
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ryota Nakano | -- | 2140 | 2022-04-30 04:51:02 | | | |
| 2 | Peter Tang | Meta information modification | 2140 | 2022-05-05 11:21:44 | | |
Video Upload Options
The indications for immune checkpoint inhibitors (ICIs) have expanded to include carcinomas of various organs. ICIs include drugs that target programmed cell death-1 (PD-1), programmed cell death ligand 1 (PDL-1), and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). The indications for these drugs have been expanded to include many types of cancer, as efficacies have been reported for malignant melanoma and lung, kidney, head and neck, stomach, liver, ovarian, and pancreatic cancers .
1. Introduction
2. Incidence
|
No. |
Ref. |
Sex |
Age |
ICI |
CT Findings |
MRI Findings |
EUS Findings |
ERCP Findings |
Imaging Type |
|---|---|---|---|---|---|---|---|---|---|
|
1 |
Ofuji et al. [13] |
M |
82 |
pembrolizumab |
diffuse enlargement |
diffuse restricted diffusion diffuse enlargement narrowing of the MPD |
hypoechoic enlargement hyperechoic spots |
NA |
autoimmune pancreatitis |
|
2 |
Dehghani et al. [14] |
M |
63 |
nivolumab |
focal enlargement fat stranding |
focal restricted diffusion late enhancement |
NA |
NA |
autoimmune pancreatitis |
|
3 |
Das et al. [15] |
M |
47 |
nivolumab |
diffuse enlargement diffuse fat stranding |
NA |
NA |
NA |
acute interstitial pancreatitis |
|
4 |
Das et al. [15] |
F |
70 |
nivolumab |
focal enlargement subtle fat stranding |
NA |
NA |
NA |
acute interstitial pancreatitis |
|
5 |
Das et al. [15] |
F |
50 |
pembrolizumab |
NA |
focal enlargement abrupt cut-off of the CBD |
NA |
NA |
autoimmune pancreatitis |
|
6 |
Das et al. [15] |
F |
64 |
nivolumab |
diffuse enlargement heterogenous enhancement fat stranding |
NA |
NA |
NA |
acute interstitial pancreatitis |
|
7 |
Das et al. [15] |
F |
56 |
ipilimumab nivolumab |
NA |
NA |
NA |
NA |
autoimmune pancreatitis |
|
8 |
Capurso et al. [16] |
F |
76 |
pembrolizumab |
MPD dilation |
MPD dilation focal restricted diffusion |
hypoechoic solid lesion stiff at elastography stenosis of the MPD |
NA |
autoimmune pancreatitis |
|
9 |
Saito et al. [17] |
M |
72 |
nivolumab |
diffuse enlargement |
NA |
NA |
NA |
acute interstitial pancreatitis |
|
10 |
Kakuwa et al. [18] |
M |
70 |
pembrolizumab |
mild diffuse enlargement MPD dilation |
NA |
NA |
NA |
autoimmune pancreatitis |
|
11 |
Tanaka et al. [19] |
F |
70 |
nivolumab |
NA |
diffuse enlargement focal restricted diffusion |
diffuse hypoechoic enlargement |
skipped narrowing of the MPD |
autoimmune pancreatitis |
Clinical characteristics and imaging findings of ICI-related pancreatitis cases in which radiographic and endoscopic images are available. Abbreviations: Ref., reference; ICI, immune checkpoint inhibitor; CT, computed tomography; MRI, magnetic resonance imaging; EUS, endoscopic ultrasonography; ERCP, endoscopic retrograde cholangiopancreatography; PET-CT, positron emission tomography combined with computed tomography; MPD, main pancreatic duct; CBD, common bile duct; NA, not available.
3. Diagnosis
3.1. Clinical Symptoms
3.2. Blood Examination
3.3. Radiology Images
4. Endoscopic Findings
4.1. EUS
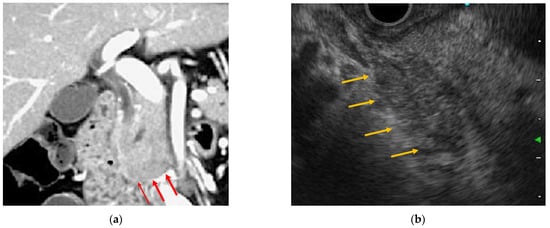
4.2. ERCP
4.3. Histopathological Findings
5. Management
References
- Michot, J.-M.; Ragou, P.; Carbonnel, F.; Champiat, S.; Voisin, A.-L.; Mateus, C.; Lambotte, O.; Annereau, M. Significance of Immune-related Lipase Increase Induced by Antiprogrammed Death-1 or Death Ligand-1 Antibodies: A Brief Communication. J. Immunother. 2018, 41, 84–85.
- Naidoo, J.; Page, D.B.; Li, B.T.; Connell, L.C.; Schindler, K.; Lacouture, M.E.; Postow, M.A.; Wolchok, J.D. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 2015, 26, 2375–2391.
- Postow, M.A. Managing Immune Checkpoint-Blocking Antibody Side Effects. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, 76–83.
- Kumar, V.; Chaudhary, N.; Garg, M.; Floudas, C.S.; Soni, P.; Chandra, A.B. Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front. Pharmacol. 2017, 8, 49.
- Hofmann, L.; Forschner, A.; Loquai, C.; Goldinger, S.M.; Zimmer, L.; Ugurel, S.; Schmidgen, M.I.; Gutzmer, R.; Utikal, J.; Göppner, D.; et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer 2016, 60, 190–209.
- Cramer, P.; Bresalier, R.S. Gastrointestinal and Hepatic Complications of Immune Checkpoint Inhibitors. Curr. Gastroenterol. Rep. 2017, 19, 3.
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D.; et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 230–241.
- Tanaka, T.; Sakai, A.; Shiomi, H.; Masuda, A.; Kobayashi, T.; Tanaka, S.; Nakano, R.; Shigeoka, M.; Koma, Y.-I.; Kodama, Y. An autopsy case of severe acute pancreatitis induced by administration of pazopanib following nivolumab. Pancreatology 2021, 21, 21–24.
- Tirumani, S.H.; Ramaiya, N.H.; Keraliya, A.R.; Bailey, N.D.; Ott, P.A.; Hodi, F.S.; Nishino, M. Radiographic Profiling of Immune-Related Adverse Events in Advanced Melanoma Patients Treated with Ipilimumab. Cancer Immunol. Res. 2015, 3, 1185–1192.
- Friedman, C.F.; Clark, V.; Raikhel, A.V.; Barz, T.; Shoushtari, A.N.; Momtaz, P.; Callahan, M.K.; Wolchok, J.D.; Chapman, P.B.; Hellmann, M.D.; et al. Thinking Critically About Classifying Adverse Events: Incidence of Pancreatitis in Patients Treated With Nivolumab + Ipilimumab. JNCI J. Natl. Cancer Inst. 2016, 109, djw260.
- Clamon, G.; Patel, R.; Mott, S. Pancreatitis associated with newer classes of antineoplastic therapies. J. Community Support. Oncol. 2017, 15, e135–e141.
- George, J.; Bajaj, D.; Sankaramangalam, K.; Yoo, J.W.; Joshi, N.; Gettinger, S.; Price, C.; Farrell, J.J. Incidence of pancreatitis with the use of immune checkpoint inhibitors (ICI) in advanced cancers: A systematic review and meta-analysis. Pancreatol. 2019, 19, 587–594.
- Ofuji, K.; Hiramatsu, K.; Nosaka, T.; Naito, T.; Takahashi, K.; Matsuda, H.; Ohtani, M.; Imamura, Y.; Ishizuka, T.; Nakamoto, Y. Pembrolizumab-induced autoimmune side effects of colon and pancreas in a patient with lung cancer. Clin. J. Gastroenterol. 2021, 14, 1692–1699.
- Dehghani, L.; Mikail, N.; Kramkimel, N.; Soyer, P.; Lebtahi, R.; Mallone, R.; Larger, E. Autoimmune pancreatitis after nivolumab anti–programmed death receptor-1 treatment. Eur. J. Cancer 2018, 104, 243–246.
- Das, J.P.; Postow, M.A.; Friedman, C.F.; Do, R.K.; Halpenny, D.F. Imaging findings of immune checkpoint inhibitor associated pancreatitis. Eur. J. Radiol. 2020, 131, 109250.
- Capurso, G.; Archibugi, L.; Tessieri, L.; Petrone, M.C.; Laghi, A.; Arcidiacono, P.G. Focal immune-related pancreatitis occurring after treatment with programmed cell death 1 inhibitors: A distinct form of autoimmune pancreatitis? Eur. J. Cancer 2018, 95, 123–126.
- Saito, H.; Ono, K. Nivolumab-induced Pancreatitis: An Immune-related Adverse Event. Radiol. 2019, 293, 521.
- Kakuwa, T.; Hashimoto, M.; Izumi, A.; Naka, G.; Takeda, Y.; Sugiyama, H. Pembrolizumab-related pancreatitis with elevation of pancreatic tumour markers. Respirol. Case Rep. 2020, 8, e00525.
- Tanaka, T.; Sakai, A.; Kobayashi, T.; Masuda, A.; Shiomi, H.; Kodama, Y. Nivolumab-related pancreatitis with autoimmune pancreatitis-like imaging features. J. Gastroenterol. Hepatol. 2019, 34, 1274.
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111.
- Di Giacomo, A.M.; Danielli, R.; Guidoboni, M.; Calabrò, L.; Carlucci, D.; Miracco, C.; Volterrani, L.; Mazzei, M.A.; Biagioli, M.; Altomonte, M.; et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: Clinical and immunological evidence from three patient cases. Cancer Immunol. Immunother. 2009, 58, 1297–1306.
- Abu-Sbeih, H.; Tang, T.; Lu, Y.; Thirumurthi, S.; Altan, M.; Jazaeri, A.A.; Dadu, R.; Coronel, E.; Wang, Y. Clinical characteristics and outcomes of immune checkpoint inhibitor-induced pancreatic injury. J. Immunother. Cancer 2019, 7, 31.
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384.
- Vissers, R.J.; Abu-Laban, R.B.; McHugh, D.F. Amylase and lipase in the emergency department evaluation of acute pancreatitis. J. Emerg. Med. 1999, 17, 1027–1037.
- Janssens, L.; Takahashi, N.; Majumder, S. Pancreatic Atrophy in Nivolumab-Associated Pancreatitis Mimics Autoimmune Pancreatitis. Pancreas 2021, 50, e28–e29.
- Song, Z.; Shih, J.; Seid, D.S. Rare Case of Nivolumab-Induced Chronic Pancreatitis. Clin. Gastroenterol. Hepatol. 2021.
- Sugumar, A.; Levy, M.J.; Kamisawa, T.; Webster, G.J.; Kim, M.; Enders, F.; Amin, Z.; Baron, T.H.; Chapman, M.H.; I Church, N.; et al. Endoscopic retrograde pancreatography criteria to diagnose autoimmune pancreatitis: An international multicentre study. Gut 2011, 60, 666–670.
- Wakabayashi, T.; Kawaura, Y.; Satomura, Y.; Watanabe, H.; Motoo, Y.; Okai, T.; Sawabu, N. Clinical and imaging features of autoimmune pancreatitis with focal pancreatic swelling or mass formation: Comparison with so-called tumor-forming pancreatitis and pancreatic carcinoma. Am. J. Gastroenterol. 2003, 98, 2679–2687.
- Suda, T.; Kobayashi, M.; Kurokawa, K.; Matsushita, E. Simultaneous occurrence of autoimmune pancreatitis and sclerosing cholangitis as immune-related adverse events of pembrolizumab. BMJ Case Rep. 2021, 14, e243360.




