
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ryszard Grenda | -- | 1526 | 2022-05-01 14:06:41 | | | |
| 2 | Beatrix Zheng | Meta information modification | 1526 | 2022-05-05 05:26:41 | | |
Video Upload Options
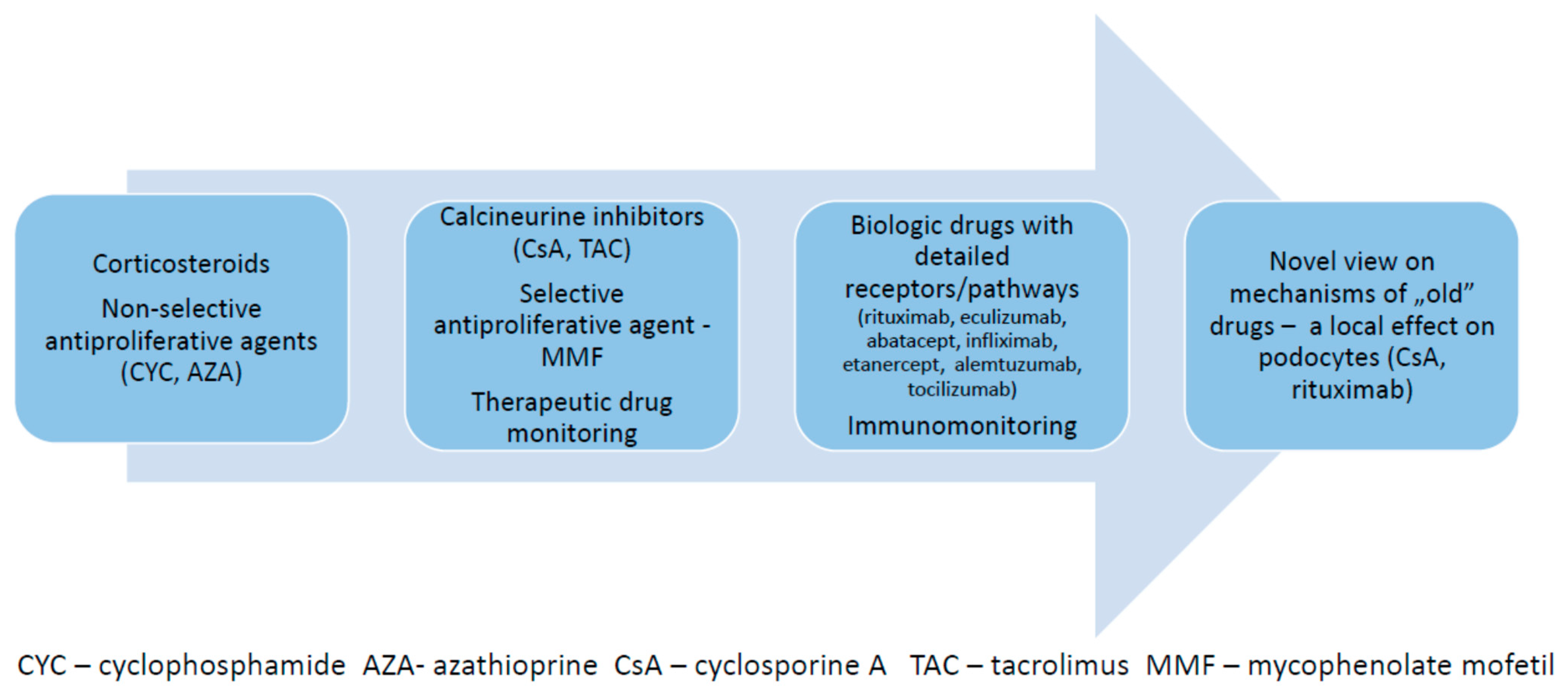
Therapy of immune-mediated kidney diseases has evolved during recent decades from the non-specific use of corticosteroids and antiproliferative agents (like cyclophosphamide or azathioprine), towards the use of more specific drugs with measurable pharmacokinetics, like calcineurin inhibitors (cyclosporine A and tacrolimus) and mycophenolate mofetil, to the treatment with biologic drugs targeting detailed specific receptors, like rituximab, eculizumab or abatacept. Moreover, the data coming from a molecular science revealed that several drugs, which have been previously used exclusively to modify the upregulated adaptive immune system, may also exert a local effect on the kidney microstructure and ameliorate the functional instability of podocytes, reducing the leak of protein into the urinary space. The innate immune system also became a target of new therapies, as its specific role in different kidney diseases has been de novo defined. Current therapy of several immune kidney diseases may now be personalized, based on the detailed diagnostic procedures, including molecular tests. However, in most cases there is still a space for standard therapies based on variable protocols including usage of steroids with the steroid-sparing agents. They are used as a first-line treatment, while modern biologic agents are selected as further steps in cases of lack of the efficacy or toxicity of the basic therapies. In several clinical settings, the biologic drugs are effective as the add-on therapy.
1. Introduction
2. Biologic Drugs in Pediatric Systemic Vasculitis
3. Biologic Drugs in Systemic Lupus Erythematosus with Renal Involvement
4. Summary
-
Upregulation of the innate and/or adaptive immune system leads to the development of a variety of immune-mediated kidney diseases in children.
-
There is an ongoing progress in pharmacotherapy of immune kidney diseases, based on scientific knowledge, which defines detailed, variable underlying disease-related mechanisms.
-
The adaptive immune system is a target of steroids, antiproliferative drugs, calcineurin inhibitors and several receptor-specific biologic agents.
-
The innate immune system is a target of specific monoclonal antibodies.
-
Surveillance of the current therapies is based on therapeutic drug monitoring and/or immunomonitoring.
-
Apart from the effect on the immune system, specific drugs (calcineurin inhibitors, rituximab, abatacept) also exert a local effect on the microstructure of the podocyte cytoskeleton, which may be clinically relevant in selected cases.
References
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13.
- Yoshikawa, N.; Nakanishi, K.; Sako, M.; Oba, M.S.; Mori, R.; Ota, E.; Ishikura, K.; Hataya, H.; Honda, M.; Ito, S.; et al. A multicenter randomized trial indicates initial prednisolone treatment for childhood nephrotic syndrome for two months is not inferior to six-month treatment. Kidney Int. 2015, 87, 225–232.
- Lombel, R.M.; Gipson, D.S.; Hodson, E.M. Treatment of steroid-sensitive nephrotic syndrome: New guidelines from KDIGO. Pediatric Nephrol. 2012, 28, 415–426.
- Haun, M.W.; Estel, S.; Rucker, G.; Friederich, H.C.; Villalobos, M.; Thomas, M.; Hartmann, M. Early palliative care for adults with advanced cancer. Cochrane Database Syst. Rev. 2017, 6, CD011129.
- Yoshikawa, N.; Honda, M.; Iijima, K.; Awazu, M.; Hattori, S.; Nakanishi, K.; Ito, H. Steroid Treatment for Severe Childhood IgA Nephropathy: A Randomized, Controlled Trial. Clin. J. Am. Soc. Nephrol. 2006, 1, 511–517.
- Moore, M.J. Clinical Pharmacokinetics of Cyclophosphamide. Clin. Pharmacokinet. 1991, 20, 194–208.
- Tejani, A.; Phadke, K.; Nicastri, A.; Adamson, O.; Chen, C.; Trachtman, H.; Tejani, C. Efficacy of Cyclophosphamide in Steroid-Sensitive Childhood Nephrotic Syndrome with Different Morphological Lesions. Nephron Exp. Nephrol. 1985, 41, 170–173.
- Zagury, A.; de Oliveira, A.L.; de Moraes, C.A.P.; Montalvão, J.A.D.A.; Novaes, R.H.L.L.; de Sá, V.M.; Carvalho, D.D.B.M.D.; Matuck, T. Long-term follow-up after cyclophosphamide therapy in steroid-dependent nephrotic syndrome. Pediatric Nephrol. 2011, 26, 915–920.
- Kamei, K.; Nakanishi, K.; Ito, S.; Saito, M.; Sako, M.; Ishikura, K.; Hataya, H.; Honda, M.; Iijima, K.; Yoshikawa, N.; et al. Long-Term Results of a Randomized Controlled Trial in Childhood IgA Nephropathy. Clin. J. Am. Soc. Nephrol. 2011, 6, 1301–1307.
- Mircheva, J.; Legendre, C.; Soria-Royer, C.; Thervet, E.; Beaune, P.; Kreis, H. Monitoring of azathioprine-induced immunosuppression with thiopurine methyltransferase activity in kidney transplant recipients. Transplantation 1995, 60, 639–642.
- Plank, C.; Nephrologie, F.A.F.P.; Kalb, V.; Hinkes, B.; Hildebrandt, F.; Gefeller, O.; Rascher, W. Cyclosporin A is superior to cyclophosphamide in children with steroid-resistant nephrotic syndrome—A randomized controlled multicentre trial by the Arbeitsgemeinschaft für Pädiatrische Nephrologie. Pediatric Nephrol. 2008, 23, 1483–1493.
- Kemper, M.J.; Kuwertz-Broeking, E.; Bulla, M.; Mueller-Wiefel, D.E.; Neuhaus, T.J. Recurrence of severe steroid dependency in cyclosporin A-treated childhood idiopathic nephrotic syndrome. Nephrol. Dial. Transplant. 2004, 19, 1136–1141.
- Choudhry, S.; Bagga, A.; Hari, P.; Sharma, S.; Kalaivani, M.; Dinda, A. Efficacy and Safety of Tacrolimus Versus Cyclosporine in Children with Steroid-Resistant Nephrotic Syndrome: A Randomized Controlled Trial. Am. J. Kidney Dis. 2009, 53, 760–769.
- Jahan, A.; Prabha, R.; Chaturvedi, S.; Mathew, B.; Fleming, D.; Agarwal, I. Clinical efficacy and pharmacokinetics of tacrolimus in children with steroid-resistant nephrotic syndrome. Pediatric Nephrol. 2015, 30, 1961–1967.
- Yang, E.M.; Lee, S.T.; Choi, H.J.; Cho, H.Y.; Lee, J.H.; Kang, H.G.; Park, Y.S.; Cheong, H.I.; Ha, I.-S. Tacrolimus for children with refractory nephrotic syndrome: A one-year prospective, multicenter, and open-label study of Tacrobell®, a generic formula. World J. Pediatric 2015, 12, 60–65.
- Dorresteijn, E.M.; Holthe, J.E.K.-V.; Levtchenko, E.N.; Nauta, J.; Hop, W.C.J.; van der Heijden, A.J. Mycophenolate mofetil versus cyclosporine for remission maintenance in nephrotic syndrome. Pediatric Nephrol. 2008, 23, 2013–2020.
- Gellermann, J.; Weber, L.; Pape, L.; Tönshoff, B.; Hoyer, P.; Querfeld, U. Mycophenolate Mofetil versus Cyclosporin A in Children with Frequently Relapsing Nephrotic Syndrome. J. Am. Soc. Nephrol. 2013, 24, 1689–1697.
- Kemper, M.J.; Valentin, L.; Van Husen, M. Difficult-to-treat idiopathic nephrotic syndrome: Established drugs, open questions and future options. Pediatric Nephrol. 2017, 33, 1641–1649.
- Purohit, S.; Piani, F.; Ordoñez, F.A.; de Lucas-Collantes, C.; Bauer, C.; Cara-Fuentes, G. Molecular Mechanisms of Proteinuria in Minimal Change Disease. Front. Med. 2021, 8, 761600.
- Iijima, K.; Sako, M.; Kamei, K.; Nozu, K. Rituximab in steroid-sensitive nephrotic syndrome: Lessons from clinical trials. Pediatric Nephrol. 2017, 33, 1449–1455.
- Sinha, R.; Agrawal, N.; Xue, Y.; Chanchlani, R.; Pradhan, S.; Raina, R.; Marks, S.D. Use of rituximab in paediatric nephrology. Arch. Dis. Child. 2021, 106, 1058–1065.
- Salvadori, M.; Tsalouchos, A. How immunosuppressive drugs may directly target podocytes in glomerular diseases. Pediatric Nephrol. 2021, 1–11.
- Fornoni, A.; Sageshima, J.; Wei, C.; Merscher-Gomez, S.; Aguillon-Prada, R.; Jauregui, A.N.; Li, J.; Mattiazzi, A.; Ciancio, G.; Chen, L.; et al. Rituximab Targets Podocytes in Recurrent Focal Segmental Glomerulosclerosis. Sci. Transl. Med. 2011, 3, 85ra46.
- Mastrangelo, A.; Serafinelli, J.; Giani, M.; Montini, G. Clinical and Pathophysiological Insights into Immunological Mediated Glomerular Diseases in Childhood. Front. Pediatric 2020, 8, 205.
- Akamine, K.; Punaro, M. Biologics for childhood systemic vasculitis. Pediatric Nephrol. 2018, 34, 2295–2309.
- Brogan, P.; Yeung, R.S.M.; Cleary, G.; Rangaraj, S.; Kasapcopur, O.; Hersh, A.O.; Li, S.; Paripovic, D.; Schikler, K.; Zeft, A.; et al. Phase IIa Global Study Evaluating Rituximab for the Treatment of Pediatric Patients with Granulomatosis with Polyangiitis or Microscopic Polyangiitis. Arthritis Rheumatol. 2021, 74, 124–133.
- Null, N. Etanercept plus Standard Therapy for Wegener’s Granulomatosis. N. Engl. J. Med. 2005, 352, 351–361.
- Langford, C.A.; Monach, P.A.; Specks, U.; Seo, P.; Cuthbertson, D.; McAlear, C.A.; Ytterberg, S.R.; Hoffman, G.S.; Krischer, J.P.; Merkel, P.A.; et al. An open-label trial of abatacept (CTLA4-IG) in non-severe relapsing granulomatosis with polyangiitis (Wegener’s). Ann. Rheum. Dis. 2014, 73, 1376–1379.
- Gopaluni, S.; Smith, R.; Goymer, D.; Cahill, H.; Broadhurst, E.; Wallin, E.; McClure, M.; Chaudhry, A.; Jayne, D. Alemtuzumab for refractory primary systemic vasculitis—A randomised controlled dose ranging clinical trial of efficacy and safety (ALEVIATE). Arthritis Res. Ther. 2022, 24, 81.
- Merrill, J.T.; Shanahan, W.R.; Scheinberg, M.; Kalunian, K.C.; Wofsy, D.; Martin, R.S. Phase III trial results with blisibimod, a selective inhibitor of B-cell activating factor, in subjects with systemic lupus erythematosus (SLE): Results from a randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2018, 77, 883–889.
- Brunner, H.I.; Abud-Mendoza, C.; Mori, M.; Pilkington, C.A.; Syed, R.; Takei, S.; Viola, D.O.; Furie, R.A.; Navarra, S.; Zhang, F.; et al. Efficacy and safety of belimumab in paediatric and adult patients with systemic lupus erythematosus: An across-study comparison. RMD Open 2021, 7, e001747.
- Wallace, D.J.; Isenberg, D.A.; Morand, E.F.; Vazquez–Mateo, C.; Kao, A.H.; Aydemir, A.; Pudota, K.; Ona, V.; Aranow, C.; Merrill, J.T. Safety and clinical activity of atacicept in the long-term extension of the phase 2b ADDRESS II study in systemic lupus erythematosus. Rheumatology 2021, 60, 5379–5389.
- Mundel, P.; Greka, A. Developing therapeutic ‘arrows’ with the precision of William Tell. Curr. Opin. Nephrol. Hypertens. 2015, 24, 388–392.




