Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Susana Llivisaca | -- | 2879 | 2022-04-30 03:47:01 | | | |
| 2 | Catherine Yang | Meta information modification | 2879 | 2022-05-05 04:35:03 | | | | |
| 3 | Catherine Yang | Meta information modification | 2879 | 2022-05-05 04:35:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Llivisaca, S.; León-Tamariz, F.; Manzano-Santtana, P.; Ruales, J.; , .; Serrano, L.; Chica, E.; Cevallos-Cevallos, J. Mortiño (Vaccinium floribundum Kunth). Encyclopedia. Available online: https://encyclopedia.pub/entry/22527 (accessed on 08 February 2026).
Llivisaca S, León-Tamariz F, Manzano-Santtana P, Ruales J, , Serrano L, et al. Mortiño (Vaccinium floribundum Kunth). Encyclopedia. Available at: https://encyclopedia.pub/entry/22527. Accessed February 08, 2026.
Llivisaca, Susana, Fabian León-Tamariz, Patricia Manzano-Santtana, Jenny Ruales, , Lizette Serrano, Eduardo Chica, Juan Cevallos-Cevallos. "Mortiño (Vaccinium floribundum Kunth)" Encyclopedia, https://encyclopedia.pub/entry/22527 (accessed February 08, 2026).
Llivisaca, S., León-Tamariz, F., Manzano-Santtana, P., Ruales, J., , ., Serrano, L., Chica, E., & Cevallos-Cevallos, J. (2022, April 30). Mortiño (Vaccinium floribundum Kunth). In Encyclopedia. https://encyclopedia.pub/entry/22527
Llivisaca, Susana, et al. "Mortiño (Vaccinium floribundum Kunth)." Encyclopedia. Web. 30 April, 2022.
Copy Citation
Mortiño is a member of the Ericaceae family native to the Andes that has been used by local communities for centuries. This species has shown potential in the areas of medicine, agronomy, and green technology. A multidisciplinary approach was used to review aspects related to the ecology, horticulture, composition and potential biotechnological applications of mortiño.
ecology
genetic diversity
domestication attempts
polyphenols
1. Origin, History, and Botany of Mortiño
Native to the tropical Andes of Colombia, Ecuador, and Perú [1][2], mortiño is commonly found at high altitudes on the edges of cold and humid paramos [3]. Mortiño commonly grows at temperatures between 7 and 18 °C [1], on rocky surfaces as a shrub reaching 2.5 m height, often prostrate or scandent [4]. It is one of the first species to grow back from root sprouts after fires in the paramos, playing a key ecological role in the regeneration of the ecosystem [5]. Mortiño leaves are small, coriaceous, elliptic, and ovate to ovate–lanceolate (Figure 1). The flowers are white to pink or red developed on racemes of 6–10 flowers, while the fruits are small (5–8 mm diameter) blue-black glabrous spherical berries at maturity [6] (Figure 2).
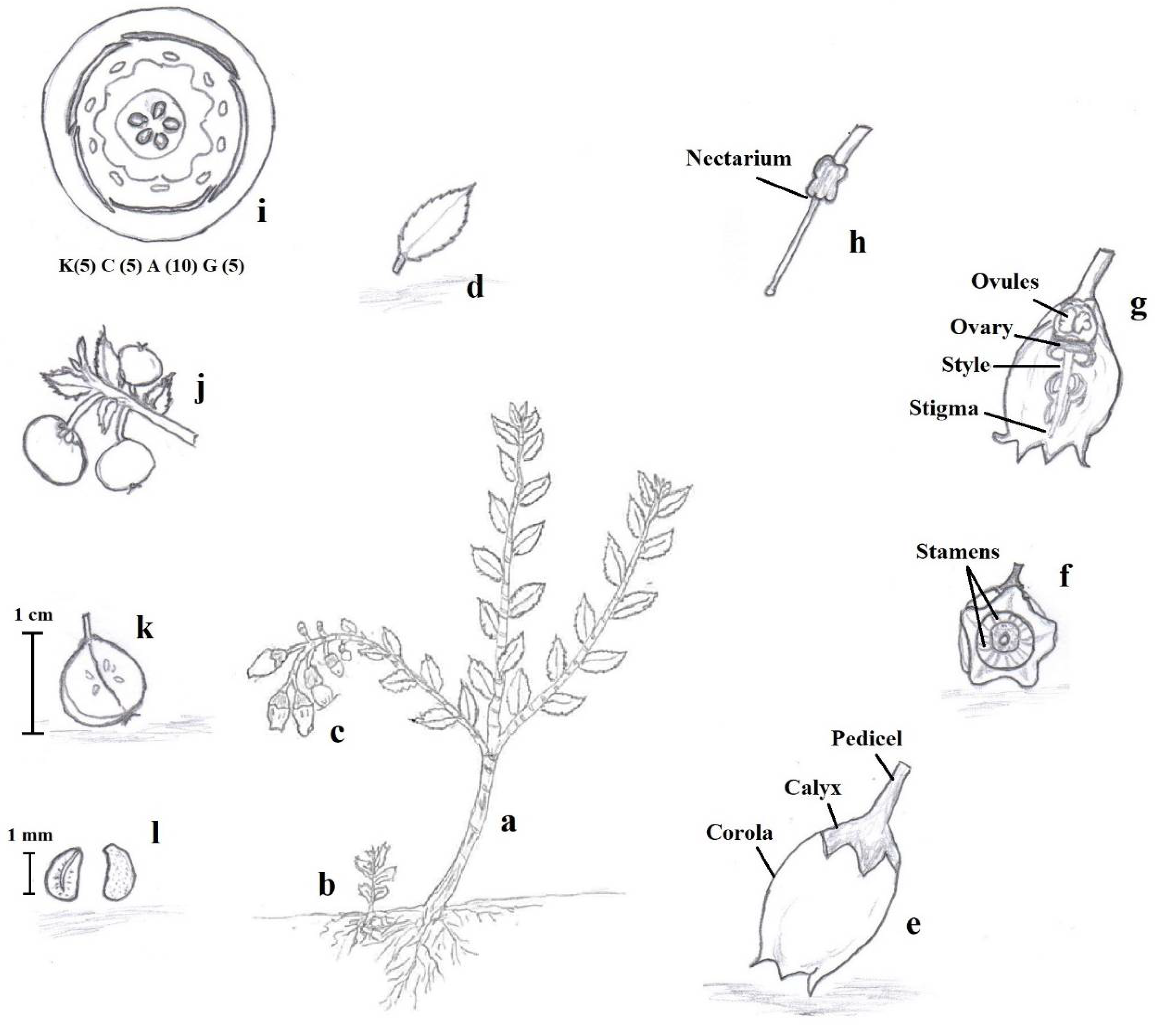
Figure 1. Description of Vaccinium floribundum Kunth: Semi-woody stem that can measure up to 2.5 m in height (a), Roots with their root hairs that, when they come to the surface, give rise to a seedling (b), White to lilac flowers in racemes of 6 to 10 (c), Elliptical, oval, or oval–lanceolate leathery leaves, with crenate-serrated edge, cuneate or round base, and slightly rounded acuminate apex (d), Corolla and Calyx (e), Vertical view of stamens (f), Gynoecium (g), Nectaries (h), Floral diagram of the Ericaceae (i), Round bittersweet berries of bluish to black color (j), Fruit in longitudinal section (k), Recalcitrant seeds of approximately 1 mm in size (l). Source: Herbarium of the Interpretation Center of the Protective Forest “La Prosperina” (BPP—Ar001E), of the Escuela Superior Politécnica del Litoral (ESPOL), Guayaquil—Ecuador, by Botanist Jaime Naranjo (Co-author).

Figure 2. Mortiño plant (Vaccinium floribundum Kunth) with its floral primordia (A), A small bunch of flowers with some pigmentation (B), Serrated leaves and its ripe berries (C). Source: This entry.
During its development, the fruit color transitions from green to white-pink, to pink, and finally to blue-black [7]. Fruit development after anthesis takes roughly between 60 and 100 days under natural conditions [8]. Taxonomically, V. floribundum has traditionally been classified within the Pyxothamnus section in the genus Vaccinium along with V. consanguineum and V. ovatum. However, phylogenetic analysis of tribe Vaccineae suggested Vaccinium is not monophyletic with V. floribundum, forming a clade along with V. consanguineum and V. meridionale, separate from other Vaccinium spp. [8].
Since it was first described in 1825 by Kunth as Vaccinium floribundum from the collections of Bonpland, the taxonomy of mortiño has varied little over the years. However, some common synonyms for the species have been reported, including: V. crenulatum, V. marginatum, V. ramosissimun, V. polystachium, V. mortinia, V. moritzianum, V. dasygynun, Metagonia crenulate, and M. marginata [3]. Common names for V. floribundum are many and often used ambiguously to identify other related species such as V. meridionale, Thibaudia floribunda, or Macleania rupestris. For instance, in Ecuador, mortiño is the most used vernacular name for the species; however, it is also known as uva de los Andes (grape from the Andes), manzanilla del cerro (chamomile from the hill), raspadura quemada (burnt panela), and uva de monte (mountain grape). In Peru, it is known as pushgay, uvitas, congama, and macha macha, whereas in Colombia it is also known as mortiño, agraz, agracejo, and chivaco; nonetheless, here, the name mortiño is more often associated with V. meridionale [1].
The consumption of this fruit was common in the Andean region before the arrival of the Europeans; later, it was assimilated into criollo traditions associated with the All Souls’ day, which continues to this day [1]. Currently, the species is mainly found in the wild, but it is also often present in smallholder farms [9].
2. Ecology and Genetic Diversity
Like many other neotropical Ericaceaes, V. floribundum predominates in belts of moist and cool montane forest preceding the transition towards the colder Paramo (cold and moist ecosystem typical of the high mountain in the Andes between the treeline and the snowline) between 3000 and 4500 m.a.s.l. [10][11]. On the slopes of the Rumiñahui volcano, near Quito (Ecuador), V. floribundum has commonly been observed in landscapes dominated by Calamagrostis intermedia and Carex jamesonii, although many other species, mostly dicots including other Ericaceae such as Pernettya prostrata, have been found growing in close spatial association to V. floribundum [12].
V. floribundum typically thrives in cold, nutrient-poor, moist though well-drained, shallow, and acidic soils [5]. The shrubs show remarkable adaptations to these conditions, such as shallow and almost horizontal root systems as well as profuse sprouting from roots and other vegetative tissues. These characteristics make V. floribundum one of the first species to regenerate damaged paramo ecosystems [5], drawing attention to ecosystem restoration programs in the Andes. Furthermore, V. floribundum has been reported as one of the species more often visited by a variety of bird and insect pollinators [13][14]. Adaptation to these environmental conditions is probably aided by association with specific soil microorganisms, as evidence of interactions between the V. floribundum rhizospheric microbiome and soil chemical properties has been reported [15]. Similarly, ericoid mycorrhiza forming fungi have been reported in V. floribundum roots, potentially contributing to the development of the species in nutrient-limited soils [12].
Currently, the conservation status of V. floribundum populations remains unknown. However, in Ecuador, mortiño is considered highly diverse—a typical characteristic of wild populations—and has shown both geographical and altitudinal patterns of diversification [16]. Still, concerns about the conservation of the species in scenarios of increasing demand of the berry and unsuccessful domestication efforts could pose risks, both to the species and to the paramos where it grows. For these reasons, interest in developing propagation techniques amenable for commercial cultivation has been recently growing in Colombia, Ecuador, and Peru [17].
3. Chemical Composition of Mortiño
The berries from Vaccinium spp. are known for their substantial amounts of sugars, polyphenols, vitamins B and C, minerals, and anthocyanins [18]. Table 1 shows the bromatological parameters and Table 2 describes works on mortiño phytochemistry.
Table 1. Physical and bromatological parameters of the Vaccinium floribundum berry.
| Parameter/Units | Value | Reference |
|---|---|---|
| Soluble solids g/100 g | 10.9 | [19] |
| Ash g/100 g | 0.4 | [19] |
| 0.4 | [20] | |
| Protein g/100 g | 0.6 | [19] |
| 0.7 | [20] | |
| Carbohydrates g/100 g | 14.5 | [19] |
| 16.9 | [20] | |
| 18.1 | [21] | |
| Fat g/100 g | 0.6 | [19] |
| 1 | [20] | |
| Calories kcal/100 g FW * | 84 | |
| 75 | [21] | |
| Water % | 80 | [20] |
| Fiber % | 7.6 | [20] |
| 2.9 | [21] | |
| pH | 3.8 | [21] |
| Fe (mg/100 g FW) | 0.64 ± 0.2 | [20] |
| K (mg/100 g FW) | 607 ± 73 | [20] |
| Ca (mg/100 g FW) | 17.0 ± 2.3 | [20] |
| Mg (mg/100 g FW) | 10.2 ± 1.1 | [20] |
| Cu (mg/100 g FW) | 0.12 ± 0.02 | [20] |
| Zn (mg/100 g FW) | 0.13 ± 0.02 | [20] |
* FW = Fresh Weight.
Table 2. Bioactive compounds from Vaccinium floribundum.
| Compounds/Analysis Technique/Units * | Reference | ||
|---|---|---|---|
| Total polyphenols By the Folin-Ciocalteu method (mg GAE/100 g) |
Fresh: | Powder: | [22] |
| 524.4 ± 4.5 | 495.6 ± 9.1 | ||
| 204.01 ± 12.50 | [23] | ||
| 882 ± 38 | [19] | ||
| 2167 ± 835 | [24] | ||
| 7254.62 ± 1209.17 | [25] | ||
| Chimborazo Province: | Pichincha Province: | [23] | |
| 107.4 ± 6.7 | 146.1 ± 13.4 | ||
| Total phenols By spectrophotometry: | |||
| Total phenols (mg GAE/100 mg) | 608.05 | [20] | |
| Total phenols (mgPgEq/g) | 1.9 ± 0.7 | [26] | |
| Total phenols (mg GAE/g) | 9.3 ± 1.4 | [26] | |
| Anthocyanins | Chimborazo Province: | Pichincha Province: | |
| Total anthocyanin (pH differential) mg/100 g | 89.9 ± 0.6 | 1095.4 ± 19.2 | [23] |
| Anthocyanin mg/g; (C3G equivalent) | 10.6 | [27] | |
| Total anthocyanin (%) | Fresh: | Powder: | [24] |
| 11.1 ± 0.5 | 2.3 ± 0.6 | ||
| Anthocyanin (mg cyanidin/100 g) | 345 | [19] | |
| Anthocyanin mg/100 g | 376.2 ± 49.9 | [28] | |
| Proanthocyanidin mg/g dry weight (epicatechin equivalent) | 5.2 | [29] | |
| Proanthocyanidin (%) | Fresh: | Powder: | [22] |
| 5.3 ± 0.5 | 4.6 ± 0.3 | ||
| Flavonoids (Spectrophotometry): | [26] | ||
| Total flavonoids (mg EC/g) | 6.5 ± 0.7 | ||
| Flavonols total content mg/100 g | 41.6 ± 10.2 | [28] | |
| Flavonols glycosides | 38 | [20] | |
| Other compounds (By spectrophotometry) | [26] | ||
| Total tannins (mg TAEq/g) | 4.2 ± 0.8 | ||
| Vit C mg/100 g | 45.9 ± 6.7 | ||
| Carotenes (μg/100 g): | |||
| -Β-carotene | 70.6 ± 2.0 | ||
| -Lutein | 866.6 ± 7.5 | ||
| Neochlorogenic acid | 1.5 ± 0.5 | [28] | |
| Chlorogenic acid | 9.5 ± 2.9 | ||
| Quercetin and myricetin | -3-O-hexosides, -3-0pentosides and -3-0-deoxyhexosides | [20] | |
| Hydroxycinnamic acids | Predominance of acids: chlorogenic, neochlorogenic and derivatives of caffeic/ferulic acid | ||
* GAE = Gallic acid equivalent; C3G = cyanidin-3-glucoside; TAEq = tannic acid equivalent.
V. floribundum berries have shown high amounts of polyphenols, with levels reaching up to 7254.62 ± 10.86 mg GAE/100 g [25]. The most representative phenolic acids and flavonols reported in mortiño were quercetin-3-O-arabinofuranoside, chlorogenic acid, and quercetin-3-O-galactoside [28].
In comparative studies, other superfruits such as Prunus serotina showed lower levels of polyphenols than those found in mortiño, with reported concentrations of 1494 ± 385 and 2167 ± 835 mg GAE/100 g for each species, respectively [24]. Similarly, a higher content of phenolic acids and flavonols was observed in V. floribundum (41.6 ± 10.2 mg/100 g FW) when compared to V. myrtillus (13.7 ± 0.2 mg/100 g FW) [28]. In another study, the content of total soluble phenolic of mortiño (882 ± 38 mg GAE/100 g FW) was almost double that of guava (462 mg GAE/100 g FW) and plum (440 mg GAE/100 g FW); furthermore, it was four times higher than the values observed in strawberry (238 mg GAE/100 g FW) but less than half the content in the Andean blackberry (2167 mg GAE/100 g FW) [20].
Anthocyanins are other important components in V. floribundum berries, accounting for up to 67% of their total phenolic compounds [20]. Delphinidin-3-arabinose and cyanidin-3-arabinose have been reported as the most abundant anthocyanins in mortiño [22]. Values from 376.2 ± 49.9 [28] to 1095.39 mg/100 g FW [23] of anthocyanin content in blueberry have been reported, exceeding that observed in V. myrtillus (568.8 ± 8.8 mg/100 g FW) [28]. However, sample processing can reduce the levels of anthocyanins, as commercial mortiño powder showed lower levels of these bioactive compounds compared to fresh berries [22]. On the other hand, the levels of proanthocyanidins observed in V. floribundum (5.2 mg/g dry weight epicatechin equivalent) were higher than those in Aristotelia chilensis berries (4.0 mg/g DW EE) but lower than the values observed in V. myrtillus (13.7 mg/g DW EE) [22].
Lastly, it is also very important to know the content of P, Mn, Se, and I of mortiño berries. Unfortunately, to date there are no studies that provide this information. Further research is needed to assess the mineral content of mortiño berries.
4. Biological Activities of Mortiño
Due to the high amounts of polyphenols, anthocyanins, and antioxidants, mortiño has shown various bioactive properties. Table 3 shows some of the antimicrobial and medicinal properties of mortiño. Several studies have shown the antimicrobial capacity of Vaccinium spp. For example, V. corymbosum extracts inhibited the growth of various pathogens such as Salmonella spp. [30] and Listeria monocytogenes [31], while V. macrocarpon prevented the growth of Bacillus cereus and Micrococcus luteus [32]. Similarly, two studies have reported the antimicrobial capacity of mortiño. In one study, mortiño extracts obtained from lyophilized fruits or leaves in 70% ethanol inhibited the growth of 12 species of human pathogens, with inhibition halos ranging from 4.3 ± 0.3 to 39.7 ± 0.2 mm. The reported inhibition halos were greater than those observed when the antibiotic ampicillin was used [23]. Similarly, aqueous pulp and peel extracts of mortiño inhibited the growth of Streptococcus mutans ATCC35668 [27].
Table 3. Medicinal properties of Vaccinium floribundum.
| Biological Activity | Main Findings | References |
|---|---|---|
| Modulatory capacity of adipogenesis |
|
[22] |
| Anti-inflammatory capacity |
|
|
| Diabetes therapy potential |
|
[29] |
| Chemopreventive activity |
|
[28] |
| Protection against oxidative stress |
|
[26] |
| Antimicrobial capacity |
|
[23] |
|
[27] |
Various medicinal properties have been attributed to V. floribundum, including potential applications in managing the symptoms of diabetes [29] and protection against oxidative stress [26]. Additionally, anti-inflammatory properties of mortiño have been suggested, as phenolic extracts of this species decreased the production of inflammatory mediators such as nitric oxide (NO), prostaglandin E2, and cycloxygenase-2 in lipopolysaccharide-stimulated RAW 264.7 macrophages [22]. Similarly, mortiño has been proposed as an inhibitor of adipogenesis, since proanthocyanidin-rich extracts of V. floribundum significantly prevented lipid accumulation in adipocytes and increased the expression of the preadipocyte factor 1 (Pref-1) by 4% in preadipocytes, a value higher than the 2.2% reached by A. chilensis but similar to the 5.9% achieved by epigallocatechin gallate used as a positive control (5.9%) [22]. Likewise, proanthocyanidins from mortiño successfully inhibited the enzymes α-glucosidase and α-amylase in vitro, with a 50% inhibition concentrations (IC50) of 35 and 25 μg/mL, respectively, suggesting a potential use of this berry for diabetes therapy [29].
The berries of V. floribundum have also shown a high antioxidant capacity ranging from 0.339 ± 0.01 g/mL to 0.69 ± 0.03 g/mL [17][22], comparable to that of V. myrtillus (0.42 ± 0.01 g/mL) [28]. Through the Trolox Equivalent Antioxidant Capacity test, 250.01 ± 2.0 μmol TEq/g FW was reported in mortiño, which was higher than the 1.52 ± 3.1 μmol TEq/g FW observed in the berries of Rubus glaucus [26]. Due to its high antioxidant capacity, mortiño has the potential to protect human cells against oxidative stress. Crude extracts of V. floribundum attenuated the damage to Human Dermal Fibroblasts (HDFa) caused by oxidative stress, providing better protection than Rubus glaucus extracts [26]. However, no chemopreventive activity of mortiño was observed in mutagenicity and genotoxicity tests against 4-nitroquinoline-1-oxide (4-NQO) using SOS Chromotests [28]. Further research is needed to assess the potential antioxidant and radical scavenging applications of V. floribundum. In vivo studies, either in clinics or other controlled environments, are still needed to confirm the health benefits of mortiño.
5. Other Uses of Mortiño
There is a growing interest on plant antioxidants for green technology applications. For this reason, mortiño has been used in the synthesis of nanoparticles and solar cells.
The synthesis of nanoparticles commonly requires the use of toxic chemicals that serve as reducing agents. As a result of its high antioxidant capacity, mortiño has the potential to replace hazardous molecules for green production of nanoparticles. Mortiño extracts with high antioxidant capacity have been used for the green synthesis of graphene and functionalization of this material, with silver nanoparticles yielding a highly efficient photocatalyst [33]. Similarly, mortiño extracts were applied as a reducing and stabilizing agent for multicomponent nanoparticles (MCNPs) [34] and zero-valent iron nanoparticles (nZVIs) for bioremediation studies [19][35]. The resulting MCNPs showed >99% removal efficiency of toxic metals in water [34], whereas the zVINs removed at least 80% of total petroleum hydrocarbons (TPH) in contaminated water and soil [35]. The researchers indicated that the efficient formation and stabilization of the nZVIs was probably related to the -OH and -COOH groups from the berry polyphenols.
Additionally, mortiño extracts have also served as sensitizers for dye-sensitized solar cells, yielding efficiencies between 0.18 and 0.26% [36]. Table 4 shows some of the applications of mortiño.
Table 4. Other applications of Vaccinium floribundum.
| Applications | Main Findings | Reference |
|---|---|---|
| Synthesis of zero-valent iron nanoparticles (nZVIs) for environmental remediation |
|
[24] |
|
[35] | |
| Production of multicomponent nanoparticles (MCNPs) for removal/immobilization of heavy metals from water and in soils |
|
[34] |
| Synthesis of silver–graphene nanocomposites with photocatalytic activity |
|
[33] |
| Biosynthesis of silver nanoparticles with photocatalytic activity |
|
[37] |
| Elaboration of dye-sensitized solar cells (DSSCs) |
|
[38] |
|
[36] |
Mortiño has also been used in the wine industry [39][40], baking [41], and even in the production of mortiño gummies [42]. The bioactive compounds of mortiño have attracted much interest in various sectors, which is why several methods have been proposed for the conservation and long-term storage of this berry, including short exposure to UV-C [43] and drying pretreatments [44] (Table 5). In a study, it was shown that after a storage period of 21 days, UV-C-treated (12.5 kJ m−2) mortiño retained 90% of the original anthocyanin levels compared to 76.85% of the untreated berries. However, the concentration of polyphenols was similar in both UV-C-treated and untreated berries [43]. Surprisingly, dry mortiño retained 93% of the anthocyanins and all the polyphenols in a storage period of 8 weeks [44]. However, other food processing technologies have been detrimental for the bioactive compounds of mortiño. Compared to lyophilization, heating in a sand bath to obtain a commercial mortiño powder yielded significantly lower levels of bioactive compounds such as anthocyanins (2.3 ± 0.6 vs. 11.1 ± 0.5%), proanthocyanidins (4.6 ± 0.3 vs. 5.3 ± 0.5%), total phenols (495.6 ± 9.1 vs. 524.4 ± 4.5 mg/g), and antioxidant capacity (3.3 ± 0.1 vs. 8.3 ± 0.4 mmol/g Trolox equivalents estimated by Oxygen Radical Absorbance Capacity (ORAC)) [22] (Table 5). In general, the processing of berries can degrade the anthocyanins naturally present in the fruits [45] (Table 3). Therefore, the pre-treatment of mortiño using UV-C represents a non-chemical approach to complement the treatment for low temperature storage, especially to maintain the anthocyanin concentration. Table 5 shows some of the preservation studies carried out on mortiño.
Table 5. Berry quality preservation studies.
| Application | Main Findings | References |
|---|---|---|
| UV-C treatments for quality preservation of V. floribundum berries |
|
[43] |
| Drying pretreatment for V. floribundum |
|
[44] |
| Winemaking |
|
[46] |
References
- Coba, P.; Coronel, D.; Verdugo, K.; Paredes, M.; Yugsi, E.; Huachi, L. Ethnobotanical Study of the Mortiño (Vaccinium fLoribundum) as Ancestral and Potentially Functional Meal. La Granja 2012, 16, 5.
- Schreckinger, M.; Lotton, J.; Lila, M.; Gonzalez, E. Berries from South America: A Comprehensive Review on Chemistry, Health Potential, and Commercialization. J. Med. Food 2010, 13, 233–246.
- Cobo, M.; Gutiérrez, B.; Torres, A.; Torres, M. Preliminary Analysis of the Genetic Diversity and Population Structure of Mortiño (Vaccinium floribundum Kunth). Biochem. Syst. Ecol. 2016, 64, 14–21.
- Pedraza, P. Vaccinium floribundum. Available online: http://tropical.theferns.info/viewtropical.php?id=Vaccinium+floribundum (accessed on 15 January 2021).
- Ramsay, P.; Oxley, E. Fire Temperatures and Postfire Plant Community Dynamics in Ecuadorian Grass Páramo. Vegetatio 1996, 124, 129–144.
- Pedraza, P. Taxon Details—Vaccinium floribundum Kunth. Available online: http://sweetgum.nybg.org/science/projects/ericaceae/taxon-details/?irn=113261 (accessed on 22 January 2021).
- Arteaga Dalgo, M.; Andrade Cuvi, M.J.; Moreno Guerrero, C. Relación Del Desarrollo Del Color Con El Contenido de Antocianinas y Clorofila En Diferentes Grados de Madurez de Mortiño (Vaccinium floribundum). Enfoque UTE 2014, 5, 14–28.
- Mendoza, F. Fenología Floral Del Mortiño (Vaccinium floribundum Kunth) Acorde a La Escala BBCH En El Páramo Andino Del Atacazo, Ecuador. Available online: https://rraae.cedia.edu.ec/Record/UDLA_1155233eaaba390cb211e874da1511b6 (accessed on 19 March 2022).
- Magnitskiy, S.; Ligarreto, G.; Lancheros, H. Rooting of Two Types of Cuttings of Fruit Crops Vaccinium floribundum Kunth and Disterigma alaternoides (Kunth) Niedenzu (Ericaceae). Agron. Colomb. 2011, 29, 197–203.
- Kron, K.A.; Powell, E.A.; Luteyn, J.L. Phylogenetic Relationships within the Blueberry Tribe (Vaccinieae, Ericaceae) Based on Sequence Data from MatK and Nuclear Ribosomal ITS Regions, with Comments on the Placement of Satyria. Am. J. Bot. 2002, 89, 327–336.
- Popenoe, W. Economic Fruit-Bearing Plants of Ecuador; Smithsonian Institution: Washington, DC, USA, 1924.
- Hidalgo, M.; Vásquez, W. Caracterización Morfológica de Microorganismos, Físico-Química Del Suelo y Arvenses Presentes En El Hábitat de Crecimiento Del Mortiño (Vaccinium floribundum Kunth) En El Páramo Del Volcán Rumiñahui, Pichincha; Universidad de las Américas: Quito, Ecuador, 2016.
- Pelayo, R.; Soriano, P.; Márquez, N.; Navarro, L. Phenological Patterns and Pollination Network Structure in a Venezuelan Páramo: A Community-Scale Perspective on Plant-Animal Interactions. Plant Ecol. Divers. 2019, 12, 607–618.
- Torres, M.; Pinos, A. Exploring the Microbiome Composition of the Rhizosphere Associated with the Wild Andean Blueberry (Vaccinium floribundum, Kunth) in the Highlands of Ecuador. Master’s Thesis, Universidad San Francisco de Quito, Colegio de Posgrados, Quito, Ecuador, 2020.
- Setaro, S.; Kottke, I.; Oberwinkler, F. Anatomy and Ultrastructure of Mycorrhizal Associations of Neotropical Ericaceae. Mycol. Prog. 2006, 5, 243–254.
- Vega, P.; Cobo, M.; Argudo, A.; Gutierrez, B.; Rowntree, J.; Torres, M. Characterizing the Genetic Diversity of the Andean Blueberry (Vaccinium floribundum Kunth.) Across the Ecuadorian Highlands. PLoS ONE 2020, 15, e0243420.
- Abreu, O.A.; Barreto, G.; Prieto, S. Vaccinium (Ericaceae): Ethnobotany and Pharmacological Potentials. Emir. J. Food Agric. 2014, 26, 577–591.
- Efferth, T.; Banerjee, M.; Paul, N.; Abdelfatah, S.; Arend, J.; Elhassan, G.; Hamdoun, S.; Hamm, R.; Hong, C.; Kadioglu, O.; et al. Biopiracy of Natural Products and Good Bioprospecting Practice. Phytomedicine 2016, 23, 166–173.
- Torrenegra Alarcón, M.E.; Villalobos Lagares, O.L.; Castellar Abello, E.A.; León Méndez, G.; Granados Conde, C.; Pajaro, N.P.; Caro Soto, M.S. Evaluation of the Antioxidant Activity of Pulps from Rubus glaucus B., Vaccinium floribundum K. and Beta Vulgaris L. Rev. Cuba. Plantas Med. 2016, 21, 1–8.
- Vasco, C.; Riihinen, K.; Ruales, J.; Kamal-Eldin, A. Chemical Composition and Phenolic Compound Profile of Mortiño (Vaccinium floribundum Kunth). J. Agric. Food Chem. 2009, 57, 8274–8281.
- Estrella, E. El Pan de America: Etnohistoria de Los Alimentos Aborigenes En El Ecuador. Publicaciones del C.S.I.C 1988, 29, 390.
- Schreckinger, M.; Wang, J.; Yousef, G.; Lila, M.; Gonzalez, E. Antioxidant Capacity and in Vitro Inhibition of Adipogenesis and Inflammation by Phenolic Extracts of Vaccinium floribundum and Aristotelia Chilensis. J. Agric. Food Chem. 2010, 58, 8966–8976.
- Llivisaca, S.; Manzano, P.; Ruales, J.; Flores, J.; Mendoza, J.; Peralta, E.; Cevallos-Cevallos, J.M. Chemical, Antimicrobial, and Molecular Characterization of Mortiño (Vaccinium floribundum Kunth) Fruits and Leaves. Food Sci. Nutr. 2018, 6, 934–942.
- Murgueitio, E.; Debut, A.; Landivar, J.; Cumbal, L. Synthesis of Iron Nanoparticles Through Extracts of Native Fruits of Ecuador, as Capuli (Prunus serotina) and Mortiño (Vaccinium floribundum). Biol. Med. 2016, 8, 1.
- Llerena, W.; Samaniego, I.; Angós, I.; Brito, B.; Ortiz, B.; Carrillo, W. Biocompounds Content Prediction in Ecuadorian Fruits Using a Mathematical Model. Foods 2019, 8, 284.
- Alarcón-Barrera, K.S.; Armijos-Montesinos, D.S.; García-Tenesaca, M.; Iturralde, G.; Jaramilo-Vivanco, T.; Granda-Albuja, M.G.; Giampieri, F.; Alvarez-Suarez, J.M. Wild Andean Blackberry (Rubus glaucus Benth) and Andean Blueberry (Vaccinium floribundum Kunth) from the Highlands of Ecuador: Nutritional Composition and Protective Effect on Human Dermal Fibroblasts Against Cytotoxic Oxidative Damage. J. Berry Res. 2018, 8, 223–236.
- Reyes, I.; Villacres, C.; Santacruz, S.; Castro, M.; Chávez, M.; Armas, A. Antibacterial and Antioxidant Effect of Ecuadorian Red Fruits on Streptococcus Mutans: In Vitro Study. Available online: https://www.scielo.sa.cr/scielo.php?pid=S1659-07752019000200023&script=sci_arttext (accessed on 28 November 2019).
- Prencipe, F.P.; Bruni, R.; Guerrini, A.; Rossi, D.; Benvenuti, S.; Pellati, F. Metabolite Profiling of Polyphenols in Vaccinium Berries and Determination of Their Chemopreventive Properties. J. Pharm. Biomed. Anal. 2014, 89, 257–267.
- Schreckinger, M.; Lila, M.; Yousef, G.; Gonzalez, E. Inhibition of α-Glucosidase and α-Amylase by Vaccinium floribundum and Aristotelia Chilensis Proanthocyanidins. ACS Symp. Ser. 2012, 1109, 71–82.
- Pervin, M.; Hasnat, M.A.; Lim, B.O. Antibacterial and Antioxidant Activities of Vaccinium corymbosum L. Leaf Extract. Asian Pac. J. Trop. Dis. 2013, 3, 444–453.
- Shen, X.; Sun, X.; Xie, Q.; Liu, H.; Zhao, Y.; Pan, Y.; Hwang, C.-A.; Wu, V.C.H. Antimicrobial Effect of Blueberry (Vaccinium corymbosum L.) Extracts Against the Growth of Listeria monocytogenes and Salmonella enteritidis. Food Control 2014, 35, 159–165.
- Viskelis, P.; Rubinskiene, M.; Jasutiene, I.; Šarkinas, A.; Daubaras, R.; Česoniene, L. Anthocyanins, Antioxidative, and Antimicrobial Properties of American Cranberry (Vaccinium macrocarpon Ait) and Their Press Cakes. J. Food Sci. 2009, 74, C157–C161.
- Vizuete, K.S.; Kumar, B.; Vaca, A.V.; Debut, A.; Cumbal, L. Mortiño (Vaccinium floribundum Kunth) Berry Assisted Green Synthesis and Photocatalytic Performance of Silver–Graphene Nanocomposite. J. Photochem. Photobiol. A Chem. 2016, 329, 273–279.
- Abril, M.; Ruiz, H.; Cumbal, L.H. Biosynthesis of Multicomponent Nanoparticles with Extract of Mortiño (Vaccinium floribundum Kunth) Berry: Application on Heavy Metals Removal from Water and Immobilization in Soils. J. Nanotechnol. 2018, 2018, 9504807.
- Murgueitio, E.; Cumbal, L.; Abril, M.; Izquierdo, A.; Debut, A.; Tinoco, O. Green Synthesis of Iron Nanoparticles: Application on the Removal of Petroleum Oil from Contaminated Water and Soils. J. Nanotechnol. 2018, 2018, 4184769.
- Taco-Ugsha, M.A.; Santacruz, C.P.; Espinoza-Montero, P.J. Natural Dyes from Mortiño (Vaccinium floribundum) as Sensitizers in Solar Cells. Energies 2020, 13, 785.
- Kumar, B.; Vizuete, K.S.; Sharma, V.; Debut, A.; Cumbal, L. Ecofriendly Synthesis of Monodispersed Silver Nanoparticles Using Andean Mortiño Berry as Reductant and Its Photocatalytic Activity. Vacuum 2019, 160, 272–278.
- Ramirez-Perez, J.; Maria, C.; Santacruz, C.P. Impact of Solvents on the Extraction and Purification of Vegetable Dyes onto the Efficiency for Dye-Sensitized Solar Cells. Renew. Wind. Water Sol. 2019, 6, 1–15.
- Albán Martínez, D.P.; Marcalla Montaguano, W.R. Estudio de Pre-Factibilidad Para La Producción Tecnificada de Vino de Mortiño (Vaccinium floribundum Kunth) En El Cantón Sigchos Comunidad Quinticusig Asociacion de Vinicultores Período 2012–2013. Bachelor’s Thesis, Universidad Técnica de Cotopaxi/UTE, Latacunga, Ecuador, 2013.
- Pastuña, G. Estudio de Factibilidad Para La Creación de Una Microempresa Dedicada a La Producción y Comercialización de Vino a Base de Mortiño, Ubicada En La Provincia de Cotopaxi, Cantón Sigchos, Año 2019. 2019. Available online: http://www.dspace.cordillera.edu.ec:8080/xmlui/handle/123456789/5166 (accessed on 24 November 2021).
- Ceron, J. Determinación de La Vida Útil Del Pan de Mortiño. 2018. Available online: http://repositorio.ute.edu.ec/xmlui/handle/123456789/18131 (accessed on 24 November 2021).
- Vicente, S.; Argüello, Y. Elaboración de Gomitas de Mortiño (Vaccinium floribundum). Bachelor’s Thesis, Universidad Técnica de Cotopaxi/UTE, Latacunga, Ecuador, 2016.
- Andrade-Cuvi, M.J.; Moreno, C.; Zaro, M.J.; Vicente, A.R.; Concellón, A. Improvement of the Antioxidant Properties and Postharvest Life of Three Exotic Andean Fruits by UV-C Treatment. J. Food Qual. 2017, 2017, 4278795.
- García, A.; Ruales, J. Study the Effect of Pre-Treatment of Drying ‘Mortiño’ (Vaccinium floribundum Kunth) with Reference to Drying Rate and Total Content of Soluble Polyphenols and Anthocyanins. Rev. Politécnica 2018, 40, 47–57.
- Yue, X.; Xu, Z. Changes of Anthocyanins, Anthocyanidins, and Antioxidant Activity in Bilberry Extract During Dry Heating. J. Food Sci. 2008, 73, C494–C499.
- Ortiz, J.; Marín-Arroyo, M.-R.; Noriega-Domínguez, M.-J.; Navarro, M.; Arozarena, I. Color, Phenolics, and Antioxidant Activity of Blackberry (Rubus Glaucus Benth.), Blueberry (Vaccinium Floribundum Kunth.), and Apple Wines from Ecuador. J. Food Sci. 2013, 78, C985–C993.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Entry Collection:
Environmental Sciences
Revisions:
3 times
(View History)
Update Date:
05 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




