez-Padilla, C.; Cruz-Suarez, L.E. Innocuity and anti-Newcastle-virus-activity of Cladosiphon okamuranus fucoidan in chicken embryos. Poult. Sci. 2016, 95, 2795–2802.
- Diaz-Resendiz, K.J.G.; Toledo-Ibarra, G.A.; Ruiz-Manzano, R.; Perez, D.A.G.; Covantes-Rosales, C.E.; Benitez-Trinidad, A.B.; Ramirez-Ibarra, K.M.; Escobedo, A.T.H.; Gonzalez-Navarro, I.; Ventura-Ramon, G.H.; et al. Ex vivo treatment with fucoidan of mononuclear cells from SARS-CoV-2 infected patients. Int. J. Environ. Health Res. 2021, 1–19.
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, E.-A.; Ahn, G.; Jee, Y.; Jeon, Y.-J. Anti-inflammatory activity of a sulfated polysaccharide isolated from an enzymatic digest of brown seaweed Sargassum horneri in RAW 264.7 cells. Nutr. Res. Pract. 2017, 11, 3.
- Amin, M.L.; Mawad, D.; Dokos, S.; Koshy, P.; Martens, P.J.; Sorrell, C.C. Immunomodulatory properties of photopolymerizable fucoidan and carrageenans. Carbohydr. Polym. 2020, 230, 115691.
- Rasin, A.B.; Silchenko, A.S.; Kusaykin, M.I.; Malyarenko, O.S.; Zueva, A.O.; Kalinovsky, A.I.; Airong, J.; Surits, V.V.; Ermakova, S.P. Enzymatic transformation and anti-tumor activity of Sargassum horneri fucoidan. Carbohydr. Polym. 2020, 246, 116635.
- Dai, Y.-L.; Jiang, Y.-F.; Lee, H.G.; Jeon, Y.-J.; Kang, M.-C. Characterization and screening of anti-tumor activity of fucoidan from acid-processed hijiki (Hizikia fusiforme). Int. J. Biol. Macromol. 2019, 139, 170–180.
- Ponce, N.M.A.; Flores, M.L.; Pujol, C.A.; Becerra, M.B.; Navarro, D.A.; Córdoba, O.; Damonte, E.B.; Stortz, C.A. Fucoidans from the phaeophyta Scytosiphon lomentaria: Chemical analysis and antiviral activity of the galactofucan component. Carbohydr. Res. 2019, 478, 18–24.
- Sun, Q.L.; Li, Y.; Ni, L.Q.; Li, Y.X.; Cui, Y.S.; Jiang, S.L.; Xie, E.Y.; Du, J.; Deng, F.; Dong, C.X. Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr. Polym. 2020, 229, 115487.
- Li, Y.; Hu, H.; Zhou, Q.; Ao, Y.; Xiao, C.; Wan, J.; Wan, Y.; Xu, H.; Li, Z.; Yang, X. α-Amylase-and redox-responsive nanoparticles for tumor-targeted drug delivery. ACS Appl. Mater. Interf. 2017, 9, 19215–19230.
- Elizondo-Gonzalez, R.; Cruz-Suarez, L.E.; Ricque-Marie, D.; Mendoza-Gamboa, E.; Rodriguez-Padilla, C.; Trejo-Avila, L.M. In vitro characterization of the antiviral activity of fucoidan from Cladosiphon okamuranus against Newcastle Disease Virus. Virol. J. 2012, 9, 1–9.
- Lee, J.-B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharmaceut. Bull. 2004, 52, 1091–1094.
- Krylova, N.V.; Ermakova, S.P.; Lavrov, V.F.; Leneva, I.A.; Kompanets, G.G.; Iunikhina, O.V.; Nosik, M.N.; Ebralidze, L.K.; Falynskova, I.N.; Silchenko, A.S. The comparative analysis of antiviral activity of native and modified fucoidans from brown algae Fucus evanescens in vitro and in vivo. Mar. Drugs 2020, 18, 224.
- Vaillant, A. Nucleic acid polymers: Broad spectrum antiviral activity, antiviral mechanisms and optimization for the treatment of hepatitis B and hepatitis D infection. Antivir. Res. 2016, 133, 32–40.
- Vaillant, A.; Juteau, J.-M.; Lu, H.; Liu, S.; Lackman-Smith, C.; Ptak, R.; Jiang, S. Phosphorothioate oligonucleotides inhibit human immunodeficiency virus type 1 fusion by blocking gp41 core formation. Antimicrob. Agents Chemother. 2006, 50, 1393–1401.
- Guzman, E.M.; Cheshenko, N.; Shende, V.; Keller, M.J.; Goyette, N.; Juteau, J.-M.; Boivin, G.; Vaillant, A.; Herold, B.C. Amphipathic DNA polymers are candidate vaginal microbicides and block herpes simplex virus binding, entry and viral gene expression. Antivir. Ther. 2007, 12, 1147.
- Lee, A.M.; Rojek, J.M.; Gundersen, A.; Ströher, U.; Juteau, J.-M.; Vaillant, A.; Kunz, S. Inhibition of cellular entry of lymphocytic choriomeningitis virus by amphipathic DNA polymers. Virology 2008, 372, 107–117.
- Bernstein, D.I.; Goyette, N.; Cardin, R.; Kern, E.R.; Boivin, G.; Ireland, J.; Juteau, J.-M.; Vaillant, A. Amphipathic DNA polymers exhibit antiherpetic activity in vitro and in vivo. Antimicrob. Agents Chemother. 2008, 52, 2727–2733.
- Cardin, R.D.; Bravo, F.J.; Sewell, A.P.; Cummins, J.; Flamand, L.; Juteau, J.-M.; Bernstein, D.I.; Vaillant, A. Amphipathic DNA polymers exhibit antiviral activity against systemic murine Cytomegalovirus infection. Virol. J. 2009, 6, 1–14.
- Basu, A.; Kanda, T.; Beyene, A.; Saito, K.; Meyer, K.; Ray, R. Sulfated homologues of heparin inhibit hepatitis C virus entry into mammalian cells. J. Virol. 2007, 81, 3933–3941.
- Chang, K.-S.; Jiang, J.; Cai, Z.; Luo, G. Human apolipoprotein e is required for infectivity and production of hepatitis C virus in cell culture. J. Virol. 2007, 81, 13783–13793.
- Noordeen, F.; Vaillant, A.; Jilbert, A.R. Nucleic acid polymers inhibit duck hepatitis B virus infection in vitro. Antimicrob. Agents Chemother. 2013, 57, 5291–5298.
- Al-Mahtab, M.; Bazinet, M.; Vaillant, A. Safety and efficacy of nucleic acid polymers in monotherapy and combined with immunotherapy in treatment-naive Bangladeshi patients with HBeAg+ chronic hepatitis B infection. PLoS ONE 2016, 11, e0156667.
- Noordeen, F.; Scougall, C.A.; Grosse, A.; Qiao, Q.; Ajilian, B.B.; Reaiche-Miller, G.; Finnie, J.; Werner, M.; Broering, R.; Schlaak, J.F. Therapeutic antiviral effect of the nucleic acid polymer REP 2055 against persistent duck hepatitis B virus infection. PLoS ONE 2015, 10, e0140909.
- Bazinet, M.; Pantea, V.; Placinta, G.; Moscalu, I.; Cebotarescu, V.; Cojuhari, L.; Jimbei, P.; Iarovoi, L.; Smesnoi, V.; Musteata, T.; et al. Safety and efficacy of 48 weeks REP 2139 or REP 2165, tenofovir disoproxil, and pegylated interferon alfa-2a in patients with chronic HBV infection naive to nucleos(t)ide therapy. Gastroenterology 2020, 158, 2180–2194.
- Blanchet, M.; Sinnathamby, V.; Vaillant, A.; Labonté, P. Inhibition of HBsAg secretion by nucleic acid polymers in HepG2. 2.15 cells. Antivir. Res. 2019, 164, 97–105.
- Blome, S.; Staubach, C.; Henke, J.; Carlson, J.; Beer, M. Classical swine fever—An updated review. Viruses 2017, 9, 86.
- Zhang, H.; Zhang, H.; Demirer, G.S.; González-Grandío, E.; Fan, C.; Landry, M.P. Engineering DNA nanostructures for siRNA delivery in plants. Nat. Protoc. 2020, 15, 3064–3087.
- Kleiboeker, S.B. Swine fever: Classical swine fever and African swine fever. Vet. Clin. Food Anim. Pract. 2002, 18, 431–451.
- Dong, H.; Song, G.; Ma, D.; Wang, T.; Jing, S.; Yang, H.; Tao, Y.; Tang, Y.; Shi, Y.; Dai, Z. Improved antiviral activity of classical swine fever virus-targeted siRNA by tetrahedral framework nucleic acid-enhanced delivery. ACS Appl. Mater. Interf. 2021, 13, 29416–29423.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
3 times
(View History)
Update Date:
05 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




