Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ralf Weiskirchen | -- | 997 | 2022-04-26 16:42:04 | | | |
| 2 | Ralf Weiskirchen | + 3 word(s) | 1000 | 2022-04-26 16:44:34 | | | | |
| 3 | Ralf Weiskirchen | + 2 word(s) | 1002 | 2022-04-26 16:45:26 | | | | |
| 4 | Catherine Yang | Meta information modification | 1002 | 2022-04-27 03:05:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Weiskirchen, R.; Singh, V.; Khurana, A.; Navik, U.; Allawadhi, P.; Bharani, K. Process of Apoptosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/22317 (accessed on 04 March 2026).
Weiskirchen R, Singh V, Khurana A, Navik U, Allawadhi P, Bharani K. Process of Apoptosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/22317. Accessed March 04, 2026.
Weiskirchen, Ralf, Vishakha Singh, Amit Khurana, Umashanker Navik, Prince Allawadhi, Kala Bharani. "Process of Apoptosis" Encyclopedia, https://encyclopedia.pub/entry/22317 (accessed March 04, 2026).
Weiskirchen, R., Singh, V., Khurana, A., Navik, U., Allawadhi, P., & Bharani, K. (2022, April 26). Process of Apoptosis. In Encyclopedia. https://encyclopedia.pub/entry/22317
Weiskirchen, Ralf, et al. "Process of Apoptosis." Encyclopedia. Web. 26 April, 2022.
Copy Citation
The term “Apoptosis” originates from Greek, which means the shedding of leaves from trees in autumn or the falling of petals from flowers. Apoptosis was firstly utilized by Kerr, Wyllie, and Currie in 1972 for explaining a morphologically discrete way of cell death. However, multiple concepts of apoptosis were precisely explained several years back. The main concept of apoptosis emerged from the knowledge of the process of programmed cell death that takes place in the developmental cycle of Caenorhabditis elegans.
apoptosis
carcinogenesis
cell models
regulation
gene expression
1. Extrinsic Pathway
The extrinsic signaling is mainly linked with transmembrane receptor-mediated interactions, which include death receptors of the tumor necrosis factor (TNF) receptor gene superfamily. The death domain of death receptors is responsible for transmitting death signals from the cell’s surface to the intracellular signaling pathways [1]. There are different death receptors, which are well characterized, such as TNF-α/TNFR1, FasL/FasR, apoptosis antigen (APO) 2L/DR4 APO3L/DR3, and APO2L/DR5 [2]. The mechanism of extrinsic pathway is well explained by the FasL/FasR and TNF-α/TNFR1 models [3]. The Fas and TNF ligands bind to the Fas and TNF receptors, respectively, which in turn allow the interaction with their respective adapter proteins, namely Fas-associated protein with death domain (FADD) and TNFR1-associated death domain protein (TRADD) [4]. The binding of the adapter protein is linked with stimulation of procaspase-8 accompanied by the autocatalytic action of caspase-8 [2]. Once caspase-8 is stimulated, this pathway converges with the execution pathway.
Importantly, the apoptosis ligand 2/tumor necrosis factor-related apoptosis-inducing ligand (APO2L/TRAIL) pathway plays an important role in the progression and development of cancer and it acts independently of p53. Further, pro-apoptotic receptor agonists (PARAs) that target DR4 and/or DR5 have potential to give significant clinical benefit by killing tumor cells that are resistant to traditional chemotherapeutic agents [5]. Hence, targeting extrinsic pathways could be a novel treatment strategy to promote apoptosis in the cancer cells.
2. Perforin/Granzyme Pathway
In this pathway, T-cell cytotoxicity is mediated by cytotoxic T lymphocytes (CTLs), which are enrolled in targeting the cells for killing through the extrinsic pathway, as shown in Figure 1 [6][7]. They show cytotoxic effects by secreting perforin and releasing cytoplasmic granules, which involve serine proteases granzyme A and granzyme B in complex with serglycin [8]. Granzyme B can activate procaspase-10 and is able to activate caspase-3 directly in such a way that bypasses the upstream signaling cascade and directly stimulates the execution pathway [9]. Granzyme A is also associated with CTL-mediated apoptosis in a caspase-independent manner. It stimulates DNA nicking leading to the degradation of apoptotic DNA [10].
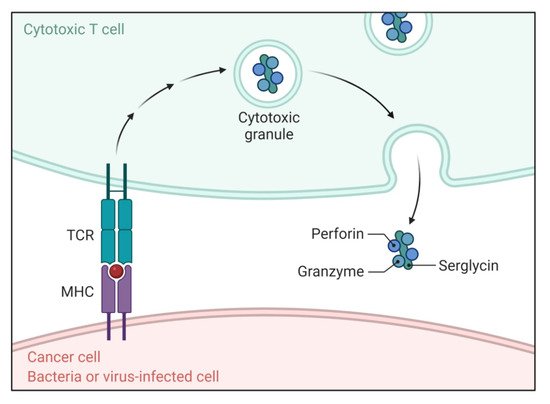
Figure 1. Function of the T cell receptor (TCR) and peptide–MHC complex in apoptosis. The TCR and peptide–MHC complex causes directed release of perforin and granzymes complexed with serglycin triggering apoptosis.
3. Intrinsic Pathway
The intrinsic pathway induces apoptosis via varied types of non-receptor-mediated stimuli, which are mainly mitochondrial-dependent events [11]. These stimuli generate intracellular signals that target the cell directly either in a positive or negative manner [10]. Positive stimuli include viral infections, radiation, hypoxia, toxins, hyperthermia and free radicals. Negative stimuli involve a lack of some growth factors, cytokines or hormones, which subsequently results in the repression of the death program, in turn, stimulating apoptosis. These mentioned stimuli bring about modifications in the inner mitochondrial membrane resulting in the liberation of pro-apoptotic proteins. One group of pro-apoptotic proteins consists of SMAC/direct IAP binding protein with low pI (DIABLO), cytochrome c and HTRA2/OMI, which activate the caspase-associated with the mitochondrial pathway [12]. Among these proteins, SMAC/DIABLO and HTRA serine peptidase 2 (HTRA2)/OMI stimulate apoptosis by preventing the functioning of IAP [12][13]. However, cytochrome c works by stimulating apoptotic protease-activating factor 1 (APAF-1) and procaspase-9 by binding to them, thereby resulting in the activation of caspase-9 [14][15]. Endonuclease G and apoptosis-inducing factor (AIF) are nucleases located in the intermembrane space of the mitochondria that together with caspase-activated DNase (CAD) lead to chromatin condensation and DNA fragmentation at later stages of apoptosis. Moreover, the members of the BCL-2 family are crucial in maintaining mitochondrial dependent apoptotic events as this family of protein regulates the permeability of the mitochondrial membrane and, hence, can act as pro-apoptotic or anti-apoptotic [16]. The pro-apoptotic genes include BAX, BCL-10, BIK, BAK, BLK, BAD, BIM, BID, PUMA and NOXA, while the anti-apoptotic genes include BCL-2, BAG, BCL-XS, BCL-XL, BCL-x and BCL-w. All these proteins decide the fate of cell, i.e., whether the cell undergoes apoptosis or aborts apoptosis [16][17][18]. Hence, modulating the expression of these proteins can regulate the apoptosis process and, thus, may halt cell proliferation in cancer cells.
4. Execution Pathway
The execution pathway connects both the intrinsic and extrinsic pathway and finally leads to apoptosis. In this cascade, the execution caspases activate and start the final process of apoptosis. Herein, activation of endonucleases and nucleases for the disintegration of nuclear material and cytoskeletal proteins is evident [10]. The main caspases of the execution pathway are caspase-3, caspase-6 and caspase-7, which degrade cytokeratins, poly (ADP-ribose) polymerase (PARP), cytoskeletal protein, namely, α-fodrin, the nuclear protein nuclear-mitotic apparatus protein (NuMA) and others, finally leading to several biochemical and morphological alterations in cells undergoing apoptosis [19]. For instance, caspase-3 is significant for cell fate following apoptosis and it activates CAD. In a normal cell, CAD remains in a binding state with its inhibitor, namely, the inhibitor of caspase-activated DNAse (ICAD), while in apoptotic cells, caspase-3 degrades ICAD, thereby releasing CAD [20]. Finally, CAD performs its function of fragmenting chromosomal DNA, resulting in the condensation of chromatin [20]. It also stimulates the process of cytoskeleton organization and cell fragmentation into apoptotic bodies.
Overall, this section discussed the involvement of different apoptosis pathways, such as the extrinsic pathway, the intrinsic pathway, and T-cell mediated and perforin/granzyme-mediated cytotoxicity, which ultimately combine with the execution pathway. Further, aberrant apoptosis is an important reason for resistance to cancer therapeutics. Additionally, cancerous cells can lead to altered expressions of several apoptotic and anti-apoptotic proteins, which ultimately may lead to increased cell proliferation. Hence, targeting these proteins opens up new possibilities for selectively eradicating cancer cells.
References
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 2001, 104, 487–501.
- Ashkenazi, A.; Dixit, V.M. Death receptors: Signaling and modulation. Science 1998, 281, 1305–1308.
- Wajant, H. The Fas signaling pathway: More than a paradigm. Science 2002, 296, 1635–1636.
- Suliman, A.; Lam, A.; Datta, R.; Srivastava, R.K. Intracellular mechanisms of TRAIL: Apoptosis through mitochondrial-dependent and-independent pathways. Oncogene 2001, 20, 2122–2133.
- Ashkenazi, A. Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev. 2008, 19, 325–331.
- Trapani, J.A.; Smyth, M. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2002, 2, 735–747.
- Russell, J.H.; Ley, T.J. Lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 2002, 20, 323–370.
- Brunner, T.; Wasem, C.; Torgler, R.; Cima, I.; Jakob, S.; Corazza, N. Fas (CD95/Apo-1) Ligand regulation in T cell homeostasis, cell-mediated cytotoxicity and immune pathology. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2003; Volume 15, pp. 167–176.
- Goping, I.S.; Barry, M.; Liston, P.; Sawchuk, T.; Constantinescu, G.; Michalak, K.M.; Shostak, I.; Roberts, D.; Hunter, A.M.; Korneluk, R.; et al. Granzyme B-induced apoptosis requires both direct caspase activation and relief of caspase inhibition. Immunity 2003, 18, 355–365.
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516.
- Joza, N.; Susin, S.A.; Daugas, E.; Stanford, W.L.; Cho, S.K.; Li, C.Y.J.; Sasaki, T.; Elia, A.J.; Cheng, H.-Y.M.; Ravagnan, L.; et al. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 2001, 410, 549–554.
- Du, C.; Fang, M.; Li, Y.; Li, L.; Wang, X. Smac, a Mitochondrial protein that promotes cytochrome c–dependent caspase activation by eliminating IAP inhibition. Cell 2000, 102, 33–42.
- Van Loo, G.; Van Gurp, M.; Depuydt, B.; Srinivasula, S.M.; Rodriguez, I.; Alnemri, E.S.; Gevaert, K.; Vandekerckhove, J.; Declercq, W.; Vandenabeele, P. The serine protease Omi/HtrA2 is released from mitochondria during apoptosis. Omi interacts with caspase-inhibitor XIAP and induces enhanced caspase activity. Cell Death Differ. 2002, 9, 20–26.
- Chinnaiyan, A.M. The Apoptosome: Heart and soul of the cell death machine. Neoplasia 1999, 1, 5–15.
- Hill, M.M.; Adrain, C.; Duriez, P.J.; Creagh, E.M.; Martin, S.J. Analysis of the composition, assembly kinetics and activity of native Apaf-1 apoptosomes. EMBO J. 2004, 23, 2134–2145.
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656.
- Liu, F.-T.; Newland, A.C.; Jia, L. Bax conformational change is a crucial step for PUMA-mediated apoptosis in human leukemia. Biochem. Biophys. Res. Commun. 2003, 310, 956–962.
- Oda, E.; Ohki, R.; Murasawa, H.; Nemoto, J.; Shibue, T.; Yamashita, T.; Tokino, T.; Taniguchi, T.; Tanaka, N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 2000, 288, 1053–1058.
- Slee, E.A.; Adrain, C.; Martin, S. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 2001, 276, 7320–7326.
- Sakahira, H.; Enari, M.; Nagata, S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature 1998, 391, 96–99.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.4K
Revisions:
4 times
(View History)
Update Date:
05 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





