Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marissa O'Callaghan | -- | 1863 | 2022-04-28 18:44:50 | | | |
| 2 | Lindsay Dong | -8 word(s) | 1855 | 2022-04-29 03:25:34 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
O'callaghan, M.; , .; Mccarthy, C. Vaping-Associated Lung Injury. Encyclopedia. Available online: https://encyclopedia.pub/entry/22464 (accessed on 16 January 2026).
O'callaghan M, , Mccarthy C. Vaping-Associated Lung Injury. Encyclopedia. Available at: https://encyclopedia.pub/entry/22464. Accessed January 16, 2026.
O'callaghan, Marissa, , Cormac Mccarthy. "Vaping-Associated Lung Injury" Encyclopedia, https://encyclopedia.pub/entry/22464 (accessed January 16, 2026).
O'callaghan, M., , ., & Mccarthy, C. (2022, April 28). Vaping-Associated Lung Injury. In Encyclopedia. https://encyclopedia.pub/entry/22464
O'callaghan, Marissa, et al. "Vaping-Associated Lung Injury." Encyclopedia. Web. 28 April, 2022.
Copy Citation
The lungs are exposed to a multitude of environmental agents with each inspiration. Some of these agents are toxic or cause damage to the lungs. Vaping or electronic cigarette (e-cigarette) use is no exception. These devices aerosolise a liquid vapour, which is then inhaled. This vapour contains chemical compounds such as nicotine, flavourings and tetrahydrocannabinol (THC). Some of these chemicals have irritative, toxic and carcinogenic properties. When inhaled these can alter the immune responses critical for normal lung function and cause lung injury. The pathological manifestation of this is diverse varying from organising pneumonia or diffuse alveolar damage to established interstitial lung disease (ILD).
vaping
e-cigarette
foamy macrophages
1. Introduction
The damaging health consequences of cigarette smoking have been widely acknowledged for years [1]. While the ability to diagnose and the management of smoking-related diseases has improved considerably, cigarette smoking, unfortunately, remains prevalent, so health services remain inundated with smoking-related illnesses [2][3]. These include, but are not limited to, coronary artery disease, cerebrovascular disease, chronic obstructive pulmonary disease, lung cancer and respiratory tract infections [4][5].
Modern e-cigarettes, commercially developed in 2003, were advertised as a novel therapy for smoking cessation [6]. These electronic devices are designed to vaporise chemical compounds [7], the term ‘vaping’ referring to the perception that the exhaled smoke is water vapour. It actually consists of fine particles of chemicals mixed in vegetable glycerin (VG) and/or propylene glycol (PG) [8]. The vaping device consists of a mouthpiece, a battery, a tank which contains the “e-liquid” or “e-juice” and a heating component for the device (Figure 1) [7][9].
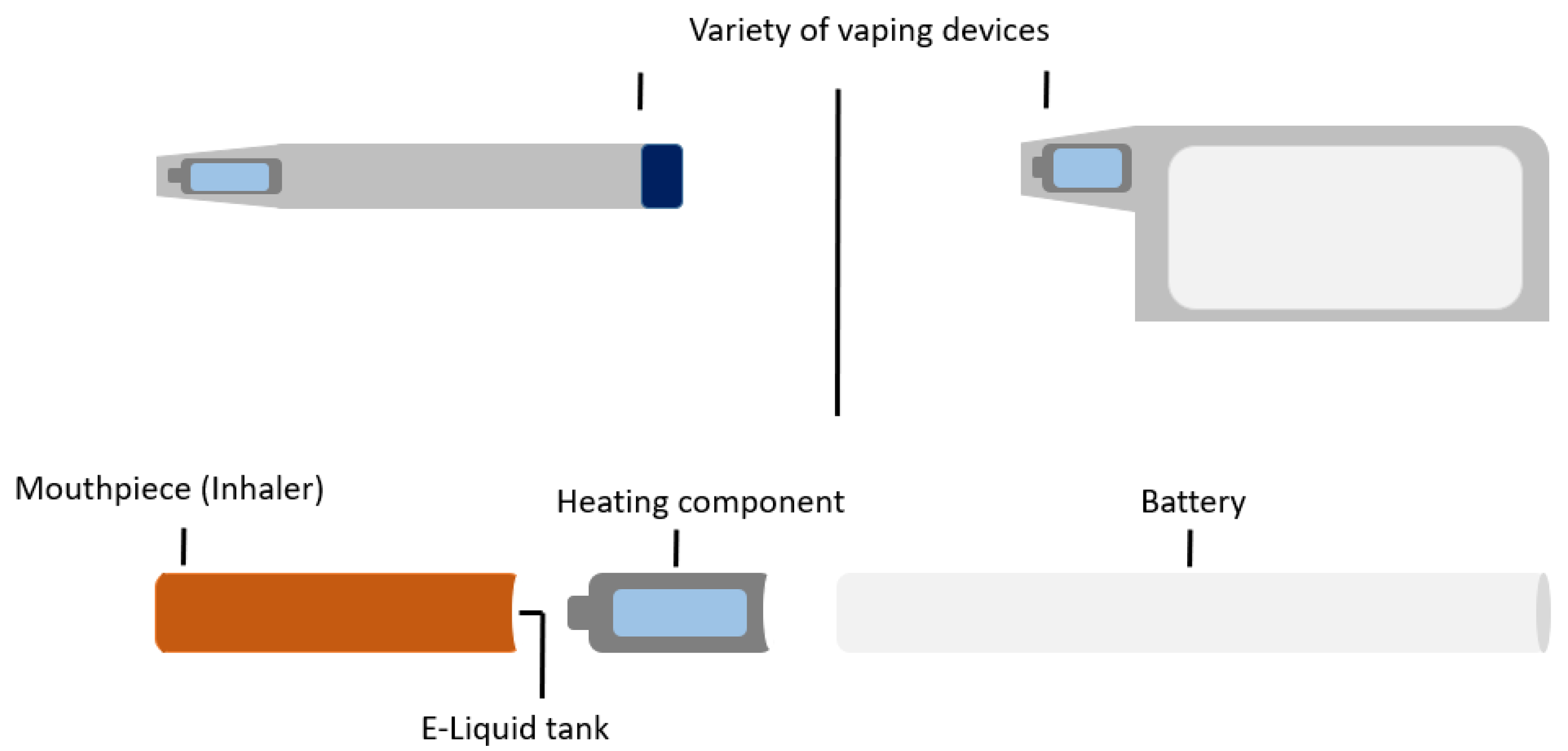
Figure 1. E-cigarette or vaping device.
E-cigarettes have since been developed in various shapes, sizes and device types. Different terminologies used to describe these devices include e-cigs, vapes, e-hookahs, vape pens, mods, tanks or electronic nicotine delivery systems (ENDS) [7]. All delivery devices work on a similar principle. Electricity, activated manually or automatically by a battery, is delivered to the device’s heating component. This, in turn, causes the e-liquid contained in the tank to evaporate and condense into a fine mist of liquid droplets (aerosols) [10]. The e-liquid or substance placed in the device or tank is user-dependant. Commonly used substances include nicotine, fruity and menthol flavouring. A minority of users use e-liquids from unauthorised sources or modify the e-liquid contents. This risks exposure to potentially harmful compounds such as heavy metals or carcinogenic chemicals [7][11].
2. Epidemiology of E-Cigarette Use
E-Cigarettes are marketed as a harm reduction tool for tobacco smokers wishing to quit [6]. They are advertised as a safe and viable alternative to cigarette smoking; however, there is a lack of evidence to prove superiority to conventional smoking cessation strategies [7][12]. E-cigarette use worldwide has grown dramatically, with a prevalence of 5.5% amongst adults in both North America and England [6][13][14][15]. The response from tobacco regulatory officials has been mixed. Some are promoting the use of e-cigarettes for harm minimisation [16], while others have requested regulation of the nicotine market, favouring proven smoking cessation techniques. Data to support any of these marketing strategies remain limited. E-Cigarette users are varied and include people who have never smoked, ex-smokers who have switched to e-cigarettes and dual-users of both conventional cigarettes and e-cigarettes [6]. The role of e-cigarettes as a smoking cessation tool is hotly contested. Trial results have been mixed and ultimately inconclusive. E-cigarettes with nicotine likely increase smoking cessation rates compared to e-cigarettes without nicotine. In addition, there is no clear evidence of harm from nicotine e-cigarettes; however, the patient numbers in the studies to date have been low, and the longest follow-up period was two years [17][18]. E-cigarettes with nicotine demonstrated improved smoking cessation rates over conventional nicotine replacement therapy in a recently published randomised control trial. The caveat was that participants in both groups had regular face-to-face meetings with clinicians, a form of support that is rarely provided to those seeking to quit in the real world [19]. Furthermore, only 18% of participants in the e-cigarette group stopped smoking entirely, suggesting that e-cigarettes are a far cry from a “cure” for tobacco smoking. Some studies even suggest that there are increased smoking relapse rates when e-cigarettes are used as a cessation tool [20][21].
Major tobacco companies entered the e-cigarette industry in 2012 and have since progressively dominated the market, buying out smaller retailers [22][23]. These companies are more likely to sell ‘cigalikes’, a form of e-cigarette with a slim cylindrical closed-system design that uses prefilled cartridges, to maximise the ease of use. These devices mimic the experience of smoking conventional cigarettes and studies suggest that smokers of cigalikes are more likely to remain dual users [23][24][25]. While other factors may also be contributory, international surveys show that one out of eight smokers have tried e-cigarettes, with the highest prevalence being amongst younger, female, higher-income smokers [26][27]. E-cigarette users view e-cigarettes as safer, healthier and less likely to cause dependency than conventional cigarettes [3][26][27][28][29][30][31]. However, without clear evidence of a role in the reduction of tobacco dependence, e-cigarettes risk renormalising and re-glamorising smoking. This is of paramount concern, potentially undoing years of effort by the public health and medical communities [3][32].
3. Mechanism of Injury with Vaping
While analysis of e-cigarette efficacy in aiding smoking cessation is ongoing, data on the overall impact of e-cigarettes on population health is limited [3]. Over 7000 compounds and at least 70 carcinogens have been identified in conventional tobacco smoke [1][3][33][34][35]. Studies comparing the toxic exposures between e-cigarettes and conventional cigarettes reported that levels of two nitrosamines and carbon monoxide were lower in e-cigarette users than in smokers but present nonetheless [36][37][38][39]. Unsurprisingly, toxic exposures were greatest in dual users [35]. Both smokers and e-cigarette users also had increased toxic metals in urine and blood, but there was some variability in the metals detected in each group [6][35][36][39].
While there are greater chemical emissions from combustible tobacco cigarette smoke than from most e-cigarette products, the chemicals in e-liquids and the additional chemicals generated during the aerosolisation of e-liquids also have potential toxic properties [40][41][42]. It has been proposed that oxidative stress is the primary driver of e-cigarette-induced toxicity at a cellular level [6][40][43][44][45][46][47][48][49][50]. While this theory is a plausible explanation for the tissue injuries reported in the literature, the impact is less than that caused by combustible tobacco smoking [51]. Another group demonstrated acute endothelial cell dysfunction following e-cigarette aerosol exposure but highlights the uncertainty surrounding the long-term consequences and outcomes with long term exposure [40][52].
Scott et al. sought to replicate the potential effects of exposure of the e-cigarette user in an acute in vitro system using a vaping-condensate technique. They showed that exposure of macrophages to e-cigarette vapour-condensate induced many of the same cellular and functional changes in alveolar macrophages seen in cigarette smokers and patients with COPD [43]. Adolescents who use e-cigarettes commonly report an increased cough and wheeze, and studies have shown an association with e-cigarette use and asthma exacerbations [2][53][54]. However, it is not yet clear if chronic e-cigarette use by itself can cause COPD in a clinical setting or if the substitution of e-cigarettes for combustible tobacco products can prevent or slow the development of COPD [40][55][56]. There is also data to support a correlation between e-cigarette use and impaired host defence [57][58]. It appears viral responses are compromised [46], and bacterial clearance by macrophages [43][59] and neutrophils appears to be reduced [60][61]. This allows increased adhesion and colonisation of bacteria [60][62] and possibly an impaired infection-fighting ability [3].
4. Electronic Cigarette and Vaping-Associated Lung Injury
A wide range of clinical presentations have been reported in the literature [63][64][65][66][67][68][69][70][71]. Respiratory symptoms are the most prevalent at hospital presentation, specifically dyspnoea, cough and chest pain. Many patients report associated gastrointestinal symptoms and constitutional symptoms, most commonly subjective fever [65][66]. Laboratory testing frequently reveals a peripheral blood eosinophilia, elevated erythrocyte sedimentation rate (ESR) and the presence of lipid-laden macrophages on bronchoalveolar lavage (BAL) assessment [68][70][72]. Most patients will have abnormal chest imaging. Typical computed tomography (CT) thorax findings are bilateral lung opacities with ground-glass changes, sometimes with subpleural sparing (Figure 2). Other reported findings include pneumomediastinum, pleural effusion and pneumothorax [65].
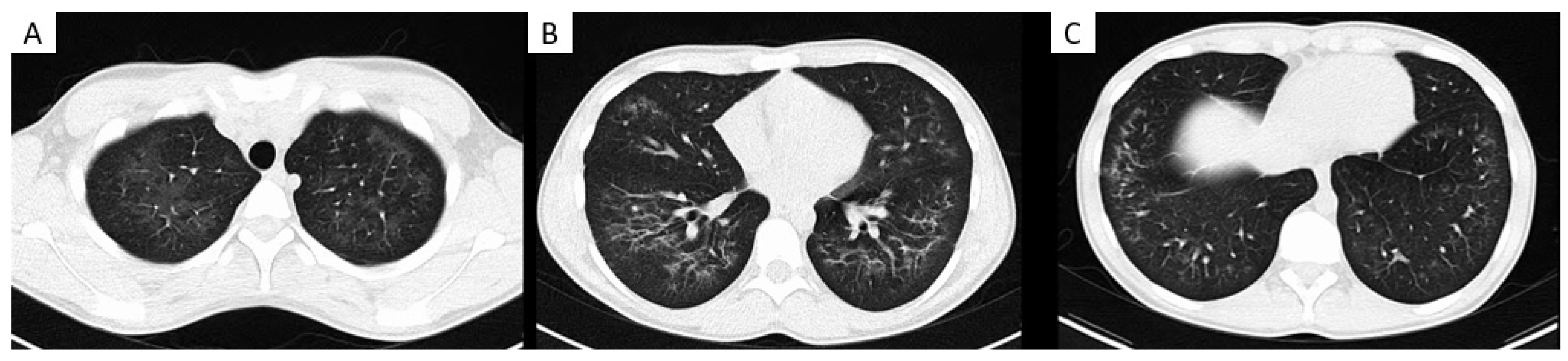
Figure 2. Images show electronic cigarette or vaping product use–associated lung injury in an 18-year-old male. Axial CT chest imaging (A–C) demonstrates extensive bilateral centrilobular and peri-bronchial ground glass opacification with subpleural sparing, slightly more confluent in the lower zones.
Although the exact mechanism of lung injury remains unclear and under investigation, exposure to products containing tetrahydrocannabinol (THC) was reported in over 80% of cases [73], and in many cases, unregulated or illicit street sources of THC were reported [74][75]. THC is the main psychoactive component in cannabis and despite THC-based oils and waxes being illegal in most American States, they remain easily accessible. THC-containing products that were seized by United States law enforcement at the peak of the EVALI outbreak, contained higher levels of vitamin E acetate than would be expected [65][76]. Furthermore, BAL samples from patients with EVALI have shown high rates of vitamin E [77] with a notable absence of vitamin E acetate in samples obtained from a healthy comparison group [78]. Vitamin E is a naturally occurring compound in surfactant, however, in contrast, vitamin E acetate is the synthetic ester of tocopherol and acetate. It is commonly added to e-liquids as a thickening agent [78].
The mechanism by which vitamin E acetate causes lung injury is not fully understood. Mice exposed to aerosols generated from vitamin E acetate have demonstrated elevated levels of BAL lipid-laden macrophages on Oil-Red-O stain [79], which is in keeping with the BAL findings in patients with EVALI [68][72] (Figure 3). The presence of lipid-laden macrophages led to studies looking at macrophage lipid metabolism. It is possible that increasing concentrations of vitamin E or vitamin E acetate could affect the physical structure and phase behaviour of surfactants [78][80]. This may then impair the ability of the surfactants to maintain alveolar surface tension, leading to respiratory dysfunction [71][78][79]. Furthermore, vitamin E acetate forms a toxic compound, ketene, when heated. Ketene is a known lung irritant and thus may also contribute to the chemical pneumonitis seen in patients with EVALI [78][81][82] (Figure 4).
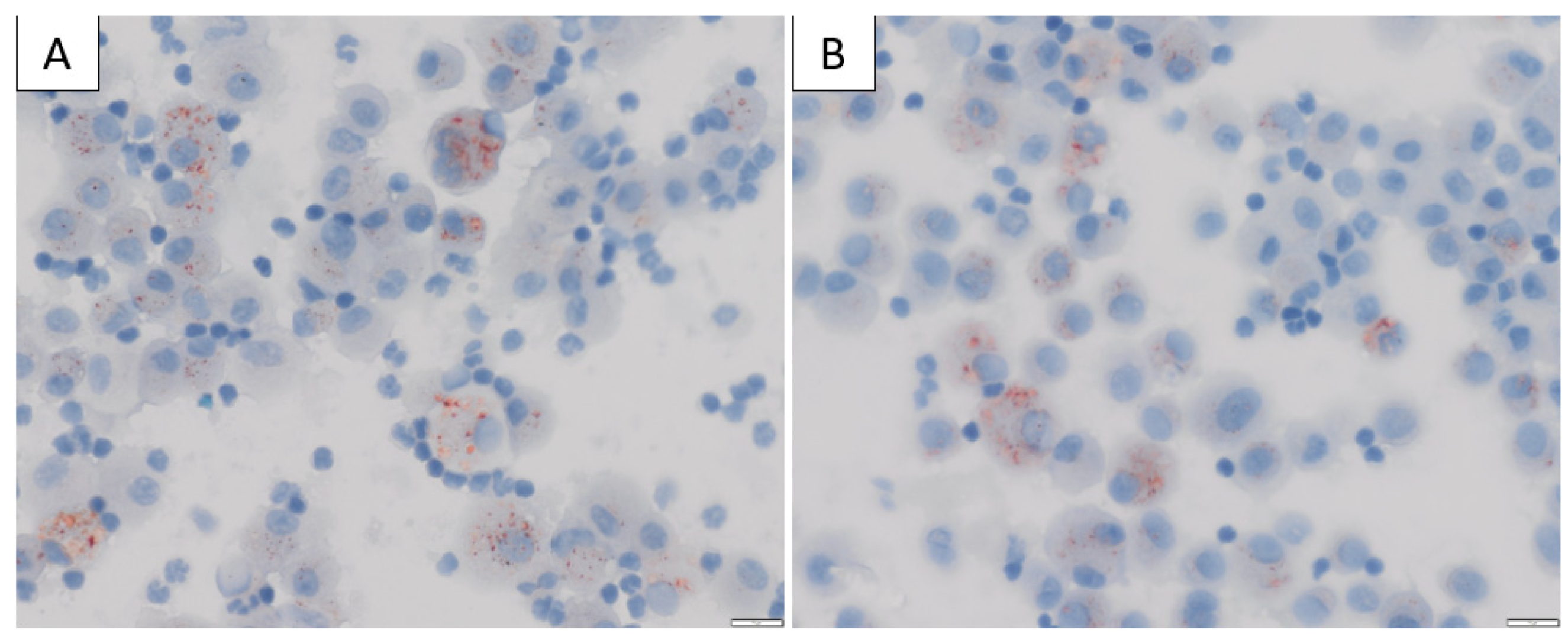
Figure 3. (A,B) Bronchoalveolar lavage (BAL) cytology from a patient diagnosed with EVALI in our institution, stained with Oil-Red-O × 400 magnification showing positive red intracytoplasmic droplets in the alveolar macrophages, consistent with excess neutral lipid.
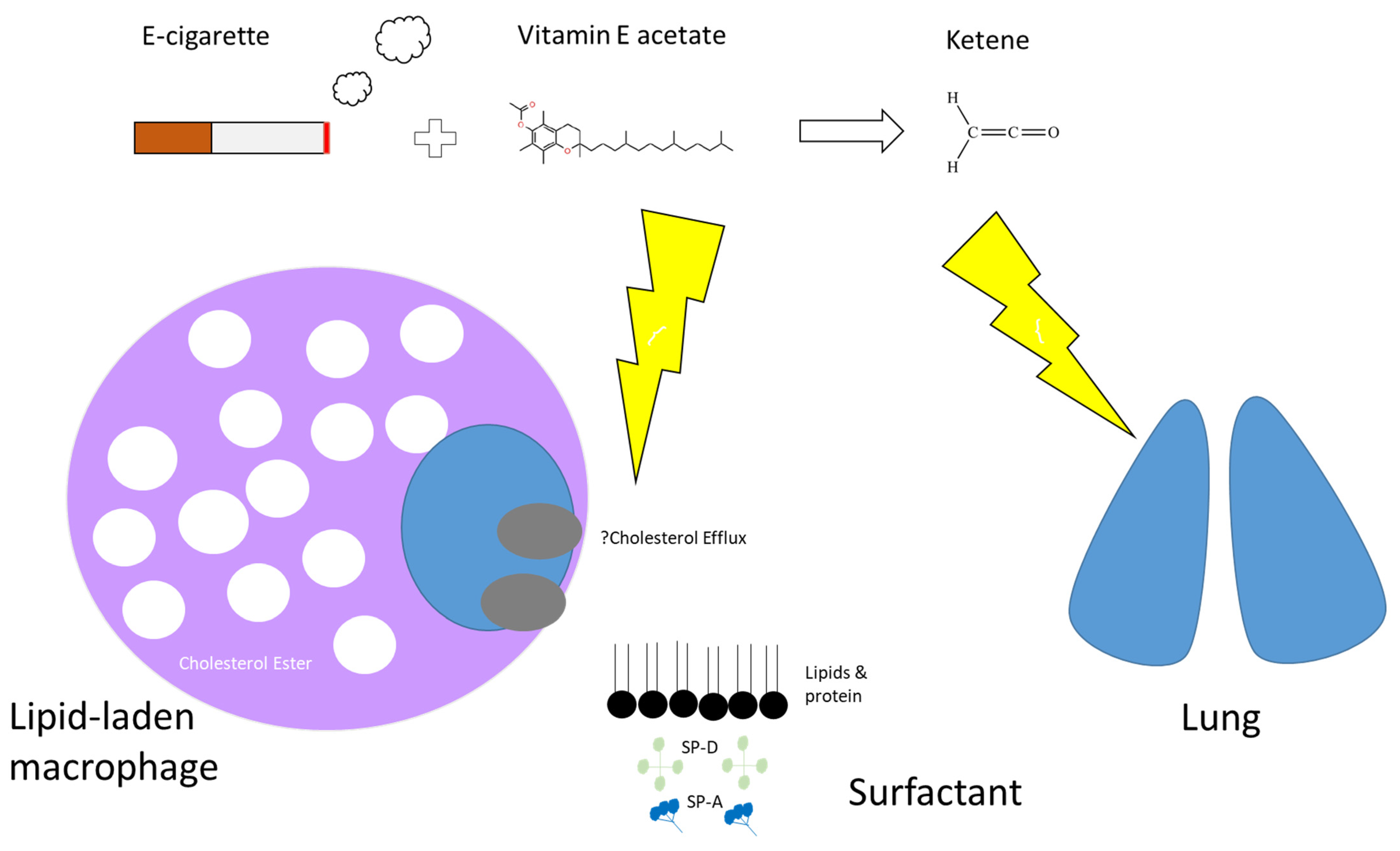
Figure 4. Proposed mechanism of action by which e-cigarettes cause lung injury. Many e-cigarettes that contain tetrahydrocannabinol (THC) have been shown to have higher levels of vitamin E acetate, commonly used as a thickening agent. It is possible that increased exposure of the lungs to Vitamin E (naturally occurring at low levels in surfactant) or Vitamin E acetate could affect the physical structure and phase behaviour of surfactant, impairing its ability to maintain surface tension leading to respiratory distress. Dysfunctional surfactant might lead to excess lipid accumulation within alveolar macrophages and that reverse cholesterol transport or cholesterol efflux might be implicated. Secondly, a known product of vaporised vitamin E acetate is ketene which is believed to be a lung irritant.
While most patients with EVALI went on to have a full recovery, over 2800 patients were hospitalised, and 68 deaths were reported throughout the outbreak [83]. Many case reports describe improvement with corticosteroid therapy [67]; however, the natural progression of this injury is not yet known, and it is possible that patients might recover without steroids or by avoiding use of e-cigarettes alone [67]. Nevertheless, because the diagnosis remains one of exclusion, empiric antimicrobial therapy might be warranted for patients with severe illness [65][84].
References
- Wellmann, K.F. Smoking and health. on the report of the advisory committee to the surgeon general of the public health service]. Dtsch. Med. Wochenschr. 1964, 89, 1085–1086.
- Thun, M.J.; Carter, B.D.; Feskanich, D.; Freedman, N.D.; Prentice, R.; Lopez, A.D.; Hartge, P.; Gapstur, S.M. 50-year trends in smoking-related mortality in the United States. N. Engl. J. Med. 2013, 368, 351–364.
- Drummond, M.B.; Upson, D. Electronic cigarettes. Potential harms and benefits. Ann. Am Thorac. Soc. 2014, 11, 236–242.
- Freeman, B.; Chapman, S. British American Tobacco on Facebook: Undermining Article 13 of the global World Health Organization Framework Convention on Tobacco Control. Tob. Control 2010, 19, e1–e9.
- West, R. Tobacco smoking: Health impact, prevalence, correlates and interventions. Psychol. Health 2017, 32, 1018–1036.
- Bozier, J.; Chivers, E.K.; Chapman, D.G.; Larcombe, A.N.; Bastian, N.A.; Masso-Silva, J.A.; Byun, M.K.; McDonald, C.F.; Alexander, L.E.; Ween, M.P. The Evolving Landscape of e-Cigarettes: A Systematic Review of Recent Evidence. Chest 2020, 157, 1362–1390.
- Oriakhi, M. Vaping: An Emerging Health Hazard. Cureus 2020, 12, e7421.
- Sosnowski, T.R.; Odziomek, M. Particle Size Dynamics: Toward a Better Understanding of Electronic Cigarette Aerosol Interactions with the Respiratory System. Front. Physiol. 2018, 9, 853.
- Salzman, G.A.; Alqawasma, M.; Asad, H. Vaping Associated Lung Injury (EVALI): An Explosive United States Epidemic. Mo. Med. 2019, 116, 492–496.
- Schier, J.G.; Meiman, J.G.; Layden, J.; Mikosz, C.A.; VanFrank, B.; King, B.A.; Salvatore, P.P.; Weissman, D.N.; Thomas, J.; Melstrom, P.C.; et al. Severe Pulmonary Disease Associated with Electronic-Cigarette-Product Use—Interim Guidance. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 787–790.
- Lampos, S.; Kostenidou, E.; Farsalinos, K.; Zagoriti, Z.; Ntoukas, A.; Dalamarinis, K.; Savranakis, P.; Lagoumintzis, G. Real-Time Assessment of E-Cigarettes and Conventional Cigarettes Emissions: Aerosol Size Distributions, Mass and Number Concentrations. Toxics 2019, 7, 45.
- Balkissoon, R. Journal Club-Electronic Cigarettes and Vaping as a Harm Reduction Alternative: Really? Chronic. Obstr. Pulm. Dis. 2019, 6, 281–291.
- Kock, L.; Shahab, L.; West, R.; Brown, J. E-cigarette use in England 2014-17 as a function of socio-economic profile. Addiction 2019, 114, 294–303.
- Hammond, D.; Reid, J.L.; Rynard, V.L.; Fong, G.T.; Cummings, K.M.; McNeill, A.; Hitchman, S.; Thrasher, J.F.; Goniewicz, M.L.; Bansal-Travers, M.; et al. Prevalence of vaping and smoking among adolescents in Canada, England, and the United States: Repeat national cross sectional surveys. BMJ 2019, 365, l2219.
- Kasza, K.A.; Ambrose, B.K.; Conway, K.P.; Borek, N.; Taylor, K.; Goniewicz, M.L.; Cummings, K.M.; Sharma, E.; Pearson, J.L.; Green, V.R.; et al. Tobacco-Product Use by Adults and Youths in the United States in 2013 and 2014. N. Engl. J. Med. 2017, 376, 342–353.
- Abrams, D.B.; Glasser, A.M.; Pearson, J.L.; Villanti, A.C.; Collins, L.K.; Niaura, R.S. Harm Minimization and Tobacco Control: Reframing Societal Views of Nicotine Use to Rapidly Save Lives. Annu. Rev. Public Health 2018, 39, 193–213.
- Hartmann-Boyce, J.; McRobbie, H.; Lindson, N.; Bullen, C.; Begh, R.; Theodoulou, A.; Notley, C.; Rigotti, N.A.; Turner, T.; Butler, A.R.; et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev. 2020, 10, CD010216.
- Ibrahim, S.; Habiballah, M.; Sayed, I.E. Efficacy of Electronic Cigarettes for Smoking Cessation: A Systematic Review and Meta-Analysis. Am. J. Health Promot. 2021, 35, 442–455.
- Hajek, P.; Phillips-Waller, A.; Przulj, D.; Pesola, F.; Myers Smith, K.; Bisal, N.; Li, J.; Parrott, S.; Sasieni, P.; Dawkins, L.; et al. A Randomized Trial of E-Cigarettes versus Nicotine-Replacement Therapy. N. Engl. J. Med. 2019, 380, 629–637.
- McMillen, R.; Klein, J.D.; Wilson, K.; Winickoff, J.P.; Tanski, S. E-Cigarette Use and Future Cigarette Initiation Among Never Smokers and Relapse Among Former Smokers in the PATH Study. Public Health Rep. 2019, 134, 528–536.
- Gomajee, R.; El-Khoury, F.; Goldberg, M.; Zins, M.; Lemogne, C.; Wiernik, E.; Lequy-Flahault, E.; Romanello, L.; Kousignian, I.; Melchior, M. Association Between Electronic Cigarette Use and Smoking Reduction in France. JAMA Intern. Med. 2019, 179, 1193–1200.
- U.S. Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion; Health OoSa. E-Cigarette Use Among Youth and Young Adults; A Report of the Surgeon General; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2016.
- Hsu, G.; Sun, J.Y.; Zhu, S.H. Evolution of Electronic Cigarette Brands From 2013–2014 to 2016–2017: Analysis of Brand Websites. J. Med. Internet Res. 2018, 20, e80.
- Zhuang, Y.L.; Cummins, S.E.; Sun, J.Y.; Zhu, S.H. Long-term e-cigarette use and smoking cessation: A longitudinal study with US population. Tob. Control 2016, 25 (Suppl. 1), i90–i95.
- Tackett, A.P.; Lechner, W.V.; Meier, E.; Grant, D.M.; Driskill, L.M.; Tahirkheli, N.N.; Wagener, T.L. Biochemically verified smoking cessation and vaping beliefs among vape store customers. Addiction 2015, 110, 868–874.
- Adkison, S.E.; O’Connor, R.J.; Bansal-Travers, M.; Hyland, A.; Borland, R.; Yong, H.H.; Cummings, K.M.; McNeill, A.; Thrasher, J.F.; Hammond, D.; et al. Electronic nicotine delivery systems: International tobacco control four-country survey. Am. J. Prev. Med. 2013, 44, 207–215.
- Mayer, M.; Reyes-Guzman, C.; Grana, R.; Choi, K.; Freedman, N.D. Demographic Characteristics, Cigarette Smoking, and e-Cigarette Use Among US Adults. JAMA Netw. Open 2020, 3, e2020694.
- Pearson, J.L.; Richardson, A.; Niaura, R.S.; Vallone, D.M.; Abrams, D.B. E-Cigarette awareness, use, and harm perceptions in US adults. Am. J. Public Health 2012, 102, 1758–1766.
- Etter, J.F.; Bullen, C. Electronic cigarette: Users profile, utilization, satisfaction and perceived efficacy. Addiction 2011, 106, 2017–2028.
- Dawkins, L.; Turner, J.; Roberts, A.; Soar, K. ‘Vaping’ profiles and preferences: An online survey of electronic cigarette users. Addiction 2013, 108, 1115–1125.
- Goniewicz, M.L.; Lingas, E.O.; Hajek, P. Patterns of electronic cigarette use and user beliefs about their safety and benefits: An internet survey. Drug Alcohol Rev. 2013, 32, 133–140.
- Collins, L.; Glasser, A.M.; Abudayyeh, H.; Pearson, J.L.; Villanti, A.C. E-Cigarette Marketing and Communication: How E-Cigarette Companies Market E-Cigarettes and the Public Engages with E-cigarette Information. Nicotine Tob. Res. 2019, 21, 14–24.
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2010.
- Counts, M.E.; Morton, M.J.; Laffoon, S.W.; Cox, R.H.; Lipowicz, P.J. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul. Toxicol. Pharmacol. 2005, 41, 185–227.
- Goniewicz, M.L.; Knysak, J.; Gawron, M.; Kosmider, L.; Sobczak, A.; Kurek, J.; Prokopowicz, A.; Jabłońska-Czapla, M.; Rosik-Dulewska, C.; Havel, C.; et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 2014, 23, 133–139.
- Goniewicz, M.L.; Smith, D.M.; Edwards, K.C.; Blount, B.C.; Caldwell, K.L.; Feng, J.; Wang, L.; Christensen, C.; Ambrose, B.; Borek, N.; et al. Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Netw. Open 2018, 1, e185937.
- Bustamante, G.; Ma, B.; Yakovlev, G.; Yershova, K.; Le, C.; Jensen, J.; Hatsukami, D.K.; Stepanov, I. Presence of the Carcinogen N′-Nitrosonornicotine in Saliva of E-cigarette Users. Chem. Res. Toxicol. 2018, 31, 731–738.
- Carroll, D.M.; Wagener, T.L.; Peck, J.D.; Brame, L.S.; Thompson, D.M.; Stephens, L.D.; Campbell, J.E.; Beebe, L.A. Biomarkers of Exposure in ENDS Users, Smokers, and Dual Users of American Indian Descent. Tob. Regul. Sci. 2018, 4, 3–15.
- Badea, M.; Luzardo, O.P.; González-Antuña, A.; Zumbado, M.; Rogozea, L.; Floroian, L.; Alexandrescu, D.; Moga, M.; Gaman, L.; Radoi, M.; et al. Body burden of toxic metals and rare earth elements in non-smokers, cigarette smokers and electronic cigarette users. Environ. Res. 2018, 166, 269–275.
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems; Public Health Consequences of E-Cigarettes; Eaton, D.L.; Kwan, L.Y.; Stratton, K. (Eds.) Public Health Consequences of E-Cigarettes; National Academies Press: Washington, DC, USA, 2018.
- Poynton, S.; Sutton, J.; Goodall, S.; Margham, J.; Forster, M.; Scott, K.; Liu, C.; McAdam, K.; Murphy, J.; Proctor, C. A novel hybrid tobacco product that delivers a tobacco flavour note with vapour aerosol (Part 1): Product operation and preliminary aerosol chemistry assessment. Food Chem. Toxicol. 2017, 106 Pt A, 522–532.
- Breheny, D.; Adamson, J.; Azzopardi, D.; Baxter, A.; Bishop, E.; Carr, T.; Crooks, I.; Hewitt, K.; Jaunky, T.; Larard, S.; et al. A novel hybrid tobacco product that delivers a tobacco flavour note with vapour aerosol (Part 2): In vitro biological assessment and comparison with different tobacco-heating products. Food Chem. Toxicol. 2017, 106 Pt A, 533–546.
- Scott, A.; Lugg, S.T.; Aldridge, K.; Lewis, K.E.; Bowden, A.; Mahida, R.Y.; Grudzinska, F.S.; Dosanjh, D.; Parekh, D.; Foronjy, R.; et al. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax 2018, 73, 1161–1169.
- Behar, R.Z.; Luo, W.; McWhirter, K.J.; Pankow, J.F.; Talbot, P. Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci. Rep. 2018, 8, 8288.
- Hua, M.; Omaiye, E.E.; Luo, W.; McWhirter, K.J.; Pankow, J.F.; Talbot, P. Identification of Cytotoxic Flavor Chemicals in Top-Selling Electronic Cigarette Refill Fluids. Sci. Rep. 2019, 9, 2782.
- Higham, A.; Bostock, D.; Booth, G.; Dungwa, J.V.; Singh, D. The effect of electronic cigarette and tobacco smoke exposure on COPD bronchial epithelial cell inflammatory responses. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 989–1000.
- Vasanthi Bathrinarayanan, P.; Brown, J.E.P.; Marshall, L.J.; Leslie, L.J. An investigation into E-cigarette cytotoxicity in-vitro using a novel 3D differentiated co-culture model of human airways. Toxicol. In Vitro 2018, 52, 255–264.
- Zhao, J.; Zhang, Y.; Sisler, J.D.; Shaffer, J.; Leonard, S.S.; Morris, A.M.; Qian, Y.; Bello, D.; Demokritou, P. Assessment of reactive oxygen species generated by electronic cigarettes using acellular and cellular approaches. J. Hazard. Mater. 2018, 344, 549–557.
- Ganapathy, V.; Manyanga, J.; Brame, L.; McGuire, D.; Sadhasivam, B.; Floyd, E.; Rubenstein, D.A.; Ramachandran, I.; Wagener, T.; Queimado, L. Electronic cigarette aerosols suppress cellular antioxidant defenses and induce significant oxidative DNA damage. PLoS ONE. 2017, 12, e0177780.
- Shaito, A.; Saliba, J.; Husari, A.; El-Harakeh, M.; Chhouri, H.; Hashem, Y.; Shihadeh, A.; El-Sabban, M. Electronic Cigarette Smoke Impairs Normal Mesenchymal Stem Cell Differentiation. Sci. Rep. 2017, 7, 14281.
- Anthérieu, S.; Garat, A.; Beauval, N.; Soyez, M.; Allorge, D.; Garçon, G.; Lo-Guidice, J.-M. Comparison of cellular and transcriptomic effects between electronic cigarette vapor and cigarette smoke in human bronchial epithelial cells. Toxicol. In Vitro 2017, 45 Pt 3, 417–425.
- Anderson, C.; Majeste, A.; Hanus, J.; Wang, S. E-Cigarette Aerosol Exposure Induces Reactive Oxygen Species, DNA Damage, and Cell Death in Vascular Endothelial Cells. Toxicol. Sci. 2016, 154, 332–340.
- Li, N.; Georas, S.; Alexis, N.; Fritz, P.; Xia, T.; Williams, M.A.; Horner, E.; Nel, A. A work group report on ultrafine particles (American Academy of Allergy, Asthma & Immunology): Why ambient ultrafine and engineered nanoparticles should receive special attention for possible adverse health outcomes in human subjects. J. Allergy Clin. Immunol. 2016, 138, 386–396.
- Dicpinigaitis, P.V. Effect of tobacco and electronic cigarette use on cough reflex sensitivity. Pulm. Pharmacol. Ther. 2017, 47, 45–48.
- Polosa, R.; Morjaria, J.B.; Prosperini, U.; Russo, C.; Pennisi, A.; Puleo, R.; Caruso, M.; Caponnetto, P. Health effects in COPD smokers who switch to electronic cigarettes: A retrospective-prospective 3-year follow-up. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2533–2542.
- Polosa, R.; Morjaria, J.B.; Caponnetto, P.; Prosperini, U.; Russo, C.; Pennisi, A.; Bruno, C.M. Evidence for harm reduction in COPD smokers who switch to electronic cigarettes. Respir. Res. 2016, 17, 166.
- Maouche, K.; Medjber, K.; Zahm, J.M.; Delavoie, F.; Terryn, C.; Coraux, C.; Pons, S.; Cloëz-Tayarani, I.; Maskos, U.; Birembaut, P.; et al. Contribution of α7 nicotinic receptor to airway epithelium dysfunction under nicotine exposure. Proc. Natl. Acad. Sci. USA 2013, 110, 4099–4104.
- Saint-Criq, V.; Gray, M.A. Role of CFTR in epithelial physiology. Cell. Mol. Life Sci. 2017, 74, 93–115.
- Soule, E.K.; Maloney, S.F.; Spindle, T.R.; Rudy, A.K.; Hiler, M.M.; Cobb, C.O. Electronic cigarette use and indoor air quality in a natural setting. Tob. Control 2017, 26, 109–112.
- Clapp, P.W.; Pawlak, E.A.; Lackey, J.T.; Keating, J.E.; Reeber, S.L.; Glish, G.L.; Jaspers, I. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L278–L292.
- Hwang, J.H.; Lyes, M.; Sladewski, K.; Enany, S.; McEachern, E.; Mathew, D.P.; Das, S.; Moshensky, A.; Bapat, S.; Pride, D.T.; et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J. Mol. Med. 2016, 94, 667–679.
- Miyashita, L.; Suri, R.; Dearing, E.; Mudway, I.; Dove, R.E.; Neill, D.R.; Zyl-Smit, R.V.; Kadioglu, A.; Grigg, J. E-cigarette vapour enhances pneumococcal adherence to airway epithelial cells. Eur. Respir. J. 2018, 51, 1701592.
- Wolf, M.; Richards, J. Acute Eosinophilic Pneumonia Due to Vaping-Associated Lung Injury. J. Crit. Care Med. 2020, 6, 259–262.
- Thota, D.; Latham, E. Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J. Emerg. Med. 2014, 47, 15–17.
- Layden, J.E.; Ghinai, I.; Pray, I.; Kimball, A.; Layer, M.; Tenforde, M.W.; Navon, L.; Hoots, B.; Salvatore, P.P.; Elderbrook, M.; et al. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin—Final Report. N. Engl. J. Med. 2020, 382, 903–916.
- Zou, R.H.; Tiberio, P.J.; Triantafyllou, G.A.; Lamberty, P.E.; Lynch, M.J.; Kreit, J.W.; McVerry, B.J.; Gladwin, M.T.; Morris, A.; Chiarchiaro, J.; et al. Clinical Characterization of E-Cigarette, or Vaping, Product Use-associated Lung Injury in 36 Patients in Pittsburgh, Pennsylvania. Am. J. Respir. Crit. Care Med. 2020, 201, 1303–1306.
- Kalininskiy, A.; Bach, C.T.; Nacca, N.E.; Ginsberg, G.; Marraffa, J.; Navarette, K.A.; McGraw, M.D.; Croft, D.P. E-cigarette, or vaping, product use associated lung injury (EVALI): Case series and diagnostic approach. Lancet Respir. Med. 2019, 7, 1017–1026.
- Maddock, S.D.; Cirulis, M.M.; Callahan, S.J.; Keenan, L.M.; Pirozzi, C.S.; Raman, S.M.; Aberegg, S.K. Pulmonary Lipid-Laden Macrophages and Vaping. N. Engl. J. Med. 2019, 381, 1488–1489.
- Schäfer, M.; Steindor, M.; Stehling, F.; Dohna-Schwake, C. EVALI (E-cigarette or vaping product use associated lung injury): First case report of an adolescent in Europe. Pediatr. Pulmonol. 2021, 56, 1274–1275.
- Adhikari, R.; Koritala, T.; Gotur, R.; Malayala, S.V.; Jain, N.K. EVALI—E-Cigarette or Vaping Product Use-Associated Lung Injury: A Case Report. Cureus 2021, 13, e13541.
- O’Carroll, O.; Sharma, K.; Fabre, A.; Murphy, D.J.; Keane, M.P.; McCarthy, C. Vaping-associated lung injury. Thorax 2020, 75, 706–707.
- Basset-Léobon, C.; Lacoste-Collin, L.; Aziza, J.; Bes, J.C.; Jozan, S.; Courtade-Saïdi, M. Cut-off values and significance of Oil Red O-positive cells in bronchoalveolar lavage fluid. Cytopathology 2010, 21, 245–250.
- Lozier, M.J.; Wallace, B.; Anderson, K.; Ellington, S.; Jones, C.M.; Rose, D.; Baldwin, G.; King, B.A.; Briss, P.; Mikosz, C.A. Update: Demographic, Product, and Substance-Use Characteristics of Hospitalized Patients in a Nationwide Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injuries—United States, December 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 1142–1148.
- Ali, M.; Khan, K.; Buch, M.; Ramos-Ramirez, M.; Sharma, M.; Patel, S.; Choudhury, S.; Anjum, H.; Khan, A.; Surani, S. A Case Series of Vaping-Induced Lung Injury in a Community Hospital Setting. Case Rep. Pulmonol. 2020, 2020, 9631916.
- McAlinden, K.D.; Eapen, M.S.; Lu, W.; Sharma, P.; Sohal, S.S. The rise of electronic nicotine delivery systems and the emergence of electronic-cigarette-driven disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L585–L595.
- Taylor, J.; Wiens, T.; Peterson, J.; Saravia, S.; Lunda, M.; Hanson, K.; Wogen, M.; D’Heilly, P.; Margetta, J.; Bye, M.; et al. Characteristics of E-cigarette, or Vaping, Products Used by Patients with Associated Lung Injury and Products Seized by Law Enforcement—Minnesota, 2018 and 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 1096–1100.
- Blount, B.C.; Karwowski, M.P.; Morel-Espinosa, M.; Rees, J.; Sosnoff, C.; Cowan, E.; Gardner, M.; Wang, L.; Valentin-Blasini, L.; Silva, L.; et al. Evaluation of Bronchoalveolar Lavage Fluid from Patients in an Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injury—10 States, August–October 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 1040–1041.
- Blount, B.C.; Karwowski, M.P.; Shields, P.G.; Morel-Espinosa, M.; Valentin-Blasini, L.; Gardner, M.; Braselton, M.; Brosius, C.R.; Caron, K.T.; Chambers, D.; et al. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N. Engl. J. Med. 2020, 382, 697–705.
- Bhat, T.A.; Kalathil, S.G.; Bogner, P.N.; Blount, B.C.; Goniewicz, M.L.; Thanavala, Y.M. An Animal Model of Inhaled Vitamin E Acetate and EVALI-like Lung Injury. N. Engl. J. Med. 2020, 382, 1175–1177.
- Attfield, K.R.; Chen, W.; Cummings, K.J.; Jacob, P.; O’Shea, D.F.; Wagner, J.; Wang, P.; Fowles, J. Potential of Ethenone (Ketene) to Contribute to Electronic Cigarette, or Vaping, Product Use-associated Lung Injury. Am. J. Respir. Crit. Care Med. 2020, 202, 1187–1189.
- Lee, H. Vitamin E acetate as linactant in the pathophysiology of EVALI. Med. Hypotheses 2020, 144, 110182.
- Wu, D.; O’Shea, D.F. Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate. Proc. Natl. Acad. Sci. USA 2020, 117, 6349–6355.
- Prevention CfDCa. Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. Available online: https://www.cdc.gov (accessed on 25 February 2021).
- Evans, M.E.; Twentyman, E.; Click, E.S.; Goodman, A.B.; Weissman, D.N.; Kiernan, E.; Adkins Hocevar, S.; Mikosz, C.A.; Danielson, M.; Anderson, K.N.; et al. Update: Interim Guidance for Health Care Professionals Evaluating and Caring for Patients with Suspected E-cigarette, or Vaping, Product Use-Associated Lung Injury and for Reducing the Risk for Rehospitalization and Death Following Hospital Discharge—United States, December 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 68, 1189–1194.
More
Information
Subjects:
Respiratory System
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
29 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




