
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fatima Domenica Elisa De Palma | + 1622 word(s) | 1622 | 2020-09-28 08:52:44 | | | |
| 2 | Catherine Yang | Meta information modification | 1622 | 2020-10-09 11:33:31 | | |
Video Upload Options
The response to neoadjuvant chemoradiation (nCRT) is a critical step in the management of locally advanced rectal cancer (LARC) patients. Only a minority of LARC patients responds completely to neoadjuvant treatments, thus avoiding invasive radical surgical resection. Moreover, toxic side effects can adversely affect patients’ survival. The difficulty in separating in advances responder from non-responder patients affected by LARC highlights the need for valid biomarkers that guide clinical decision-making. In this context, circulating tumor biomarkers (i.e., microRNAs, circulating tumor cells and cell-free nucleic acids), as well as single nucleotide polymorphisms (SNPs) associated with miRNAs (miR-SNPs)) seem to be promising candidates for predicting LARC prognosis and/or therapy response.
1. Introduction
Rectal cancer (RC) is one among the top four most deadly neoplasms worldwide [1]. In the last three decades, high-income countries experienced a reduction in the incidence of RC, as well as a decrease of its mortality, maybe due to both diagnostic/therapeutic improvements and secondary prevention. However, middle- and low-income countries have a constantly increasing incidence but lower if compared to the rest of the world.
Clinical presentation of RC includes a change in bowel habits (diarrhoea, constipation, frequents bowel movements), presence of haematochezia, rectal tenesmus, abdominal pain and systemic symptoms such as iron-deficiency anaemia, weight loss, and weakness.
The management of RC requires an effective coordination between healthcare professionals due to the interdisciplinary nature of its treatment pathway. The multidisciplinary team should be made of referral oncologists, colorectal surgeons, radiotherapists, radiologists, pathologists and endoscopists. The absence of any one of these figures may compromise the oncological outcome [2][3]. In fact, a retrospective analysis showed that unsuccessful multidisciplinary discussion was one of the predictive factors for positive resection margins, as well as the absence of radiotherapy [4].
2. Circulating Biomarkers to Predict Response to nCRT in LARC
In the era of precision medicine, nevertheless the several advantages of liquid biopsy derived from non-invasiveness, and rapidity as well as less cost of the test, the pre-analytical variability, and particularly the low detection sensitivity limited its clinical utility in the management of LARC, which is always captained by the gold standard, the tissue biopsy.
Liquid biopsy consists of the non-invasive detection and analysis of the circulating components that are present in body fluids, also defined as “tumor circulome” [5]. Human bloodstream, for instance, is not only composed of different types of circulating cells, which embrace circulating tumor cells (CTCs), but can also include extracellular vesicles (i.e., exosomes), circulating derived tumor-proteins as well as a small amount of circulating cell-free nucleic acids, such circulating DNA (ctDNA) or circulating RNA (ctRNA), originating from primary and metastatic lesions (Figure 1) [5]. ctRNAs comprise extracellular vesicles-associated circulating RNA and a variety of RNA classes (i.e., mRNA, long non-coding RNAs and miRNAs). Differently from ctRNAs, to date, ctDNAs and CTCs are the only circulating analytes that are approved as clinical biomarkers by the US Food and Drug Administration (FDA) [5][6]. However, only a few studies have focused on the potential role of ctDNA and CTCs as well as circulating derived tumor-proteins in LARC.
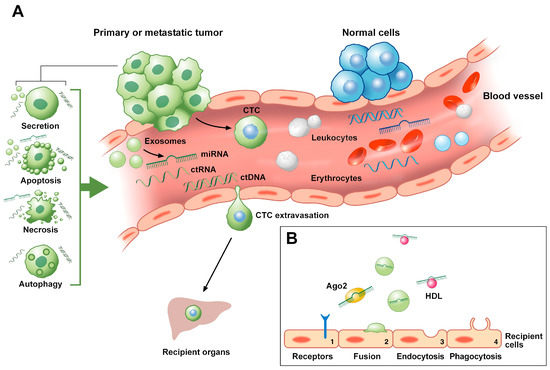
Figure 1. Mechanisms of release into the bloodstream and of migration to recipient cells of circulating tumor biomarkers. (A) CTCs usually detach from the primary or metastatic tumor and transmigrate through the vessel wall barrier to circulate into the bloodstream of LARC patients. During their travel in blood, CTCs become entrapped into microvessels. As a consequence, such travelers establish metastasis in recipient tissues either cause the rupture of the microvasculature or through extravasation. ctDNAs and ctRNAs release includes apoptosis, autophagy, necrosis, lysis of CTCs and active secretion from tumor cells. ctRNAs include extracellular vesicles-associated circulating RNA as well as a variety of RNA classes (i.e., lncRNAs, miRNAs). Among them, miRNAs can be released into the blood circulation from the lysis of tumor cells (i.e., necrosis, apoptosis) or from active secretion (i.e., exosomes or apoptotic bodies). (B) miRNAs floating in the blood can be present as cell-free miRNAs, being associated with RNA-binding proteins (i.e., Ago2) or lipoproteins (i.e., HDL), or be packaged inside microvesicles, such as exosomes. Circulating miRNAs are internalized by recipient cells through different mechanisms, including (1) the capture by specific cell receptors, (2) direct fusion with the plasma membrane of the receiving cells, (3) endocytosis and (4) phagocytosis. Ago2, protein argonaute 2; CTC, circulating tumor cell; ctDNA, circulating tumor DNA; ctRNA, circulating tumor RNA; HDL, high-density lipoprotein; LARC, locally advanced rectal cancer; lncRNA, long non-coding RNA; miRNA, microRNA. Circulating tumor analytes are represented in green color, while the non-cancerous ones in blue color.
3. Single Nucleotide Polymorphisms (SNPs) at miRNAs in LARC
Single nucleotide polymorphisms (SNPs), as the name suggests, are caused by a single nucleotide variation in the DNA sequence [7]. Being the most common type of heritable genetic variation in humans, SNPs are used to study genetic differences between individuals and populations [7].
Many SNPs doesn’t affect gene functions, being they naturally present at DNA level [8]. However, in some cases, such sequence variations can also impact gene expression and be implicated in the development of cancer [7]. Additionally, they have been described also as potential prognostic and predictive biomarkers [9].
SNPs can occur in coding or non-coding regions of the genome, such as in miRNAs (miR-SNPs) [10]. It has been demonstrated that SNPs could be located in the miRNA sequences, in miRNA genes or in miRNAs binding sites. miR-SNPs might affect the individual’s susceptibility by causing loss or gain of miRNA functions, or by altering the epigenetic regulation of miRNA encoding genes, or again by affecting the pri- and pre-miRNA processing or the interaction between miRNAs and their target mRNAs [10].
Few studies focused on the involvement of miR-SNPs in LARC [11][12][13]. For instance, the prognostic role of the SNP rs4919510, that is characterized by the guanine to cytosine (G > C) base substitution in the sequence of the mature miRNA-608, has been explored in LARC patients subjected to neodjuvant capecitabine and oxaliplatin (CAPOX) treatment followed by CRT, surgery, or to adjuvant CAPOX ± cetuximab treatment [11]. This retrospective study showed that the CC genotype was associated with a worse 5-year progression-free survival (PFS) in LARC patients treated with chemotherapy with respect to the CG/GG genotype patients. Furthermore, the administration of cetuximab to the chemotherapy and CRT, significantly improves the 5-year PFS and OS in CC carriers when compared to CG/GG genotypes [11]. This study evidenced not only the role of miR-SNPs in the risk of cancer, but also their impact in drug response.
The rs61764370 SNP (thymine to guanine (T > G) base substitution) has been identified as potential biomarker of response to neoadjuvant treatment and of a favorable outcome for LARC patients [12]. This polymorphism, harbored in the complementary site 6 (LCS-6) of the tumor suppressor miRNA let-7, alters the affinity between let-7 and its target oncogene KRAS, and consequently increasing cancer proliferation [12]. In particular, it has been demonstrated that LARC individuals with LCS-6 TG genotype reached complete response after neoadjuvant treatment and showed a better 5-years PFS and OS when compared to the TT genotype group [12].
In another study, 265 Caucasian LARC patients (divided in two subgroups depending on the radiation dose) subjected to neoadjuvant CRT based on 5-FU were screened for a panel of 114 miR-SNPs [13]. A total of five SNPs were identified in miRNAs target genes. In detail, two SNPs in SMAD Family Member 3 (SMAD3) (rs744910 and rs745103) and one SNP in transactivation response element RNA-binding protein (TRBP) (rs6088619) were predictive of pCR. In contrast, the SNPs rs10719 (in Drosha) and rs17228212 (in SMAD3) had an unfavorable chance of pCR [13].
4. Conclusions
Interlinked disciplines collaborated to improve the management of RC that, in the past years, has been dominated by the dramatic surgery approach. At present, neoadjuvant chemoradiation in LARC care has the potential to reduce the tumor size for an effective oncological resection and the local risk of recurrence. Unfortunately, the responsiveness to nCRT has a wide range of variability. Thus, the assessment of the variation of levels of single or signatures of miRNAs in tumor specimens or, even better circulating miRNAs and/or tumor cells in body fluids, could improve the stratification of LARC patients according to the nCRT response, consequently facilitating the clinical decision-making.
Alterations in the abundance of miRNAs and circulating tumor markers are involved in the pathogenesis of various types of human cancers, including LARC. Moreover, they show a great potential as non-invasive biomarkers; miRNAs, for instance, due to their stability as tumor-derived cell-free molecules. Their clinical relevance in human diseases as diagnostic, prognostic, and therapeutic biomarkers is also evidenced by the number of clinical trials that at present have been completed (clinicaltrials.gov). However, only a few potential biomarkers are currently used in the clinical practice [14][15][16]. To date, the European medicines agency (EMA) and FDA approved the detection of mutations of the EGFR gene from ctDNA in order to select patients affected by non-small cell lung cancer who are eligible for treatment with erlotinib (FDA), afatinib (FDA), gefitinib (EMA), or osimertinib (EMA and FDA), thus avoiding biopsies for some patients [14][15][16]. The FDA has also approved a blood-based test for the early detection of CRC patients, namely the EpiproColon® test [17]. This latter is a qualitative assay based on the ctDNA analysis for the detection of methylated Septin9 DNA, whose hypermethylated status is associated with CRC [17]. Concerning CTCs analysis, the CellSearch® test is the only one that has been approved by the FDA for the prognosis of CRC, breast and prostate tumors [18].
In summary, it still remains to be determined whether LARC patients can be advantageously stratified based on the levels of expression of miRNAs and other circulating tumor markers. We envision that this gap of knowledge will be filled by future studies that will use next-generation technologies, ultimately improving the quality of life and therapeutic outcome of rectal cancer patients.
References
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. The Lancet 2019.
- Peltrini, R.; Luglio, G.; Cassese, G.; Amendola, A.; Caruso, E.; Sacco, M.; Pagano, G.; Sollazzo, V.; Tufano, A.; Giglio, M.C.; et al. Oncological outcomes and quality of life after rectal cancer surgery. Open Med. 2019.
- Dodaro, C.A.; Calogero, A.; Tammaro, V.; Pellegrino, T.; Lionetti, R.; Campanile, S.; Menkulazi, M.; Ciccozzi, M.; Iannicelli, A.M.; Giallauria, F.; et al. Colorectal cancer in the elderly patient: The role of neo-adjuvant therapy. Open Med. Pol. 2019.
- Al-Sukhni, E.; Attwood, K.; Gabriel, E.; Nurkin, S.J. Predictors of circumferential resection margin involvement in surgically resected rectal cancer: A retrospective review of 23,464 patients in the US National Cancer Database. Int. J. Surg. 2016.
- De Rubis, G.; Rajeev Krishnan, S.; Bebawy, M. Liquid Biopsies in Cancer Diagnosis, Monitoring, and Prognosis. Trends Pharmacol. Sci. 2019, 40, 172–186.
- Marcuello, M.; Vymetalkova, V.; Neves, R.P.L.; Duran-Sanchon, S.; Vedeld, H.M.; Tham, E.; van Dalum, G.; Flügen, G.; Garcia-Barberan, V.; Fijneman, R.J.A.; et al. Circulating biomarkers for early detection and clinical management of colorectal cancer. Mol. Aspects Med. 2019, 69, 107–122.
- Haraksingh, R.R.; Snyder, M.P. Impacts of Variation in the Human Genome on Gene Regulation. J. Mol. Biol. 2013, 425, 3970–3977.
- Brody, T. Chapter 19—Biomarkers. In Clinical Trials (Second Edition); Brody, T., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 377–419. ISBN 978-0-12-804217-5.
- Horvat, M.; Potočnik, U.; Repnik, K.; Kavalar, R.; Štabuc, B. Single Nucleotide Polymorphisms as Prognostic and Predictive Factors of Adjuvant Chemotherapy in Colorectal Cancer of Stages I and II. Available online: https://www.hindawi.com/journals/grp/2016/2139489/ (accessed on 28 August 2020).
- Ryan, B.M.; Robles, A.I.; Harris, C.C. Genetic variation in microRNA networks: The implications for cancer research. Nat. Rev. Cancer 2010, 10, 389–402.
- Sclafani, F.; Chau, I.; Cunningham, D.; Lampis, A.; Hahne, J.C.; Ghidini, M.; Lote, H.; Zito, D.; Tabernero, J.; Glimelius, B.; et al. Sequence variation in mature microRNA-608 and benefit from neo-adjuvant treatment in locally advanced rectal cancer patients. Carcinogenesis 2016, 37, 852–857.
- Sclafani, F.; Chau, I.; Cunningham, D.; Peckitt, C.; Lampis, A.; Hahne, J.C.; Braconi, C.; Tabernero, J.; Glimelius, B.; Cervantes, A.; et al. Prognostic role of the LCS6 KRAS variant in locally advanced rectal cancer: Results of the EXPERT-C trial. Ann. Oncol. 2015, 26, 1936–1941.
- Dreussi, E.; Pucciarelli, S.; De Paoli, A.; Polesel, J.; Canzonieri, V.; Agostini, M.; Friso, M.L.; Belluco, C.; Buonadonna, A.; Lonardi, S.; et al. Predictive role of microRNA-related genetic polymorphisms in the pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer patients. Oncotarget 2016, 7, 19781–19793.
- Kazandjian, D.; Blumenthal, G.M.; Yuan, W.; He, K.; Keegan, P.; Pazdur, R. FDA Approval of Gefitinib for the Treatment of Patients with Metastatic EGFR Mutation-Positive Non-Small Cell Lung Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 1307–1312.
- Solassol, I.; Pinguet, F.; Quantin, X. FDA- and EMA-Approved Tyrosine Kinase Inhibitors in Advanced EGFR-Mutated Non-Small Cell Lung Cancer: Safety, Tolerability, Plasma Concentration Monitoring, and Management. Biomolecules 2019, 9, 668.
- Greig, S.L. Osimertinib: First Global Approval. Drugs 2016, 76, 263–273.
- Lamb, Y.N.; Dhillon, S. Epi proColon® 2.0 CE: A Blood-Based Screening Test for Colorectal Cancer. Mol. Diagn. Ther. 2017, 21, 225–232.
- Riethdorf, S.; O’Flaherty, L.; Hille, C.; Pantel, K. Clinical applications of the CellSearch platform in cancer patients. Adv. Drug Deliv. Rev. 2018, 125, 102–121.




