
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dariusz Rakus | + 1860 word(s) | 1860 | 2020-09-25 11:49:57 | | | |
| 2 | Rita Xu | -450 word(s) | 1410 | 2020-09-29 11:47:18 | | |
Video Upload Options
Astrocyte-neuron crosstalk is a phenomenon in which both of those cell types depend on each other and support their development, genes expression, metabolism, excitability and plasticity. Astrocyte–neuron crosstalk incontrovertibly plays a crucial role in shaping neuronal metabolism. It has been shown that it substantially affects the expression of basal metabolic enzymes in both types of cells, by essentially unknown factor(s) which are released to extracellular space directly and using extracellular vesicles-packed molecules and by cell-to-cell contacts. Additionally, astrocytes support neurons with lactate, which (when secreted during enhanced neuronal activity events) stimulates a formation and maintenece of long-term plastycity phenomena in neurons.
1. Introduction
Fbp2 (muscle isozyme of fructose 1,6-bisphosphatase) is a ubiquitously expressed enzyme of glycogen synthesis from non-carbohydrates, e.g., lactate (glyconeogenesis). However, it plays also a variety of non-enzymatic functions. Fbp2 is involved in the regulation of hypoxia-inducible factor 1α (Hif1α) stability, cell cycle-dependent events, biogenesis of mitochondria, and protection of the organelles against high reactive oxygen species (ROS)- and high Ca2+-induced stress [1][2][3][4]. This diversity of functions results from the ability of Fbp2 to adopt different oligomeric forms in solution, from monomeric to octameric. In a cell, Fbp2 exists as the mixture of mainly dimers and tetramers. Only the dimeric form of Fbp2 interacts with mitochondria, and only its tetrameric form is retained in the nucleus. The ratio of these oligomeric forms depends on the presence of physiological inhibitors of Fbp2 enzymatic activity — AMP and NAD+, which also tetramerize the protein [5].
Recently, we have demonstrated that the presence of Fbp2, presumably its dimeric form, in hippocampal neurons is indispensable for N-methyl-D-aspartate receptor (NMDAR)-dependent long-term potentiation (LTP) formation in the hippocampus [6]. LTP, the most studied example of synaptic plasticity, is regarded as the neuronal basis of learning [7]. However, the precise mechanism underlying the persistent synaptic enhancement is not fully understood. It is, for example, widely accepted that astrocytic glycogen degradation followed by lactate transport to neurons (in the process called astrocyte–neuron lactate shuttle) is indispensable for LTP formation [8], but how the astrocytic glycogen-derived lactate stimulates LTP is essentially unknown. Lactate influences the redox state of neurons reducing the NAD+/NADH ratio, and it has been shown that such a reduction (or, in other words, elevation of the NADH/NAD+ ratio) stimulates activity of NMDAR by modifying the neuronal redox state [9]. However, this also leads to reduction of the pool of tetrameric Fbp2 and increment of the fraction of dimeric Fbp2 in neurons. Dimeric Fbp2, in turn, can interact with neuronal calcium/calmodulin-dependent protein kinase 2 (Camk2), which correlates with Camk2 autophosphorylation. Silencing of Fbp2 expression or simultaneous inhibition and tetramerization of the enzyme in neurons abolishes Camk2 autoactivation and blocks formation of the early phase of LTP and expression of the late-phase LTP markers [6]. We have thus hypothesized that the NAD+ level-dependent change in the neuronal Fbp2 dimer/tetramer ratio might be a crucial mechanism by which astrocyte–neuron lactate shuttle regulates LTP formation, making Fbp2 a hub linking neuronal signaling with redox and/or energetic state of brain during the formation of memory traces [6].
Astrocyte–neuron crosstalk incontrovertibly plays a crucial role in shaping neuronal metabolism. It has been shown that it substantially affects the expression of basal metabolic enzymes in both types of cells, by essentially unknown factor(s) released to extracellular space and by cell-to-cell contacts [10]. Although it has been demonstrated that in astrocytes, the expression of regulatory enzymes of glycolysis (phosphofructokinase P and pyruvate kinase M1/M2 isoforms) is stimulated by neuron-derived transthyretin [11], mechanisms regulating the levels of other glucose metabolism enzymes in astrocytes and neurons remain uncharted.
2. Fbp2 Expression in Hippocampal Cells Depends on Cell Culture Conditions
To explore the potential regulators of Fbp2 expression in hippocampal astrocytes and neurons, we used murine primary cell cultures and immunofluorescent techniques.
We found that the 48 h co-culture of these two cell types led to over 40% reduction of the Fbp2-related fluorescent signal in astrocytes and, simultaneously, to about 50% increase in the signal in neurons, as compared to monocultures of these cells (Figure 1).
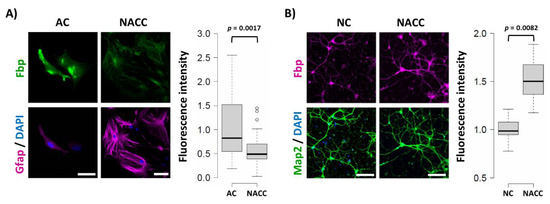
Figure 1. Co-culture-induced changes in the muscle isozyme of fructose 1,6-bisphosphatase (Fbp2) protein-related fluorescence in astrocytes and neurons. (A) Co-culturing of neurons and astrocytes (NACC) reduces the Fbp2 protein level in astrocytes (identified by glial fibrillary acidic protein (Gfap) staining) but (B) increases it in neurons (identified by microtubule-associated protein 2 (Map2) staining), compared to monocultures of these cells (AC—astrocytic culture; NC—neuronal culture). Box plots present the quantification of the Fbp-related fluorescent signal normalized to control group. Each experiment was repeated at least in triplicate. Bar = 40 µm in (A) and 80 µm in (B).
3. Fbp2 Activity and Protein Level Reduction Is Not Reflected by mRNA Changes
Surprisingly, the observed changes in the Fbp2 protein level in astrocytes did not reflect the changes in mRNA quantity, after 48 h of the co-culturing of astrocytes with neurons (Figure 2). Moreover, in neurons co-cultured with astrocytes, the mRNA level for Fbp2 was even lower (~20%) than in neurons from monocultures (Figure 2). This suggests that the reciprocal regulation of Fbp2 level in both types of cells is not related to changes in the expression of the protein but relies on modifications of its stability.
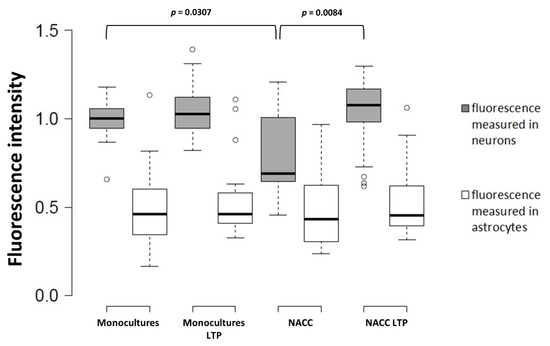
Figure 2. Quantification of the changes in the Fbp2 mRNA level in neuron–astrocyte co-culture under control conditions and after induction of long-term potentiation (LTP). NACC—neuron–astrocyte co-culture, control conditions; NACC LTP—neuron–astrocyte co-culture after induction of LTP. Fluorescence intensity was normalized to control group. The experiment was repeated in triplicate.
Both neuronal Fbp2 and astrocytes are indispensable parts of neuronal plasticity machinery (LTP) [6][8]; thus, we asked if LTP induction affects the expression of mRNA for Fbp2 in neurons and astrocytes. We found that, in the co-culture, the chemical induction of LTP significantly elevated mRNA for Fbp2 in neurons, but not in astrocytes (Figure 2).
This implies that different mechanisms are responsible for the regulation of Fbp2 levels in these two cell types, under resting conditions and during memory formation. While astrocytes in the co-cultures kept under resting conditions (i.e., without LTP induction) appear to slightly reduce the neuronal amount of mRNA for Fbp2 but increase the stability of the protein, the induction of LTP elevates neuronal expression of mRNA for the enzyme. Under the same resting conditions, neurons decrease the amount of Fbp2 protein in astrocytes by a mechanism independent of mRNA expression, but the induction of synaptic plasticity has no further effect on the Fbp2 level in these cells.
4. Reduction of Fbp2 Expression Is Correlated with Decrease in the Proliferative Potential of Astrocytes
We have shown that, in some cell types, Fbp2 is engaged in cell cycle regulation [10]. Thus, we tested the effect of co-culturing on the proliferative potential of astrocytes. It appeared that such a treatment resulted in a significant reduction of Ki-67-related fluorescence in astrocytes as compared to astrocytic monocultures (Figure 3). This was in line with the results of previous experiments on the Ki-67 expression in astrocytes [10][12]. Additionally, we observed changes in the astrocytic morphology from round-shaped to star-shaped, process-bearing (Figure 3), which is in agreement with results of previous studies (our own and other groups) [10][13] showing that neurons affect the morphology of these glial cells as soon as after 24 h of their co-culture.
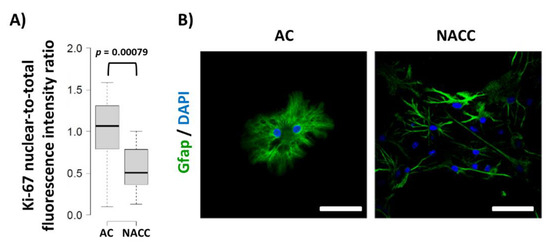
Figure 3. Changes in Ki-67 protein-related nuclear-to-total fluorescence ratio and morphology of astrocytes under different culture conditions. (A) Quantification of changes in Ki-67 protein-related fluorescence normalized to control group. (B) Morphology of astrocytes stained with anti-Gfap antibodies. DAPI counterstains nuclei. Bar = 50 µm. AC—astrocytic monoculture; NACC—neuron–astrocyte co-culture. The experiment was repeated in triplicate.
It has been suggested that proliferation and differentiation of astrocytes are regulated by the c-Fos protein [14]. c-Fos can heterodimerize with c-Jun to form the transcription factor activator protein 1 (AP-1) and control the expression of target genes involved in adaptive responses in the nervous system [15]. Thus, we checked the level of nuclear c-Fos protein in astrocytes, and observed that 48 h of their co-culture with neurons triggered a marked reduction of c-Fos-related fluorescence in this compartment (Figure 4). A similar picture of postnatal maturation-related proliferative potential reduction correlating with Fbp2 expression decrease was recently observed after the addition of cardiac fibroblasts to a monoculture of cardiomyocytes [16].
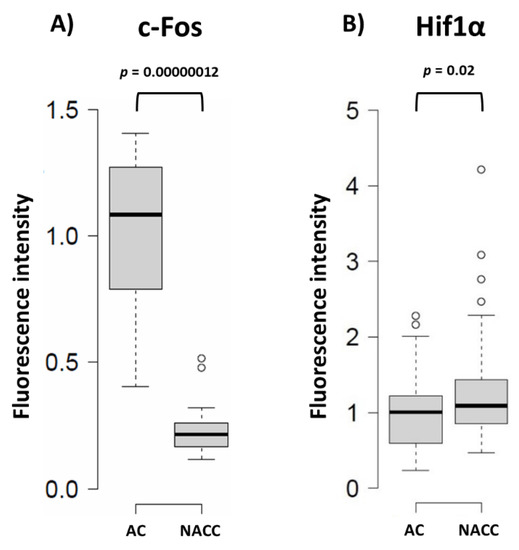
Figure 4. Quantification of the changes in the c-Fos and Hif1α protein-related signal in astrocytes under different conditions. (A) Neuron–astrocyte co-culture (NACC) reduces c-Fos-related fluorescence in astrocytes compared to astrocytic monoculture (AC); (B) Neuron–astrocyte co-culture increases Hif1α-related fluorescence in astrocytes compared to AC. Fluorescence intensity was normalized to control group.
References
- Duda, P.; Janczara, J.; McCubrey, J.A.; Gizak, A.; Rakus, D. The Reverse Warburg Effect Is Associated with Fbp2-Dependent Hif1α Regulation in Cancer Cells Stimulated by Fibroblasts. Cells 2020, 9, 205, doi:10.3390/cells9010205.
- Mamczur, P.; Sok, A.J.; Rzechonek, A.; Rakus, D. Cell cycle-dependent expression and subcellular localization of fructose 1,6-bisphosphatase. Cell Biol. 2012, 137, 121–136, doi:10.1007/s00418-011-0884-1.
- Huangyang, P.; Li, F.; Lee, P.; Nissim, I.; Weljie, A.M.; Mancuso, A.; Li, B.; Keith, B.; Yoon, S.S.; Celeste Simon, M. Fructose-1,6-Bisphosphatase 2 Inhibits Sarcoma Progression by Restraining Mitochondrial Biogenesis. Cell Metab. 2020, 31, 174–188, doi:10.1016/j.cmet.2019.10.012.
- Gizak, A.; Pirog, M.; Rakus, D. Muscle FBPase binds to cardiomyocyte mitochondria under glycogen synthase kinase-3 inhibition or elevation of cellular Ca 2+ level. FEBS Lett. 2012, 586, 13–19, doi:10.1016/j.febslet.2011.11.032.
- Wisniewski, J.; Piróg, M.; Holubowicz, R.; Dobryszycki, P.; McCubrey, J.A.; Rakus, D.; Gizak, A. Dimeric and tetrameric forms of muscle fructose-1,6- bisphosphatase play different roles in the cell. Oncotarget 2017, 8, 115420–115433, doi:10.18632/oncotarget.23271.
- Duda, P.; Wójtowicz, T.; Janczara, J.; Krowarsch, D.; Czyrek, A.; Gizak, A.; Rakus, D. Fructose 1,6-Bisphosphatase 2 Plays a Crucial Role in the Induction and Maintenance of Long-Term Potentiation. Cells 2020, 9, 1375, doi:10.3390/cells9061375.
- Bliss, T.V.P.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39, doi:10.1038/361031a0.
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 2011, 144, 810–823, doi:10.1016/j.cell.2011.02.018.
- Yang, J.; Ruchti, E.; Petit, J.-M.; Jourdain, P.; Grenningloh, G.; Allaman, I.; Magistretti, P.J. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Natl. Acad. Sci. USA 2014, 111, 12228–12233, doi:10.1073/pnas.1322912111.
- Mamczur, P.; Borsuk, B.; Paszko, J.; Sas, Z.; Mozrzymas, J.; Wiśniewski, J.R.; Gizak, A.; Rakus, D. Astrocyte-neuron crosstalk regulates the expression and subcellular localization of carbohydrate metabolism enzymes. Glia 2015, 63, 328–340, doi:10.1002/glia.22753.
- Zawişlak, A.; Jakimowicz, P.; McCubrey, J.A.; Rakus, D. Neuron-derived transthyretin modulates astrocytic glycolysis in hormone-independent manner. Oncotarget 2017, 8, 106625–106638, doi:10.18632/oncotarget.22542.
- Hatten, M.E. Neuronal inhibition of astroglial cell proliferation is membrane mediated. Cell Biol. 1987, 104, 1353–1360, doi:10.1083/jcb.104.5.1353.
- Matsutani, S.; Yamamoto, N. Neuronal regulation of astrocyte morphology in vitro is mediated by GABAergic signaling. Glia 1997, 20, 1–9, doi:10.1002/(SICI)1098-1136(199705)20:1<1::AID-GLIA1>3.0.CO;2-E.
- Hisanaga, K.; Sagar, S.M.; Hicks, K.J.; Swanson, R.A.; Sharp, F.R. c-fos proto-oncogene expression in astrocytes associated with differentiation or proliferation but not depolarization. Brain Res. 1990, 8, 69–75, doi:10.1016/0169-328X(90)90011-2.
- Sheng, M.; Greenberg, M.E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 1990, 4, 477–485, doi:10.1016/0896-6273(90)90106-p.
- Gizak, A.; Mccubrey, J.A.; Rakus, D. Cell-to-cell lactate shuttle operates in heart and is important in age-related heart failure. Aging (Albany NY). 2020, 12, 3388–3406, doi:10.18632/aging.102818.




