Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joana Antunes | -- | 3711 | 2022-04-28 22:00:29 | | | |
| 2 | Dean Liu | Meta information modification | 3711 | 2022-04-29 03:14:49 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Antunes, J.; Moreira, I.; Gomes, F.; Cunha, F.; Henriques, M.; Fangueiro, R. Protective Textiles against Biological Threats. Encyclopedia. Available online: https://encyclopedia.pub/entry/22474 (accessed on 08 February 2026).
Antunes J, Moreira I, Gomes F, Cunha F, Henriques M, Fangueiro R. Protective Textiles against Biological Threats. Encyclopedia. Available at: https://encyclopedia.pub/entry/22474. Accessed February 08, 2026.
Antunes, Joana, Inês Moreira, Fernanda Gomes, Fernando Cunha, Mariana Henriques, Raul Fangueiro. "Protective Textiles against Biological Threats" Encyclopedia, https://encyclopedia.pub/entry/22474 (accessed February 08, 2026).
Antunes, J., Moreira, I., Gomes, F., Cunha, F., Henriques, M., & Fangueiro, R. (2022, April 28). Protective Textiles against Biological Threats. In Encyclopedia. https://encyclopedia.pub/entry/22474
Antunes, Joana, et al. "Protective Textiles against Biological Threats." Encyclopedia. Web. 28 April, 2022.
Copy Citation
The development of protective clothing is crucial nowadays, as there are increased levels of harmful biological threats, both for military forces and civilians.
advanced protection
protective textiles
biological warfare agents
antimicrobial
1. Biological Protective Textiles
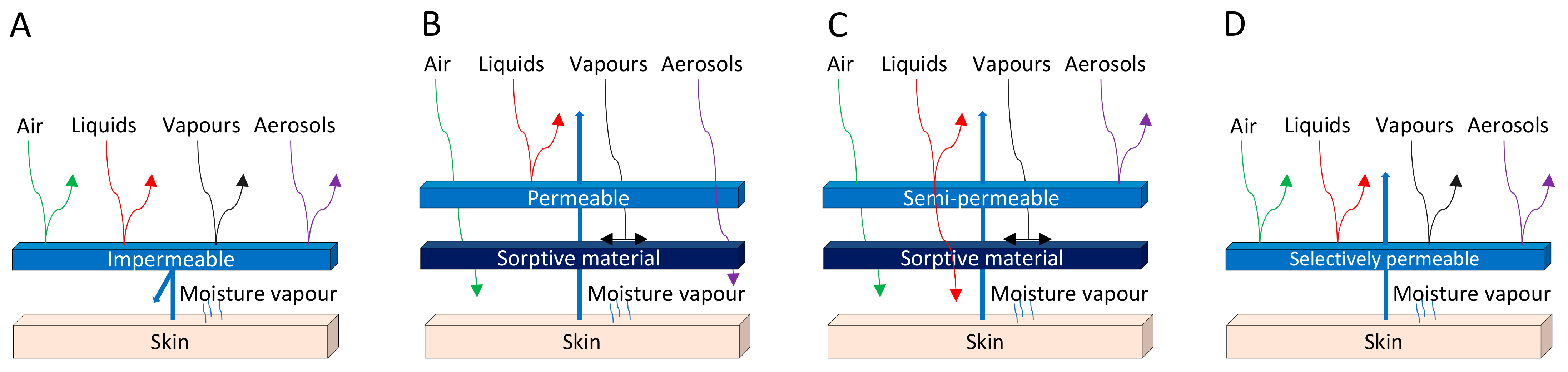
The development of protective clothing is crucial nowadays, as there are increased levels of harmful biological threats, both for military forces and civilians [1]. The main purpose of barrier textiles is to protect the user against external hazards such as BWAs while maintaining safety and comfort next to the skin [2]. Figure 1 illustrates, in a simple manner, the different types of conventional biological protection, namely an impermeable membrane (A), an air-permeable shell layer (B), a semipermeable shell layer (C), and a selectively permeable membrane (D). However, most of the available protective clothing systems rely on passive protection, acting as a full barrier against air, vapors, and liquids, as in hazardous materials (HAZMAT) suits (Figure 1A) [1]. Materials that are chemically or mechanically unresponsive to the environment must be engineered to meet performance specifications under worst-case-scenario conditions, often sacrificing performance for the sake of other parameters [3]. Air-permeable overgarments are most frequently composed of an activated-carbon layer to adsorb toxic vapors, designed to be worn over battledress duty uniforms (Figure 1B) [1][2]. Although activated-carbon adsorption material has protective properties, it is limited by a nonselective adsorption, poor protection performance against large toxic liquid droplets, and secondary pollution. Hence, current needs, new materials, and new technologies are acting together to promote the advances of permeable protective suits in pursuit of high performance, multifunctionality, lightweightness, and comfort [4]. The development of new protective clothing with different features that can adsorb hazardous agents is envisioned, which can be accomplished by using different fibrous materials and by following a specific design. Selectively permeable fabrics are important to improving the user’s comfort by reducing the airflow through the fabric layers while keeping a high water-vapor permeability [4]. As an example, the integration of electrospun nanofiber membranes in textile fibrous structures produces a high aerosol filtration efficiency, good air permeability, low surface density, and low-pressure loss, thanks to the small but highly interconnected pores and large surface area of built nanofibers [4][5]. In addition, active protection appeared as a promising concept to detect and inactivate/degrade microorganisms and BWAs, while considering that materials capable of responding to their environment may achieve optimal performance under a much wider set of conditions [3][5]. This can be achieved either by using fibers such as the ones prepared via electrospinning or by functionalizing textiles with nanomaterials that possess those capabilities.
The development of biological protective clothing depends on a combination of different requirements, such as a barrier to liquids, water vapor permeability, and stretch properties. However, it also depends on parameters such as weight and comfort for the wearer, which will ultimately influence the level and durability of the protection. The type of biological threat also impacts this selection and constitutes one of the reasons why the requirements must be established beforehand [6].
2. Fibrous Materials
Protective clothing can be achieved through the usage of several different fibrous materials, which are listed in this subsection with regard to the current solutions and the new developments.
3. Fibrous Structures
Different fibrous structures can be developed, and these are presented in this subsection with respect to conventional and active innovative protection.
4. Bioactive Agents
The latest research has directed its efforts at the study of metal–organic frameworks (MOFs); quantum dots; and inorganic particles integrating silver (Ag), copper (Cu), zinc (Zn), and titanium (Ti) cations. Glimpses of the potential of natural polymer chitosan (CS) or derivatives as BWA-counteracting agents, applied as a coating layer or in the form of organic particles (loaded or not with plant-derived compounds such as plant extracts and essential oils (EOs)) can be perceived. Hydrogen-bonded organic frameworks (HOFs), which emerged recently, are also showing high potential to act as self-cleaning materials. The following sections will describe the aforementioned bioactive agents, unveiling the details of their biocidal potential, mechanisms of action, and known limitations.
5. Textile Fabric Functionalization Methods
Textiles can carry microorganisms and also promote their survival, proliferation, and endurance. When a fabric is used for clothing, an infestation may create infections and constitute a biological threat. Antimicrobial functional finishes are therefore applied to textiles to protect the wearer and the fabric itself [7]. Various techniques exist to immobilize bioactive agents onto textile fibers, each one carrying its specifications, advantages, and limitations, with the fabric being previously treated and functionalized in order to improve the impregnation of the selected bioactive agents, as well as their durability within the textile. The dip-pad-cure method, the dip-and-dry method, the exhaustion method, the spray-dry method, the spray-cure method, the pad-batch method, and sol-gel and sonochemical coatings are a few relevant examples of the impregnation methods of bioactive agents [8][9]. However, coating and laminating procedures are increasingly important techniques for adding value to textiles, including coating approaches such as the lick-roll method; direct coating (knife on air, knife over table, knife over roller, knife over rubber blanket); foam coating; foam and crushed-foam coating; transfer coating; kiss-roll coating; rotary-screen printing; spray coating; calendar coating; hot-melt extrusion coating; and rotogravure [10].
Starting with MOFs, recent antimicrobial stars, some interesting studies have been performed. The work of Cheung and colleagues [11] stands out, as PET textiles had UiO-66-NH2 MOFs grown in situ following chlorination with a hypochlorite bleach solution to obtain regenerable N–chlorine MOFs coating the textile. The same occurred elsewhere [12], but this time ZIF(Ni), ZIF-8(Zn), and ZIF-67(Co) were the MOFs synthesized into cotton fabrics. A silicate modification acted as a crosslinker between cotton on one side and ZIF-MOFs on the other, thereby increasing the number of MOFs adsorbed onto the fabrics. The fabrics were scoured for dirt removal or even bleached for discoloration [11][12], and sometimes functionalized to gain functional dopamine moieties [13] or the previously mentioned silicate modification [12] to reinforce binding with the bioactive agents through covalent bridges.
The same trend has been observed with inorganic NPs, with most of the NPs being grown in situ following textile incubation with metallic precursors. Despite their well-known handicaps, Ag NPs continue to be the most studied inorganic NPs in protective textiles, although often in combination with other microbicidal enhancers. Textile functionalization with the bioactive agents occurs mostly via the in situ formation of NPs [8][14][15][16][17]. As an example, El-Naggar and colleagues [8] showed that bleached and mercerized (an alkaline treatment to improve affinity towards subsequent chemical modifications) cotton fabric was rendered more hydrophilic through plasma treatment, then washed with a nonionic detergent to remove impurities and silanized to encourage metal–ligand binding with the Ag NPs. Silanization treatment forms silane groups that act as fiber–NP coupling agents, creating a siloxane bridge between the two components [18]. Finally, the treated fabric was immersed in a solution carrying metallic precursors, sonicated, padded, squeezed, and cured for thermal reduction to form Ag NPs. Görgülüer et al. [19] washed rayon fabric in an acetic acid solution and in a wet surfactant so that any chemical finishing, such as silicon, and softening on the fabric could be efficiently removed. Afterwards, the fabric was immersed in TiO2 NPs; poly(dimethylsiloxane) (PDMS) to functionalize the later NPs with hydrophobic moieties; AgNO3 and NaBH4 as metallic precursor and reducing agent, respectively; and finally, tetrahydrofuran (THF) to assist in the production of compact and spherical Ag NPs. Samples were ready for characterization following a drying step. While using ZnO NPs to guarantee bacterial cell death in desized and bleached cotton fabrics, Noorian et al. [20] also washed the fabric in nonionic detergent, before performing oxidization by periodate and treatment with 4-aminobenzoic acid ligands (PABA). NPs were similarly built in situ after the immersion of the fabric in a ZnO precursor, ultrasonication, and chemical reduction.
The integration of CQDs into cotton fabric that had been scoured, bleached, and cationized with 3–chloro–2–hydroxypropyl trimethyl ammonium chloride (C6H15Cl2NO), was indeed very simple [21]; it was achieved by dissolving previously prepared CQDs, impregnating the fabric with them while stirring, and drying. The addition of an rGO coating through a dip-dry process onto fabrics composed of cotton or silk [15] that had been previously washed with acetone and hot water and functionalized with a silane derivative allowed increased quantities of Ag and Cu NPs to be added subsequent to the composition, particularly with cotton, which is richer in hydroxyl groups than silk.
Botelho and team [14] washed PA taffeta and submitted it to plasma treatment. CS was then added through the dip-dry method, followed by the already prepared Ag NPs. Dip-pad-dry was the immobilization technique also selected by Verma et al. [22] to integrate dissolved CS, along with citric acid (C₆H₈O₇) to act as a linker to the enzymatically desized and scoured cotton fabric, with sodium hypophosphite (NaPO2H2) as the catalyst; this worked as a mordant to enhance the dyeability of the cotton. Samples were then padded, dried, and cured. A final step included a dyeing process with onion-skin dye. Some studies have additionally integrated plant-derived molecules into/onto CS-based small-scale particles [23][24][25]. Singh et al. [25] used the emulsification of gelatin and rosemary EO followed by ionic gelation between gelatin and CS to encapsulate the EO and produce a stable shell. Linen fabric was dipped in a microcapsule (MC) dispersion and low-temperature curable acrylic binder, padded, and dried. Verma and colleagues [23] encapsulated cinnamon EO within CS MCs produced by simple complexation with Tween 20. Dense taffeta cotton fabrics, which had been desized, bleached, and mercerized, were dipped into an MC dispersion and a binding agent (dimethyloldihydroxyethylene urea, DMDHEU), padded, dried, and cured; they were then autoclaved and stored. In another study, Wang and colleagues [26] explored HOFs that carried building units incorporating CH3-, F-, or NH2-groups on the ortho-position of the phenyl ring of the benzoic acid and were produced via a sol-gel method. These were spray-coated onto woven and knitted cotton fabric, as well as commercial chirurgical disposable face masks; dried; washed in acetone to remove unbound agent and solvent; and then dried again.
As mentioned above, multiple bioactive agents have been tested with textiles, alone or combined in order to obtain synergistic effects in the fight against pathogens. Many authors are also aware of the need to obtain durable bioactive effects, namely by retaining the bioactive compounds attached to fibers [11][19][26]. It is, however, noticeable that the past two years, during the COVID-19 pandemic situation, have been key for attempts to control the washing durability of finished fabrics, thereby responding to a major concern of the textile finishing industry [23]. Some authors have even followed standardized protocols to assess such features (the KS K ISO 6330 [15], IS: 3361-979 [22], AATCC-61 [27], or AATCC 2010 [21] standards), thus proving that the required bioactivity is present even after laundering activity. Table 1 summarizes the main, and representative, antimicrobial protective textiles designed for military purposes or for general use.
Table 1. Recent trends (2020–2022) in antimicrobial protective textiles designed for military purposes or for general use.
| Fabric | Bioactive Agent | Impregnation Method | AM Testing | Protective Textile | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Details | Cleaning and/or Pretreatment | Cell | Method | Main Results | Durability | ||||
| Woven and knitted cotton fabric, plus commercial chirurgical disposable face masks | - | HOF-101-R (R=H, CH3, F, NH2), obtained by sol-gel method | Spray coating: HOF-101 tecton derivatives (1 mg/mL in DMF) were sprayed on various fiber materials (1 × 1 cm2) for 10 s and dried (100 °C, 1 h). The procedure was repeated enough times until the sprayer was empty. Fibers were washed by acetone 3 times and dried (100 °C, 1 h). | S. aureus, E. coli, K. pneumoniae, and M. marinum | Shake-flask method, under simulated daylight and dark conditions | After illumination under simulated daylight for 2.5 min, the HOF-101-F/fiber killed 95% of E. coli. Following 12 h of solar irradiation and exposure to bacteria for 2 h, cell death was ≈46%. Performance maintained after light irradiation and dark treatments for 5 cycles. Over 99.99% of bacteria was eliminated after daylight treatment for 30 min. Antibacterial performance under complete dark conditions without preirradiation was much slower. |
Washed in water without observable HOF loss. |
Face masks | [26] |
| PET | Scoured in 3% NaOH solution (90 °C, 20 min), then washed with water | Regenerable N-chlorine, loaded into Zr-MOF UiO-66-NH2 | In situ MOF synthesis: PET textile (20 cm × 20 cm), BDC-NH2 (90 mmol, 16.2 g) and ZrOCl2·8H2O (60 mmol, 19.4 g) mixed in water (400 mL) and TFA (200 mL) in a sealed 1 L Schott bottle, sonicated for 0.5 h, placed at 100 °C for 6 h, cooled to RT, washed by water (2 × 500 mL) and acetone (3 × 500 mL), dried at RT, and activated at 110 °C for 24 h under dynamic vacuum. | S. aureus, E. coli, and SARS-CoV-2 | Modified AATCC 100–2004 (with textile “sandwiched” using another identical sample for full contact), SEM of harvested bacteria, anti-SARS-CoV-2 virus test | Bacteria: 7-log reduction within 5 min. SARS-CoV-2: 5-log reduction within 15 min. |
23% loss in chlorine content after 40 days storage, sealed, under ambient conditions, still enabling total sterilization. | Cloth against BWAs and CWAs | [11] |
| 100% plain-woven cotton, 185 gm/ m2 |
Scoured, bleached, then cationized with C6H15Cl2NO (50 °C, 2 h) | CQDs clustered from synthesized TM | Dip-dry: 0.25 g of prepared components (TM or CQDs) dissolved in 25 mL of CHCl3. Fabric (0.25 g) impregnated in 0.25 g of TM or CQDs (1 h, continuous stirring), then air-dried. |
S. aureus, E. coli, and C. albicans | Kirby–Bauer disk diffusion technique, MIC determination | 82%, 71%, and 62% growth inhibition, respectively, in 24 h. | 68%, 63%, and 67% growth inhibition, respectively, after 10 washing cycles. | Military clothing | [21] |
| Pristine CNWs fabricated from pulp and lyocell fibers |
Drying (90 °C, 5 h) and hydrofobization with CI, plus UV-induced grafting of PTB | PHMG or NEO | Outer layer: grafting of antiviral/antibacterial agents by the ring-opening reaction of the PTB with -NH2 of PHMG or NEO onto hydrophobic CI-functionalized CNWs. Middle layer: the same onto pristine CNWs. | S. aureus, E. coli, HcoV-229E virus, and SARS-CoV-2 virus | Colony count method and antiviral testing | Bacteria: >99.99%, 99.99 ± 0.01% growth inhibition rate after 10 min of incubation with CNWs-PTB-PHMG. Sars-Cov-2: 16.23 ± 1.69% survival after ~0.1 min with CNWs-PTB-NEO, 99.84% ± 0.14% after 30 min with CNWs-PTB-PHMG. |
- | Face masks | [28] |
| 100% plain-weave cotton fabric: 80 ends/inch, 75 picks/inch, and 168 (g/m2) |
Scoured, bleached, and C8H11NO2-modified (immersion in C8H11NO2.HCl solution at pH 8.5, 24 h) | Ag NPs | Dip-dry: immersion in 10 mM AgNO3 (continuous stirring, 30 °C, 8 h) and vacuum-drying (12 h, 40 °C). | S. aureus and E. coli | ASTM E2149-01 | Bacterial reduction of 86% for S. aureus and 93% for E. coli following 1 h of incubation, 100% after 24 h. | ~98% bacterial reduction after 20 washes. | Functional textiles | [17] |
| Woven viscose (120 g/m2) | Fabric phosphorylation: immersion in DAPH at a molar ratio of 1:1; urea was also included as 3 equiv of DAHP, then rinse with water |
ZPT | Dip-pad-dry: padding with 0.5 wt % aqueous solution of N2O6Zn·6H2O via the 2-dip-2-nip method. Then, water-soluble NaZPT was added at a molar ratio of 1:2 with respect to the metal precursor. Immersion in a ZPT ligand solution (2 h, 40 °C, orbital shaking at 120 rpm). Drying (80 °C, 10 min), curing (150 °C, 2 min), and rinsing with water. |
S. aureus, E. coli, and C. albicans | Qualitative Kirby−Bauer disk diffusion method; quantitative AATCC-100, OD600, and bacteria survival (CFU) measurement methods; SEM and quantitative antifungal assay |
Viscose-ZPT induced high ZoI (48 or 53 mm, respectively, against S. aureus or E. coli). | Viscose-ZPT induced high ZoI after 20 washes (38 or 43 mm, respectively, against S. aureus or E. coli). 96–97% growth inhibition (20 washes). | Protective clothing | [27] |
| 100% cotton or silk | Acetone and hot water (60 °C) washed; air-dried; soaking in coupling-agent solution (pH 4–5, C9H20O5Si:water = 1:15) for 4 h at 60 °C; air-dried | rGO and Ag/Cu NPs |
Immersion in 0.25 mg/mL rGO suspension (RT, 4 h), air drying (3 times), separately soaked in 0.05 M AgNO3 and CuSO4·5H2O solutions (2 h), air-dried, immersion in 2% wt/V Na2S2O4 solution (chemical reduction, 4 h, 80 °C, 100 rpm), washed in water, dried (hotplate at 60 °C), and heat-treated in a vacuum oven (20 min, 175 °C). |
S. aureus, E. coli, P. aeruginosa, and C. albicans | CFU counts | 69–99% (S. aureus), 92–100% (E. coli), and 97–100% (P. aeruginosa) growth inhibition, especially with Ag NPs after 24 h; 63–69% C. albicans growth inhibition with Cu NPs (50% with Ag NPs), namely using cotton. | 85−99% growth inhibition against Gram-negative bacteria; 62 to 90% against S. aureus after 10 washing cycles. | Protective clothing | [15] |
| Woven cotton fabric (areal mass density: 280 g/m2; threads/cm: warp 48 ± 2; weft 37 ± 1; and CIE whiteness 80) |
Desized, bleached, and mercerized |
CS MCs, prepared by simple emulsion (with Tween 20) and loaded with cinnamon bark EO | Immersed in MCs (80 g/L) and the binding agent (40 g/L, DMDHEU), padded (wet pick up of 80%), dried (90 °C, 15 min), cured (150 °C, 5 min), autoclave-sterilized, and stored at RT. | S. aureus and E. coli | Diffusion assay method | 90% (S. aureus) and 97% (E. coli) growth inhibition. | 69% MC remaining after 5 washes, 12.5% after 10 washes. | Protective textiles | [23] |
| 100% cotton knitted fabric (194 g/m2) with (1 x 1) interlock structure |
Cleaned with acetone and water, mercerized |
Ag NPs | Immersed into a solution of C6H8O6 (5 min), dried (5 min, 80 °C); immersed into AgNO3 solution (5 min), dried (5 min, 80 °C); 1–3 cycles. Encapsulation in a silicone binder solution in acetone at a ratio of 1:7 for 5 min (1 time), dried (10 min, 80 °C). |
S. aureus and E. coli | AATCC 147, agar diffusion assay | Higher ZoI for 1-cycle samples after 24 h (0.531 mm with S. aureus, 0.25 mm with E. coli). | - | Protective textiles | [16] |
| Woven cotton fabric | Enzymatic desizing and scouring | CS and onion-skin dye | Dip-pad-dry: dip within CS (4%), C₆H₈O₇ (6%), and NaH2PO2 (5%) at 1:30 material:liquor ratio (pH 5, 90 °C, 45 min), pad (P = 2 kg/cm, expression of 70–75%), dry (100 °C, 5 min), and cure (140 °C, 4 min). Dyeing with onion-skin dye (exhaustion method): 6% dye, pH 5.5, 90 °C, 75 min, 1:30 material:liquor ratio. | S. aureus and E. coli | AATCC Test Method100, shake-flask | S. aureus (98.03%) and E. coli (97.20%) growth reduction after 24 h. | Reduction in S. aureus growth from 96.84 to 80.14% and E. Coli from 93.20 to 80.74% after 5–20 washing cycles. |
Protective textiles | [22] |
| Rayon fabric | Acetic acid (3 g/L) and TEGO® wet surfactant (2 g/L) (Evonik) solution in DW (pH 3.5, 20 min), oven-drying |
TiO2, Ag NPs | Dip-dry: immersion in coating mixture (60 mL of 5% TiO2 NPs + 9.7 mL PDMS + 8 mL of 1 M AgNO3 + 10 mL 0.017 M NaBH4 + 30 mL THF) 10 min, drying (70 °C, 4 h). | S. aureus and E. coli | Agar diffusion assay | ZoI of 14.44 mm (S. aureus) and 13.12 mm (E. coli) after 24 h. | Water contact angle remained nearly constant (152.3°) after 20 laundering cycles. | Multifunctional textiles | [19] |
| Polyamide taffeta (52 warp and 32 weft yarns, 100 g/m2) | Washing, plasma treatment (RT, atmospheric pressure, width of 50 cm, gap distance of 3 mm, 10 kV, 40 Hz, 5 times, both sides) |
Ag NPs, CS | Dip-dry: dip in each solution (5 min, RT) and dry (50 °C, 20 min). | S. aureus and P. aeruginosa | ASTM-E2149-01, shake-flask | S. aureus (80%) and P. aeruginosa (60%) growth reduction after 2 h. | - | Face masks | [14] |
| Bleached and mercerized cotton fabric | O2 plasma treatment (13.56 MHz, 3 min, 400 W, 200 cm3/min, 0.003 mbar); washing with nonionic detergent (C₃₂H₆₆O₉, 10 mmol); sonication (30 min); air-drying and washing with water; dipping in acetone solution of C9H22O3SSi (1%, 24 h); curing (75 °C, 30 min); rinsing with water |
Ag NPs | In situ synthesis of Ag NPs: dip in 0.1–4 wt % CH3AgNO2, sonication (15 min), padding, squeezing, and curing (130 °C, 5 min). | S. aureus, E. coli, and C. albicans | Agar diffusion assay | Clear and large ZoI after 24–48 h. | - | Multifunctional textiles | [8] |
| Plain cotton fabric (135 g/m2) | Immersion in 4 mg/mL C8H11NO2. HCl (pH 8.5) |
ZIF-8 | Immersion in Zn(NO3)2.6H2O (0.893 g, 15 mL) solution + C4H6N2 (0.985 g, 15 mL) solution, autoclaving (100 °C, 12 h), washing, and drying (60 °C). | E. coli | Disc diffusion method | Defined ZoI after 24 h. | - | Multifunctional textiles | [13] |
| Cotton fabrics (shibeka, honeycomb, and crepe) |
Bleached | CS or Ag NPs | Dip-dry: immersion in CS solution (10 min), squeezing for 100% wet pickup (constant pressure), drying (80 °C, 4 min), and curing (140 °C, 2 min); immersion in Ag NP dispersion (100–300 ppm), squeezing for 100% wet pickup (constant pressure), drying (80 °C, 3 min), and curing (140 °C, 2 min). | S. aureus, P. aeruginosa, C. albicans, and A. niger | Disc diffusion method | 20 or 13 (S. aureus), 15 or 11 (P. aeruginosa), 13 or 21 (C. albicans), and 12 or 11 mm (A. niger) with 6% CS (Crepe) or 300 ppm Ag NPs (Shebika), respectively, after 24 h. | - | Protective textiles | [29] |
| Desized and bleached cotton fabric (100% cellulose, 117.5 g/m2) | Washed (30 min, 50 °C, nonionic detergent Adrasil HP P-836, 1 g/L, 1:60 L:G), water-rinsed, dried at RT; periodate oxidation in phosphate buffer (pH 8, L:G 1:50, dark), addition of NaIO4 (5 g/L, 30 min, ultrasonication at 20 kHz, 750 W at 70% efficiency), water-washed, dried at RT; PABA treatment (10 g/L, 2 h) using acetate buffer solution (pH 5.5, ultrasonication), water-washed, dried at RT |
ZnO NPs | In situ synthesis of ZnO NPs: immersion in 1 mM ZnCl2 solution (30 min) and ultrasonication (pH 10 for 30 min by adding 4 g/L NaOH). Ultrasonication (extra 30 min, 60 °C), water washing, and drying (120 min, 110 °C). | S. aureus and E. coli | AATCC 100-2004, 24 h | 99.9% (S. aureus) and 99.4% (E. coli) growth inhibition. | 93.7% or 95.3% (S. aureus) and 93.4% or 95.4% (E. coli) after abrasion or washing process, respectively. | Protective textiles | [20] |
| Scoured and bleached plain-woven 100% cotton fabrics (165 gm/m2) |
Silicate modification: immersion in 100 mL of 5% NaOH (50 °C, 5 h, stirring), addition of 6 mL C3H5ClO (5 h reaction), water and anhydrous ethanol washing, drying (60 °C); silicate mixture synthesized by dropwise addition of SiC8H20O4 (12 mL) and methanol (80 mL) to a flask with 30 mL of ammonia and 320 mL of methanol; stirring 3 h, curing (110 °C, 1 h) | ZIF(Ni), ZIF-8(Zn), and ZIF- 67(Co) MOFs |
In situ synthesis of MOFs: immersion, separately, in 50 mL of methanol with metal salts (0.736 g of Ni(NO3)2, 0.758 g of Zn(NO₃)₂, and 0.733 g of Co(NO3)2), stirring 1 h at RT; pour three solutions individually from C4H6N2 (1.623 g in 50 mL of methanol) above the three mixtures, stir 8 h; ethanol-wash and dried (vacuum, 60 °C, 12 h). | S. aureus, B. cereus, E. coli, and C. albicans | Kirby−Bauer disk diffusion method, overnight | ZoI: 25 (S. aureus), 23 (B. cereus), 15 (E. coli), 22 (C. albicans) for cotton–silicate–ZIF(Ni). | ZoI: 19 (S. aureus), 18 (B. cereus), 12 (E. coli), 18 (C. albicans) for cotton–silicate–ZIF(Ni) after 5 washing cycles. | Protective textiles | [12] |
| Inner layer: polystyrene fiber 3-ply twisted yarns (tex: 0.058, 0.115, or 0.230); outer layer: 3-ply twisted single yarns with PCMs, including use of functional fibers Resistex® Silver |
Washed with 2.5 g/L nonionic detergent Felosan RG-N, 2.0 g/L Na2CO3, 3.0 g/L water softener CalgonVR Power (60 °C, 60 min), rinsed with 1 g/L acetic acid solution, centrifuged, air-dried |
Silver | None | S. aureus, E. coli, and K. pneumoniae | EN ISO 20645 | Low bacterial growth. | - | Multifunctional socks | [30] |
PET: poly(ethylene terephthalate);TFA: trifluoroacetic acid; RT: room temperature; AATCC (American Association of Textile Chemists and Colorists); TM: 4–(2,4–dichlorophenyl)–6–oxo–2–thioxohexahydropyrimidine–5–carbonitrile; MIC: minimum inhibitory concentration; CNWs: cellulose nonwovens; CI: cyclohexyl isocyanate; UV: ultraviolet; PTB: poly(thiiran-2-yl methyl methacrylate-2-(4-benzoyl phenoxy)ethyl methacrylate; PHMG: polyhexamethyleneguanidine; NEO: neomycin sulfate; DAPH: diammonium hydrogen phosphate; ZPT: zinc pyrithione; ZoI: zone of inhibition; CFU: colony-forming units; DMDHEU: dimethyloldihydroxyethylene urea; ZIF-8: zeolite imidazole skeleton-8; PABA: 4-aminobenzoic acid ligand; L:G: liquor-to-fabric ratio; PCMs: phase-change materials.
References
- Schreuder-Gibson, H.L.; Truong, Q.; Walker, J.E.; Owens, J.R.; Wander, J.D.; Jones Jr, W.E. Chemical and biological protection and detection in fabrics for protective clothing. MRS Bull. 2003, 28, 574–578.
- Bhuiyan, M.A.R.; Wang, L.; Shaid, A.; Shanks, R.A.; Ding, J. Advances and applications of chemical protective clothing system. J. Ind. Text. 2019, 49, 97–138.
- Lundberg, D.J.; Brooks, A.M.; Strano, M.S. Design Rules for Chemostrictive Materials as Selective Molecular Barriers. Adv. Eng. Mat. 2022, 24, 2101112.
- Zhao, X.; Liu, B. Permeable Protective Suit: Status Quo and Latest Research Progress. Cailiao Daobao/Mater Rev 2018, 32, 3083–3089.
- Araújo, J.C.; Fangueiro, R.; Ferreira, D.P. Protective multifunctional fibrous systems based on natural fibers and metal oxide nanoparticles. Polymers 2021, 13, 2654.
- Truong, Q.; Wilusz, E. 13—Advances in chemical and biological protective clothing. In Smart Textiles for Protection; Chapman, R.A., Ed.; Woodhead Publishing: Cambridgeshire, UK, 2013; pp. 364–377.
- Al-Balakocy, N.G.; Shalaby, S.E. Imparting antimicrobial properties to polyester and polyamide fibers-state of the art. J. Text. Assoc. 2017, 78, 179–201.
- El-Naggar, M.E.; Khattab, T.A.; Abdelrahman, M.S.; Aldalbahi, A.; Hatshan, M.R. Development of antimicrobial, UV blocked and photocatalytic self-cleanable cotton fibers decorated with silver nanoparticles using silver carbamate and plasma activation. Cellulose 2021, 28, 1105–1121.
- Natarajan, G.; Rajan, T.P.; Das, S. Application of Sustainable Textile Finishing Using Natural Biomolecules. J. Nat. Fibers 2020, 1–18.
- Basuk, M.; Kherdekar, G. A synopsis on Coating and lamination in textiles: Process and applications. Colourage 2018, 65, 43–55.
- Cheung, Y.H.; Ma, K.; Van Leeuwen, H.C.; Wasson, M.C.; Wang, X.; Idrees, K.B.; Gong, W.; Cao, R.; Mahle, J.J.; Islamoglu, T.; et al. Immobilized Regenerable Active Chlorine within a Zirconium-Based MOF Textile Composite to Eliminate Biological and Chemical Threats. J. Am. Chem. Soc. 2021, 143, 16777–16785.
- Emam, H.E.; Darwesh, O.M.; Abdelhameed, R.M. Protective cotton textiles via amalgamation of cross-linked zeolitic imidazole frameworks. Ind. Eng. Chem. Res. 2020, 59, 10931–10944.
- Ran, J.; Chen, H.; Bi, S.; Guo, Q.; Deng, Z.; Cai, G.; Cheng, D.; Tang, X.; Wang, X. One-step in-situ growth of zeolitic imidazole frameworks-8 on cotton fabrics for photocatalysis and antimicrobial activity. Cellulose 2020, 27, 10447–10459.
- Botelho, C.M.; Fernandes, M.M.; Souza, J.M.; Dias, N.; Sousa, A.M.; Teixeira, J.A.; Fangueiro, R.; Zille, A. New textile for personal protective equipment—Plasma chitosan/silver nanoparticles nylon fabric. Fibers 2021, 9, 3.
- Bhattacharjee, S.; Joshi, R.; Yasir, M.; Adhikari, A.; Chughtai, A.A.; Heslop, D.; Bull, R.; Willcox, M.; Macintyre, C.R. Graphene- And Nanoparticle-Embedded Antimicrobial and Biocompatible Cotton/Silk Fabrics for Protective Clothing. ACS Appl. Bio. Mat. 2021, 4, 6175–6185.
- Islam, M.T.; Mamun, M.A.A.; Hasan, M.T.; Shahariar, H. Scalable coating process of AgNPs-silicone on cotton fabric for developing hydrophobic and antimicrobial properties. J. Coat. Technol. Res. 2021, 18, 887–898.
- Tania, I.S.; Ali, M.; Azam, M.S. Mussel-Inspired Deposition of Ag Nanoparticles on Dopamine-Modified Cotton Fabric and Analysis of its Functional, Mechanical and Dyeing Properties. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4065–4076.
- Tavares, T.D.; Antunes, J.C.; Ferreira, F.; Felgueiras, H.P. Biofunctionalization of natural fiber-reinforced biocomposites for biomedical applications. Biomolecules 2020, 10, 148.
- Görgülüer, H.; Çakıroğlu, B.; Özacar, M. Ag NPs deposited TiO2 coating material for superhydrophobic, antimicrobial and self-cleaning surface fabrication on fabric. J. Coat. Technol. Res. 2021, 18, 569–579.
- Noorian, S.A.; Hemmatinejad, N.; Navarro, J.A.R. Ligand modified cellulose fabrics as support of zinc oxide nanoparticles for UV protection and antimicrobial activities. Int. J. Biol. Macromol. 2020, 154, 1215–1226.
- Emam, H.E.; El-Shahat, M.; Hasanin, M.S.; Ahmed, H.B. Potential military cotton textiles composed of carbon quantum dots clustered from 4–(2,4–dichlorophenyl)–6–oxo–2–thioxohexahydropyrimidine–5–carbonitrile. Cellulose 2021, 28, 9991–10011.
- Verma, M.; Gahlot, N.; Singh, S.S.J.; Rose, N.M. UV protection and antibacterial treatment of cellulosic fibre (cotton) using chitosan and onion skin dye. Carbohydr. Polym. 2021, 257, 117612.
- Bouaziz, A.; Dridi, D.; Gargoubi, S.; Zouari, A.; Majdoub, H.; Boudokhane, C.; Bartegi, A. Study on the grafting of chitosan-essential oil microcapsules onto cellulosic fibers to obtain bio functional material. Coatings 2021, 11, 637.
- Antunes, J.C.; Domingues, J.M.; Miranda, C.S.; Silva, A.F.G.; Homem, N.C.; Amorim, M.T.P.; Felgueiras, H.P. Bioactivity of chitosan-based particles loaded with plant-derived extracts for biomedical applications: Emphasis on antimicrobial fiber-based systems. Mar. Drugs 2021, 19, 359.
- Singh, N.; Sheikh, J. Novel Chitosan-Gelatin microcapsules containing rosemary essential oil for the preparation of bioactive and protective linen. Ind. Crops Prod. 2022, 178, 114549.
- Wang, Y.; Ma, K.; Bai, J.; Xu, T.; Han, W.; Wang, C.; Chen, Z.; Kirlikovali, K.O.; Li, P.; Xiao, J.; et al. Chemically Engineered Porous Molecular Coatings as Reactive Oxygen Species Generators and Reservoirs for Long-Lasting Self-Cleaning Textiles. Angew. Chem. Int. 2022, 61, e202115956.
- Kumari, N.; Bhattacharya, S.N.; Das, S.; Datt, S.; Singh, T.; Jassal, M.; Agrawal, A.K. In Situ Functionalization of Cellulose with Zinc Pyrithione for Antimicrobial Applications. ACS Appl. Mater. Interfaces 2021, 13, 47382–47393.
- Deng, C.; Seidi, F.; Yong, Q.; Jin, X.; Li, C.; Zhang, X.; Han, J.; Liu, Y.; Huang, Y.; Wang, Y.; et al. Antiviral/antibacterial biodegradable cellulose nonwovens as environmentally friendly and bioprotective materials with potential to minimize microplastic pollution. J. Hazard Mater. 2022, 424, 127391.
- Ramadan, M.A.; Taha, G.M.; El- Mohr, W.Z.E.A. Antimicrobial and uv protection finishing of polysaccharide -based textiles using biopolymer and agnps. Egypt. J. Chem. 2020, 63, 2707–2716.
- Stygienė, L.; Varnaitė-Žuravliova, S.; Abraitienė, A.; Sankauskaitė, A.; Skurkytė-Papievienė, V.; Krauledas, S.; Mažeika, V. Development, investigation and evaluation of smart multifunctional socks. J. Ind. Text. 2020, 1528083720970166.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
837
Revisions:
2 times
(View History)
Update Date:
29 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




