Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bruno Miranda | -- | 5947 | 2022-04-26 09:20:21 | | | |
| 2 | Rita Xu | -6 word(s) | 5941 | 2022-04-26 09:51:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Miranda, B.; Nocerino, V.; Tramontano, C.; Chianese, G.; Dardano, P.; Rea, I.; De Stefano, L. Plasmonic Nanosensors. Encyclopedia. Available online: https://encyclopedia.pub/entry/22275 (accessed on 08 February 2026).
Miranda B, Nocerino V, Tramontano C, Chianese G, Dardano P, Rea I, et al. Plasmonic Nanosensors. Encyclopedia. Available at: https://encyclopedia.pub/entry/22275. Accessed February 08, 2026.
Miranda, Bruno, Valeria Nocerino, Chiara Tramontano, Giovanna Chianese, Principia Dardano, Ilaria Rea, Luca De Stefano. "Plasmonic Nanosensors" Encyclopedia, https://encyclopedia.pub/entry/22275 (accessed February 08, 2026).
Miranda, B., Nocerino, V., Tramontano, C., Chianese, G., Dardano, P., Rea, I., & De Stefano, L. (2022, April 26). Plasmonic Nanosensors. In Encyclopedia. https://encyclopedia.pub/entry/22275
Miranda, Bruno, et al. "Plasmonic Nanosensors." Encyclopedia. Web. 26 April, 2022.
Copy Citation
Current advances in the fabrication of smart nanomaterials and nanostructured surfaces find wide usage in the biomedical field. Nanosensors based on localized surface plasmon resonance exhibit unprecedented optical features that can be exploited to reduce the costs, analytic times, and need for expensive lab equipment. Moreover, they are promising for the design of nanoplatforms with multiple functionalities (e.g., multiplexed detection) with large integration within microelectronics and microfluidics.
localized surface plasmon resonance
plasmonic nanoparticles
metal-enhanced fluorescence
surface-enhanced Raman scattering
1. Plasmonic Colloids for Biomedicine
1.1. LSPR-Based Colorimetric Biosensors
The composition, size, and shape of plasmonic NPs are all parameters that strongly affect their LSPR response and introduce themselves as excellent optical transducers for the development of innovative sensors [1]. The interaction between the recognition element and the analyte of interest results in a shift of the LSPR related to the concentration of the analyte of interest. The recognition element is responsible for the specificity and selectivity of the sensor. In recent years, the real challenge was to produce simple and low-cost diagnostic devices able to show results in a short time [2]. In this context, colorimetric sensors, providing a visible response to the naked eye, turned out to be excellent candidates. In this section, colorimetric sensors for different applications are presented. Size and shape, the limit of detection (LOD), and the selectivity of the NPs are detailed. It is well known that the LSPR spectral shape and the wavelength of the peak maximum depend on the material, the size, and the shape of the NPs [3][4]. The material is the crucial choice in the development of the sensor. Gold is preferred to obtain a platform with high chemical stability and resistance to oxidation, whereas silver is used to have sharper resonances and higher sensitivity to refractive index variations [5][6]. For example, gold NPs of 50–60 nm in diameter have a plasmon resonance at 530 nm and a refractive index sensitivity of 60 nm/RIU, while silver NPs of 50–60 nm have a plasmon resonance at 435 nm and a refractive index sensitivity of 160 nm/RIU [6][7]. Shape is also an important parameter to evaluate; 20 nm spherical gold NPs have an LSPR at a wavelength of 520 nm, whereas gold nanorods of 10–20 nm in diameter and 10–100 nm in length show two plasmonic bands at 520 nm and 700 nm due to the longitudinal and transverse resonance, respectively; in addition, gold nanocubes of 44 nm in size have an LSPR peak at 538 nm and a sensitivity of 83 nm/RIU, whereas silver nanocubes of 30 nm in size have an LSPR peak at 510 nm with a high sensitivity of 146 nm/RIU [4][8][9][10] (Figure 1a). Over the years, LSPR-based sensors with nanoparticles of different shapes or sizes for the detection of a large variety of molecules involved in diagnostics have been proposed. The relevance of LSPR sensing in nanomedicine was also demonstrated in early stages of cancer diagnosis [11] (Figure 1b). The detection of specific prostatic antigen (PSA) was performed through 10 nm-gold particles covalently bound to the anti-PSA monoclonal antibody [12]. Parameters such as pH, temperature, and concentration ratio between the gold NPs and the anti-PSA were optimized to facilitate the covalent bond. The presence of the anti-PSA generated a 28 nm-redshift in the gold absorption peak from 510 nm, confirming the immobilization of the antibodies on NPs, and the presence of the analyte PSA in the test sample caused a further redshift (up to 578 nm). The LOD, in this case, was 0.2 ng/mL, and the selectivity was assessed with two different tumor markers, generating no spectral change. Velotta et al. proposed a colorimetric sensor to detect the presence of human immunoglobulin G (IgG) in simulated fluids (Figure 1c) [13]. In this work, 40-nm gold NPs, synthesized and stabilized with sodium citrate, were used to immobilize the antibody by the photochemical immobilization technique (PIT) [14][15]. The presence of the analyte of interest caused an aggregation process of the colloidal solution in combination with an increase in the average size of the NPs. Therefore, a redshift of the plasmon of about 3.4 nm from the peak of LSPR of gold NPs at 540 nm is visible both to the naked eye and via spectrophotometric analysis. Despite the LOD of the sensor being just 100 ng/mL, the rapid response and the practical use make the sensor an efficient tool for mass screening. To develop sensors with better performances, researchers also used a combination of different noble metals such as gold and silver. In this regard, Di et al. developed a coupled system built on gold/silver NPs for the colorimetric detection of glucose (Figure 1d) [16]. In this case, gold NPs were used as a catalyzer to oxidate glucose, and silver NPs acted as an alternative to chromogenic agents for detecting glucose. Briefly, gold and silver NPs were synthesized by using sodium citrate as both reducing agent and stabilizer, and their dimensions were about 4 nm and 10.8 nm, respectively. When glucose was added to a buffer solution containing gold NPs, they catalyzed the oxidation of the glucose and produced H2O2, which caused dissolution of the silver NPs, inducing a change in the plasmon resonance. This change was visible to the naked eye, since the solution color shifted from yellow to red. Therefore, it was possible to determine the presence of glucose in a quicker and lower-cost way with an LOD of 3 μM.
In another study for glucose monitoring, the properties of a small amphiphilic protein (Vmh2) were exploited: it could spontaneously interact with glucose [17]. In this work, 8 nm gold NPs were synthesized with polyethylene glycol (PEG), and the obtained solution appeared in a shade of red and pink [18]. The NPs were functionalized with hydrophobins (HFB), and the solution changed color from red/pink to purple [19]. The HFB-functionalized NPs were mixed with glucose at different concentrations, and the variation of the gold LSPR was monitored by spectrophotometry. The main purpose of this work was to produce hybrid NPs to improve biocompatibility. The experimental results were obtained through UV–Vis spectroscopy, dynamic light scattering measurements, polarization modulation–infrared reflection–adsorption spectroscopy (PM-IRRAS), and X-ray photoelectron spectroscopy (XPS).
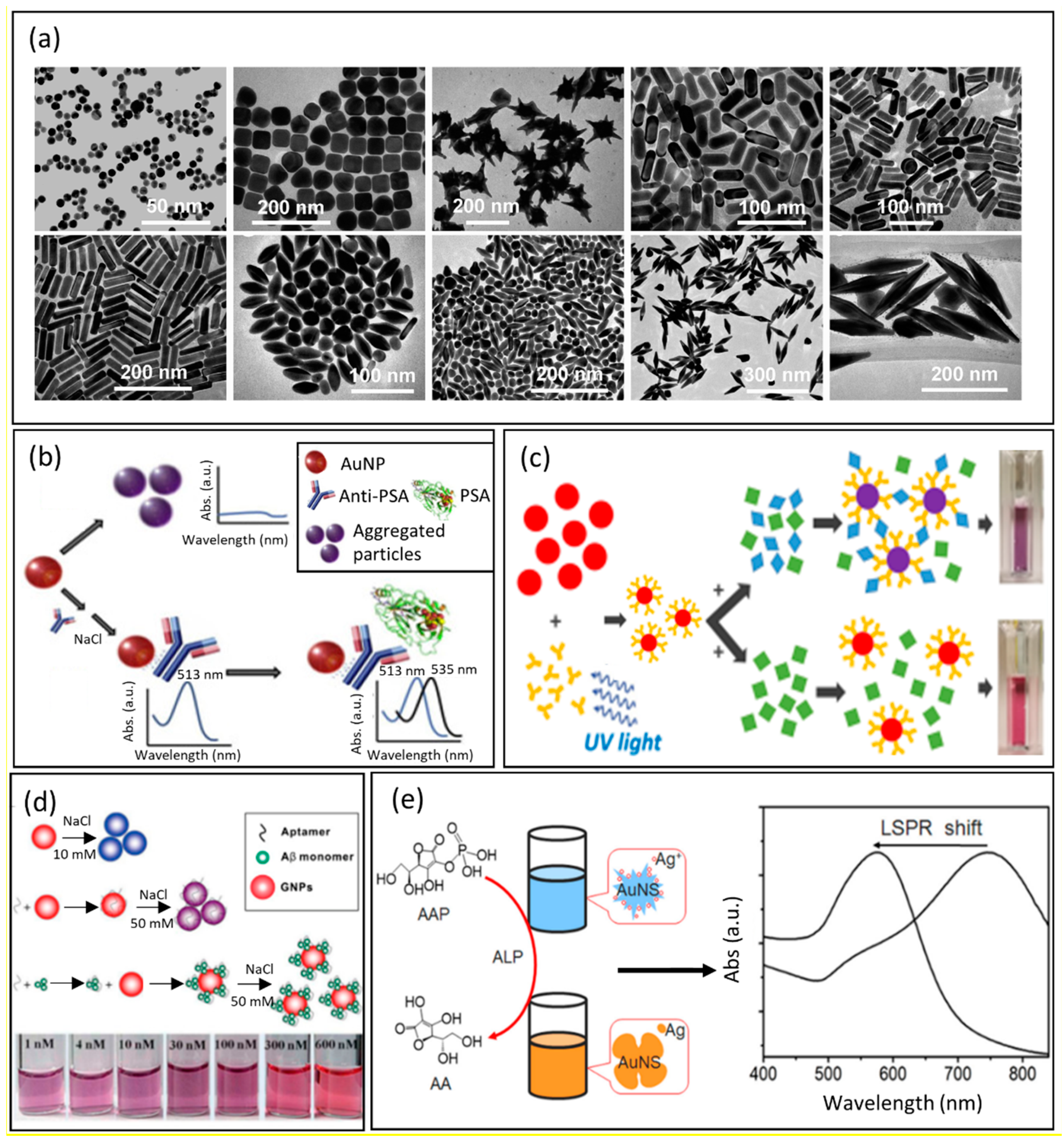
Figure 1. (a) From left to right, representative TEM images of Au nanoparticles of different shapes: on the top, nanospheres, nanocubes, nanobranches, nanorods (aspect ratio = 2.4 ± 0.3), and nanorods (aspect ratio = 3.4 ± 0.5); on the bottom, nanorods (aspect ratio = 4.6 ± 0.8), nanobipyramids (aspect ratio = 1.5 ± 0.3), nanobipyramids (aspect ratio = 2.7 ± 0.2), nanobipyramids (aspect ratio = 3.9 ± 0.2), and nanobipyramids (aspect ratio = 4.7 ± 0.2). Reproduced Adapted with permission from Ref. [9]. Copyright (2008), American Chemical Society. (b) Representation of the mechanism of the optical nanosensor for the detection of PSA. Colloidal AuNPs change color from red to purple in the absence of antibody proteins. The conjugation of antibodies to AuNPs stabilizes them and enables the detection of PSA. Adapted with permission from Ref. [11]. Copyright (2021), Elsevier. (c) Colorimetric detection scheme. AuNPs are functionalized with Abs by the photochemical immobilization technique (PIT), and then the presence of specific antigen induces the colorimetric variation of the colloidal solution from red to purple. In this case, there is the controlled aggregation of nanoparticles. Adapted with permission from Ref. [13]. Copyright (2018), American Chemical Society. (d) Detection of DNA-aptamer based on the colloidal solution of gold nanoparticles. Adapted with permission from Ref. [20], Copyright (2018), MDPI-open access article under the Creative Commons Attribution License. (e) Schematic representation of colorimetric detection of ALP. The blue shift of LSPR depends on the amount of enzyme that reduces silver nanoparticles on the surface of AuNSs. Adapted with permission from Ref. [21]. Copyright (2016), Elsevier.
The flexibility to modify the surface of NPs has also made LSPR-based nanosensors very for the detection of bacteria. Nemati’s group developed an immunodiagnostic sensor able to detect the presence of V. cholerae O1, a gram-negative bacterium involved in cholera disease [22]. In this work, the synthesis of gold NPs with a size of 40 nm was performed using trisodium citrate, and their surface was functionalized by a monoclonal antibody able to recognize bacteria [20]. Monitoring the LSPR shift, it was possible to evaluate the concentrations of V. cholerae down to an LOD of 10 CFU/mL. A different strategy for biosensing through enzyme-guided growth of silver NPs on gold nanostars was reported by Ju et al. [21]. Once again, they combined the properties of gold and silver to obtain better performances. Alkaline phosphatase (ALP), in presence of ascorbic acid 2-phosphate (AAP), produced a reductant acting on the silver NPs available on the 55 nm gold nanostars (AuNSs). The reduction of the silver NPs (AgNPs) resulted in a blue shift of the LSPR and a change of the AuNSs’ color from blue to purple, and then to orange. According to this, it was possible to quantify the amount of enzyme that reduced the silver NPs on the surface of the AuNSs (Figure 1d). The biosensor LOD was 0.5 pM, and it is well to underline that this method can be easily combined to detect other biomolecules by the naked eye. Since the surrounding of the NPs affects the LSPR, and the LSPR peak wavelength shift is proportional to the changes in the refractive index of the surrounding, this property could be useful to estimate the thickness of a biopolymer wrapping NPs, as proposed by Tramontano et al. [23]. The authors monitored the gelatin thickness on the surface of diatomite NPs due to the AuNPs grown in situ on their surface. The formation of a gelatin shell around the NPs resulted in a change of the refractive index and a redshift proportional to the thickness of the gelatin. Moreover, when enzymes or other conditions degraded the gelatin shells, a blue shift occurred, and it could be related to the released drug. In recent years, the COVID-19 pandemic has increased the request of assays with results easily interpretable by naked-eye or by a simple UV–Vis spectrometer. In this context, various LSPR biosensors have been presented [24][25][26][27], among which Liv et al. realized a colorimetric biosensor to detect the presence of the SARS-CoV-2 spike antigen [28]. 15 nm gold NPs (AuNPs) were synthesized by the standard citrate reduction method, and then the citrate groups were displaced by 11-mercaptoundecanoic acid (MUA). The SARS-CoV-2 spike antibody (Mab) was covalently bound to the NPs. After functionalization, the AuNPs appeared dimensionally larger (34 nm), and the LSPR peak redshifted to 526 nm. In the presence of the SARS-CoV-2 spike antigen, the LSPR peak underwent a further redshift of about 25 nm, detectable by the naked eye, since the color of the colloidal solution quickly shifted from red to purple. In this case, the LOD was 48 ng/mL, and the selectivity was demonstrated using two different spike antigens. A summary of the reported LSPR-based colorimetric biosensors is provided in Table 1.
Table 1. Examples of LSPR-based colorimetric biosensors.
| Material | Shape | Size (nm) | Analyte | Linear Range | Sensitivity | LOD | Ref. |
|---|---|---|---|---|---|---|---|
| Gold | Spherical | 9 | PSA | 0.2–1 ng/mL | 43.75 nm/(ng mL−1) | 0.2 ng/mL | [11] |
| Gold/Silver | Spherical | 4 | Glucose | 5–70 µM | 3 µM | [16] | |
| Gold | Spherical | 8 ± 3 | Glucose | 0.3–1.2 mg/mL | 0.13 ± 0.06 a.u./(mg mL−1) |
7.3 ± 0.3 mg/mL | [17] |
| Gold | Spherical | 43 | V. cholerae O1 | 10–104 CFU/mL | 10 CFU/mL | [22] | |
| Gold/Silver | Nanostars | 55 ± 5 | Alkaline phosphatase (ALP) | 1.0 pM to 25 nM | 0.5 pM | [21] | |
| Gold | Spike-like nanoparticles | Hemagglutinin | 1 pM to 10 nM | 1 pM | [25] | ||
| Gold | Spherical | 55 ± 5 | SARS-CoV-2 viral RNA | 0.2–3 ng/μL | 0.18 ng/μL | [27] | |
| Gold | Spherical | 16 | SARS-CoV-2 spike antigen | 0–1000 ng/mL | 48 ng/mL | [28] |
1.2. SERS-Based Colloidal Sensors for Bioimaging and Biomedical Applications
The direct analysis of biological processes and functions is crucial for understanding both physiological cell activity and pathological abnormalities. The direct study of living cells, tissues, or organs through in situ non-invasive methodologies is necessary for understanding the complexity of the processes underlying diseases, and testing strategies for clinical treatment. The modern analytical techniques (fluorescence [29], chromatography [30], spectroscopy [31]) used in biomedicine show ultrahigh sensitivity and high-performance levels for imaging, drug release monitoring, cell mapping [32], and other medical purposes. However, they have certain inevitable limitations. For instance, when it comes to tracking molecules or NPs inside cells, the most implemented approach is based on fluorescence [33]. This approach requires labeling the molecule with fluorophores or dyes that might affect the pharmacological effect of the drug. Moreover, fluorescence is sensible to photobleaching, causing under or over-estimation in the sample quantification.
Other strategies employed in disease diagnosis, pathogenic environmental monitoring, and healthcare screening are expensive and require extensive manipulation of the sample before analysis, hindering the real-time investigation of biological processes. In the request for rapid and non-destructive platforms for biomedical purposes, SERS-active platforms optimally adapted in shape have been demonstrated to complement traditional approaches, offering real-time in vitro and in vivo analyses [34]. SERS arises from the coherent oscillation of the electrons in noble metals and the excitation of LSPRs at specific wavelengths of incident light. When a molecule is adsorbed at the hot spot, the excitation and Raman field are both enhanced, giving rise to a giant amplification of the scattering signal up to 1010 [35]. Since most of the chemical and biological compounds have unique Raman fingerprint patterns, SERS enables sensitive and label-free investigations of a wide array of interesting analytes. Noble metal particles, such as gold (Au) or silver (Ag) in suspensions or immobilized on a substrate [36], are by far the most used substrates, since their LSPR response can be easily excited producing enhancement on the order of 103–104 [37]. Managò et al. showed that gold-decorated silica NPs can be used as a SERS platform for monitoring and quantifying the amount of Galunisertib released in living colorectal cancer (CRC) cells without needing external fluorophores or markers [38]. In this work, the AuNPs grown on the surface of DNPs by an in-situ approach produced an enhancement of the Raman drug signal of 105 and allowed a quantification down to a sub-femtogram scale resolution. The author reconstructed a false Raman map (Figure 2a) to study the internalization of the hybrid delivery system. Then, thanks to the strong Raman drug enhancement provided by AuNPs, the drug release was monitored at different time intervals in vitro (Figure 2b) and CRC cells (Figure 2c), making it easy to correlate the amount of released drug to the observed biological outcome. SERS represented a precise and rapid tool to understand the release behavior of the drug delivery system in the cell, overcoming traditional spectroscopic or fluorescent issues for drug monitoring [31]. The possibility to analyze samples using a fast, sensitive, and non-destructive method is thus fundamental when handling sensitive samples such as cells during in vitro investigations. For instance, the cell uptake of NPs can be monitored by different techniques, including TEM [39], but most of them can damage cells or allow for analysis of only a small portion of the cell volume. SERS, instead, can be used as an excellent tool for the study of the NPs’ cell uptake and their mechanism of internalization. The main advantage of using SERS rather than TEM relies on the time and costs required for the analysis. Kapara et al. functionalized AuNPs with an anti-ERα (Estrogen Receptor-alpha) antibody and a Raman reporter to investigate the internalization and localization of ERα-AuNPs in breast cancer cells [40]. ERα-AuNPs were incubated with cells for a shorter time and without the use of fluorescent staining, which needs expensive primary and secondary antibodies. The authors realized 3D SERS images of the entire cell volume and showed that modified AuNPs cannot penetrate breast cancer cells by passive targeting, but with a receptor-mediated pathway driven by their binding to the ERα receptor in the plasma membrane. The internalization of AuNPs is a crucial factor determining their application in nanomedicine, and the possibility to investigate their cell uptake with non-invasive techniques is mandatory to discriminate between the toxicity caused by either AuNPs or the technique. The safety of AuNPs, such as any material for human use, needs accurate evaluation and reliable techniques as well. SERS found application also in the detection of toxic contaminants (bacteria, endotoxins, toxins, and viruses [41] in AuNPs, since this material can be contaminated by a bacterial endotoxin known as “liposaccharide”, a potent cause of immunoreactivity in mammals [42]. The detection of LPS in AuNPs can be challenging because they can interfere with the most common assays (limulus amoebocyte lysate (LAL), monocyte activation test (MAT) [43]), altering the final readout of the analysis. Verde et al. showed that SERS can complement the traditional techniques to detect LPS directly on AuNPs with chemical specificity among different types of LPS or bacteria, such as Escherichia coli and Klebsiella pneumonia. They developed a sensitive platform with a detection limit of 5 LPS molecules per AuNPs, high-reproducibility, and without modifying the AuNP surface through functionalization approaches [44]. Furthermore, the authors showed that AuNPs did not produce biologically significant toxic effects in macrophages and that most of the inflammatory response is driven by the presence of LPS. Therefore, this study supported the use of AuNPs as safe imaging material for biomedical applications and offered a reliable technique for assessing the absence of toxic LPS before using AuNPs in medicine. To obtain higher enhancement of the Raman scattering, noble metal films can be realized on three-dimensional (3D) SERS substrates that are promising platforms for the analysis of complex samples [45]. The 3D SERS substrates show excellent sensing performance, since they allow for 3D plasmonic coupling both in-plane and out-of-plane, expanding the hotspot of the 3D volume compared to the 2D substrates [46]. These 3D structures can be found in nature, such as insects, birds, and flowers, which possess periodic micro and nanostructures with efficient light manipulation and plasmonic properties [47]. In recent years, the dielectric nanoarchitecture of diatom shells served as low-cost platforms for SERS sensing of biological compounds [48]. Managò et al. showed that a silica/metal hybrid structure composed of diatom valve and Au can be applied for the chemical analysis of both red blood and leukemia cells thanks to a robust SERS amplification (EF) of 4.6 × 106 [49]. The metalized frustule was developed using Au thermal evaporation to grow a metal layer on the 3D silica nanoarchitecture. The main advantage of diatom-based SERS platforms is their low-cost production and 3D natural periodic and sub periodic architecture, which make them valid alternatives to costly and complex 3D geometries [50]. Indeed, the metalized frustule offered the possibility to analyze the cell membrane thanks to the unique cell spectral signature from Raman scattering, supporting the use of SERS for the detection of several oncological disorders. In recent years, there has been an increasing number of publications reporting the employment of SERS for in vivo investigations, especially for cancer imaging and detection [51]. Sers-encoded particles (SEP) made of a plasmonic NP core (silver or Au) coated with a SERS probe have shown a high photostability and optical contrast when working with cells and near-infrared (NIR) excitations [52].
SEP can be functionalized with a wide range of biological moieties [53][54][55] to promote the preferential accumulation of NPs in tumors compared to normal tissues. Nicolson et al. developed a SEP-based biosensor for visualizing the diffusion of brain main tumor and delimiting the margins, isolating distant tumor clusters of fewer than five cells in vivo [56]. The author synthesized Au nanostars (AuNSs) coated by silica and further functionalized with a SERS probe and the tripeptide RGD (arginyl glycyl aspartic acid) for targeting integrin, which is predominantly expressed on tumor endothelial cells (Figure 2d). To avoid background interference with the Raman signatures of interest, the authors combined spatially offset Raman spectroscopy (SORS) with the SERS technique, making it possible to obtain excellent tumor localization with a power density five times less than that used for conventional Raman spectroscopy. Thanks to their unique Raman fingerprint, RGD-AuNPs allowed for the identification of metastasis located at about 350 μm from the main tumor by Raman imaging. Integrin targeted RGD-AuNPs-SEP enabled an accurate outline of the brain tumor and localization of microscopic extensions invading the brain, in agreement with the magnetic resonance imaging (MRI) (Figure 2e,f). It was also possible to detect isolated glioblastoma cells on their migratory path. These results, among others reported in the literature and this section, hold promise to allow high precision visualization and mapping of tumors [57] and the extents of metastasis. The attention that SERS has gained in the last decade for in vitro/vivo applications is supported by the opportunity to overcome limitations held by traditional techniques that are time-consuming, costly, and not as sensitive and accurate as SERS for performing real-time analysis in complex samples.
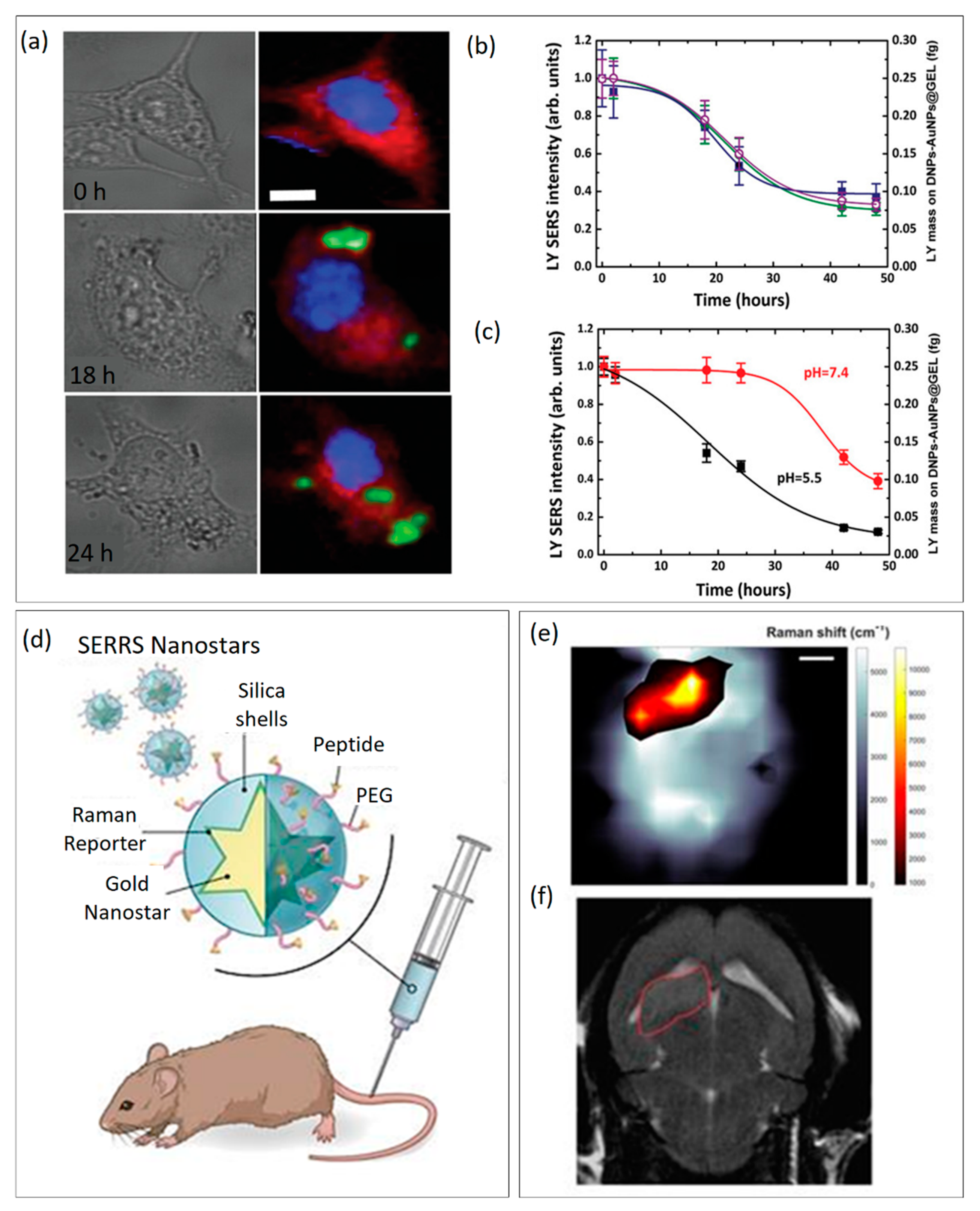
Figure 2. (a) False map reconstructed by Raman imaging through the MCH approach for analyzing the internalization of hybrid DNPs in cells. (b) Drug release studies monitored in CRC cells by real-time SERS. (c) Drug release studies monitored in phosphate buffered saline (PBS) pH 7.4 and 5.5 by real-time SERS. Adapted with permission from Ref. [38]. Copyright (2021), John Wiley & Sons. (d) Functionalization of active-targeted nanostars for in vivo imaging of brain tumors. (e) The SORS heat map confirming the presence of a brain tumor is in good agreement with the MRI image (f). Adapted with permission from Ref. [56]. Copyright (2019), Ivyspring International Publisher.
2. Plasmonic Nanoarrays for Biomedicine
One of the best ways to improve the LSPR sensors’ performances is to enhance the interactions between light and matter. In this regard, in addition to the growing development of colorimetric sensors, based on solution assays, new nanotechnologies have been developed that can effectively improve the light–matter interaction [58]. Although the potentiality of colloidal plasmonic NPs has been elucidated, with a particular focus on their application in biosensing and bioimaging applications, some of the major issues associated with them are the chemical stability and signal reproducibility, which could hinder their application at the industrial level [59][60][61]. Moreover, the quest for easy-to-read, rapid, and portable devices has pushed scientists towards the design of optical substrates based on plasmonic NPs, whose response could be easily monitored by simple spectroscopy. Several techniques have been proposed to produce plasmonic periodic, quasi-periodic, and non-periodic nanoarrays with precise optical properties, which could be exploited for biochemical sensing and whose performance strongly depended on the size, shape, material, and interparticle distance of the proposed nanoplatform [62]. Three methods are generally recognized for the fabrication of plasmonic nanoarrays: top-down, bottom-up, and mixed approaches [63][64][65][66][67]. The first one is generally the most expensive and time-consuming and leads to the fabrication of periodic nanoarrays with an easily predictable and tunable optical response. “Hard” lithography (e.g., E-beam lithography) is generally employed for this type of approach, providing arrays with always renewed optical performance, which can be exploited in biomedicine to achieve ultra-low limits of detection and extremely high sensitivities [68]. Vice versa, the bottom-up approach promises low-cost and large-scale substrates starting from chemically synthesized plasmonic NPs, which are self-assembled on an optically transparent or reflecting substrate. This technique does not require expensive instruments and offers the opportunity to have access to plasmonic arrays, which are generally non-periodic or quasi-periodic, and which exhibit reduced analytical performance in the biosensing field but could be in principle applied on a large scale. Generally, plasmonic nanoarrays assembled via this technique show great potential in external signal applications for MEF and SERS-based biosensors [53]. Finally, a mixed fabrication approach takes advantage of the two previously mentioned fabrication approaches and offers a good trade-off between large-scale fabrication, costs, and performance [69]. In this section, researchers summarize the most recent plasmonic nanoarrays for intrinsic LSPR sensing and external signal amplification (SERS and MEF). Plasmonic nanoarrays can be engineered starting from structural parameters and generally exhibit better performance, better reproducibility, and multi-target detection compared to colloidal nanoparticles [70].
2.1. LSPR-Based Plasmonic Nanoarrays
The worldwide recognition of the main applications of plasmonic nanoarrays exploits the shift of the LSPR exhibited by noble-metal nanoparticles upon changes in the refractive index (RI) of the surrounding environment for bulk refractive index sensing and biochemical sensing [5]. This means that when properly functionalized with a biorecognition element, plasmonic NP absorption peaks undergo a redshift, which is generally proportional to the concentration of the used bioprobe. Unlike colloidal NPs, whose red-shift is generally due to NP aggregation, which can be monitored by the naked eye, when dealing with plasmonic nanoarrays, the tiny red-shifts measured by spectroscopic techniques are only due to the variation of the surrounding refractive index. The sensitivity (S) of a plasmonic nanoarray is a parameter that describes its intrinsic capability of undergoing a redshift upon variation of the refractive index in the proximity of the plasmonic nanoarray surface:
S is measured in nm per refractive index unit (nm/RIU). This parameter is not sufficient to describe the overall sensing capabilities of an LSPR-based optical transducer. Given the full width at half maximum (FWHM) parameter to describe the sharpness/broadness of the plasmon resonance, it is possible to introduce the figure of merit (FOM) as
This parameter describes the performance of an LSPR-based optical transducer. More generally, in the biosensing community, gold is generally preferred to silver due to its chemical stability. However, silver exhibits sharper resonances, which boost the FOM of silver-based plasmonic nanoarrays. The oxidative susceptibility of silver hinders its application in LSPR-based biosensors; however, several strategies have been implemented to protect it from chemical oxidation [71][72]. The applications of LSPR-based plasmonic devices, in the diagnostic field, can be manifold. As an example, the detection and quantification of IgG can be a reliable means for the determination of some diagnoses such as hepatitis B virus, renal failure, hyperstimulation of the immune system, and some types of cancer [73]. Moreover, IgG could be used as biorecognition elements for biomarkers, cells, viruses, as well as pesticides and pollutants.
Both periodic and non-periodic plasmonic nanoarrays have been proposed for IgG-based sensing. Vestri et al. recently proposed a plasmonic 2D nanostructure based on a periodic arrangement of iso-Y gold NPs (Figure 3a). The array was fabricated by EBL lithography, its optical response was numerically evaluated, and a sensitivity of 412 nm/RIU was measured. The proposed platform was functionalized with IgG and allowed for the detection of a pesticide (namely, the imidacloprid) in the dynamic range of 1–1000 ng/mL achieving an LOD of 1 ng/mL [74]. Chen et al. proposed instead a multiplexed detection based on IgGs using large-scale plasmonic nanoarrays based on thermal dewetting of gold films of 10 nm thickness. They achieved a sensitivity of 104 nm/RIU and still a good LOD of multiple biomarkers, including IgG [75]. Another interesting application of LSPR-based plasmonic nanoarrays in the diagnostic field concerns the detection of cancer biomarkers, such as the prostate cancer-specific antigen (PSA). In [76], the specific binding between PSA-specific DNA aptamers and the LSPR optical response of gold nano-disks arrays on glass slides were exploited. DNA-aptamers were attached to the glass substrates with gold nano-discs, which before immobilization were first heated to 90° and then cooled to room temperature to maintain the flexibility of the aptamers, which was critical for PSA binding. Two different substrates were used to consider two different concentrations of aptamers. The binding between aptamer and gold nano-disks occurs between SH groups and Au atoms. The proposed platform exhibited a sensitivity of 113 nm/RIU and achieved an LOD of 1.49 ng/mL (Figure 3b). These results show the potentiality of plasmonic nanoarrays combined with aptamers, which could find large applications for pre-biopsy testing for cancer and partly avoid invasive exams [77]. Another example of PSA detection by plasmonic nanoarrays was achieved by using silver nano-columns [78]. The nano-columns with a diameter between 5 nm and 10 nm were immobilized on a glass substrate. They were stabilized by a self-assembled monolayer (SAM) of 11-mercaptoundecanoic acid (MUA) and 6-mercaptohexanol (MCH), and then the substrates were immersed in solutions containing different concentrations of anti-PSA. In this case, the achieved LOD was 850 pg/mL with clinically acceptable specificity, confirming the better performance of silver compared to gold when properly stabilized. Plasmonic nanoarrays can be therefore employed in the monitoring of the state of health and wellness of human beings. Recently, there have been numerous studies that have shown that vitamin D deficiency can be associated with various diseases, even serious ones, such as cardiovascular disease and osteoporosis; on the other hand, an excess can lead to kidney failure [79]. Recently, a sensor based on bottom-up synthesized gold nanorods immobilized on a glass substrate was proposed for the detection of 25-hydroxyvitamin D3, whose level in the blood can be directly traced back to that of vitamin D3 [80]. The gold nanorods were synthesized directly with citrate, used as a stabilizer, and subsequently functionalized with an aptamer capable of binding specifically only with 25-hydroxyvitamin D3 (Figure 3c). In this case, 1,6hexanedithiol was used as a blocking agent to promote specific binding, which improves LSPR and thus detection performance. This aptasensor showed a large dynamic range (0.1–105 ng/mL), which was considered clinically relevant to evaluate a deficiency or an excess of vitamin D. The achieved LOD was 0.1 ng/mL. A last interesting application of LSPR-based plasmonic nanoarrays concerns virus sensing. As an example, the hepatitis B virus (HBV), which is the culprit of hepatitis B disease, can lead to severe consequences as well as hepatocellular carcinoma (HCC) [81]. Gold NPs were synthesized using a gold nanoseed growth method. and then conjugation with the anti-HBsAg antibody on a glass substrate was performed [82]. The solution was later inoculated with the target analyte. To increase the sensitivity and enhance the optical response of the device, a second layer of AuNPs conjugated with anti-HbsAg was created to obtain a heteroassembled AuNP sandwich–immunoassay chip format. Three different sizes of gold nanoparticles were synthesized, namely 15 nm, 30 nm, and 50 nm, respectively, and using the 15 nm ones for both the first and second layer, a lower LOD of 100 fg/mL in 10–15 min was obtained. With a single layer of 15 nm AuNPs, the LOD was 10 pg/mL. One of the most recent applications of plasmonic nanoarrays is related to the COVID-19 pandemic outbreak, whose early diagnosis still represents the most powerful tool to prevent its spread. In this regard, a biosensor combining plasmonics and microfluidics-based on Au nanospikes fabricated by gold electrodeposition was proposed [83]. Optical sensing of anti-SARS-CoV-2 spike protein antibodies was performed in diluted human plasma without any labeling agents, reaching an LOD of 0.08 ng/mL, exhibiting complementing performances to the existing serological COVID-19 tests. Being based on a large-scale fabrication approach, the proposed platform could find application as a multiplexed biosensing platform for disease monitoring. Depending on the application and the desired performance, periodic as well as non-periodic structures of different shapes and sizes and materials can be fabricated and applied in the rapid diagnosis of diseases. A summary of LSPR-based plasmonic nanoarrays is provided in Table 2.
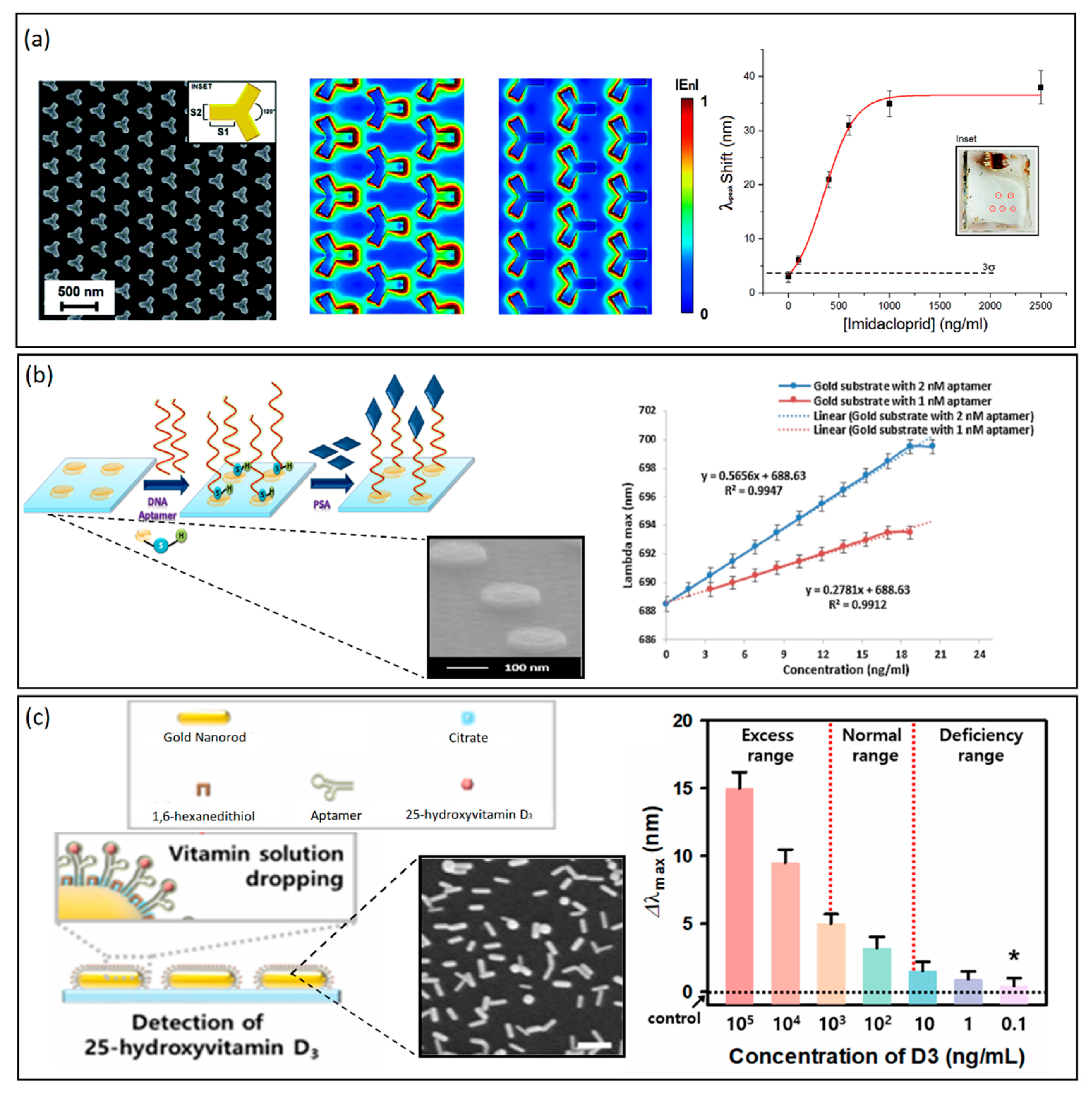
Figure 3. (a) From left to right: a plasmonic 2D nanostructure based on a periodic arrangement of iso-Y gold NPs, electric field enhancement simulations, and detection of imidacloprid pesticide achieving an LOD of 1 ng/mL. Adapted with permission from Ref. [74]. Copyright (2021), Royal Society of Chemistry. (b) From left to right: detection scheme of PSA through aptamers covalently bound to gold atoms. SEM image of gold nano-disks on a glass substrate. A linear relationship between lambda max shifts and the change of PSA concentration for two gold substrates having different concentrations of DNA aptamers. The dotted lines are the theoretical curves, while the continuous lines are the experimental curves. Adapted with permission from Ref. [76], Copyright (2018), Elsevier. (c) From left to right: detection scheme of 25-hydroxyvitamin D3, by gold nanorods (AuNRs) immobilized on a glass substrate. SEM image of AuNRs. 25-Hydroxyvitamin D3 detection by AuNRs based on lambda max shifts for a wide range of concentrations (0.1–105 ng/mL). Adapted with permission from Ref. [80], Copyright (2021), Elsevier.
Table 2. Examples of LSPR-based plasmonic nanoarrays.
| Material | Array Structure | Fabrication Method | Analyte | Linear Range | Sensitivity | LOD | Ref. |
|---|---|---|---|---|---|---|---|
| Gold | Periodic iso-Y NPs | EBL lithography | Imidacloprid | 1–1000 ng/mL | 412 nm/RIU | 1 ng/mL | [74] |
| Gold | Single-layer four-channel microfluidic device | Physical vapor deposition + rapid thermal annealing | IgG and CRP | 108.9 ± 1.3 nm/RIU | [75] | ||
| Gold | Nanodisk arrays | Deposition on glass slides | PSA | 1.7–20.4 ng/mL | 113 nm/RIU | 1.49 ng/mL | [76] |
| Silver | Nano-columns | Glancing angle deposition | PSA | 0.5–24 ng/mL | 134 nm/RIU | 850 pg/mL | [78] |
| Gold | Nanorods | Seed-mediated growth method | 25-hydroxyvitamin D3 | 0.1–105 ng/mL | 0.1 ng/mL | [80] | |
| Gold | Heteroassembled nanoparticles | Nanoseed growth on glass substrate | Hepatitis B virus | 100 fg/mL | [81] | ||
| Gold | Nanospikes | Electrodeposition | Anti-SARS-CoV-2 spike protein antibodies | 183 ± 10 nm/RIU | 0.08 ng/mL | [83] |
2.2. Plasmonic Nanoarrays for MEF-and SERS-Based Biosensors
Nanostructured plasmonic arrays promise the advantage of being highly controllable, optically stable, and easy to tune via advanced photolithographic techniques or controlled self-assembly [66][69][84]. The use of LSPR for refractive index sensing offers promising opportunities in terms of limit of detection and sensitivities, which can be further enhanced by exploiting the large electromagnetic field enhancement in the surroundings of the NPs to amplify external signals, such as Raman scattering and fluorescence, by serving as nanoantennas. Several SERS- and MEF-based biosensors have been recently proposed in the form of both periodic and non-periodic nanoarrays, achieving very promising results in the biosensing field [41][85]. Although plasmonic nanoarrays for SERS-based biosensors have been extensively reviewed in recent years [86][87], it is worth mentioning some of the most recent SERS-based nanostructured surfaces for cancer biomarker detection. In this frame, Muhammad et al. [88] reported on a gold NP array functionalized with a DNA aptamer for SERS detection of interleukin-6 (IL-6), an important inflammatory and cancer progression cytokine. The recognition of the target IL-6 in serum is achieved by the conformational of the aptamer, resulting in the corresponding change of the output Raman intensity ratio (I660/I736). The proposed nanoarray is reported to work in the IL-6 dynamic range of 10−12–10−7 M (Figure 4a). This example further elucidates the applicability of SERS substrates for real samples (blood, serum, urine) with no nonspecific signal detection. Most of the published top-down fabrication strategies allow regular, densely packed, and periodic geometries with strong electromagnetic field confinement, which represent the ideal candidates for plasmonic sensing. Despite this, large efforts have been the method to obtain similar performance starting from the self-assembly of monolayers of bottom-up synthesized plasmonic NPs. For example, Kang et al. [89] developed a closely-packed and ordered Au octahedra array as a highly active and reliable SERS substrate for miRNA detection, which is another hot topic in the biosensing community. The synthesis of Au octahedra was performed via bottom-up chemical synthesis, and the monolayer was achieved by exploiting interfaces between two immiscible liquids. The proposed sensor achieved an miRNA concentration down to 5.3 aM (LOD), a broad dynamic range (from 10 aM to 10 nM) without any signal amplification strategies, and discriminated targets differing from one another by only a single nucleotide. Among the many available SERS nanoarrays, more exotic nanostructures for the amplification of the Raman signals are not missing. For example, Chen et al. proposed a 3D nanopopcorn plasmonic substrate fabricated by a thermal evaporation method. After functionalization with a specific aptamer DNA, it was exploited for the highly sensitive and reproducible detection of the influenza A/H1N1 virus. They exploit the surface energy difference between the perfluorodecanethiol (PFDT) spacer and Au layer to self-assemble gold NPs in a highly uniform way.
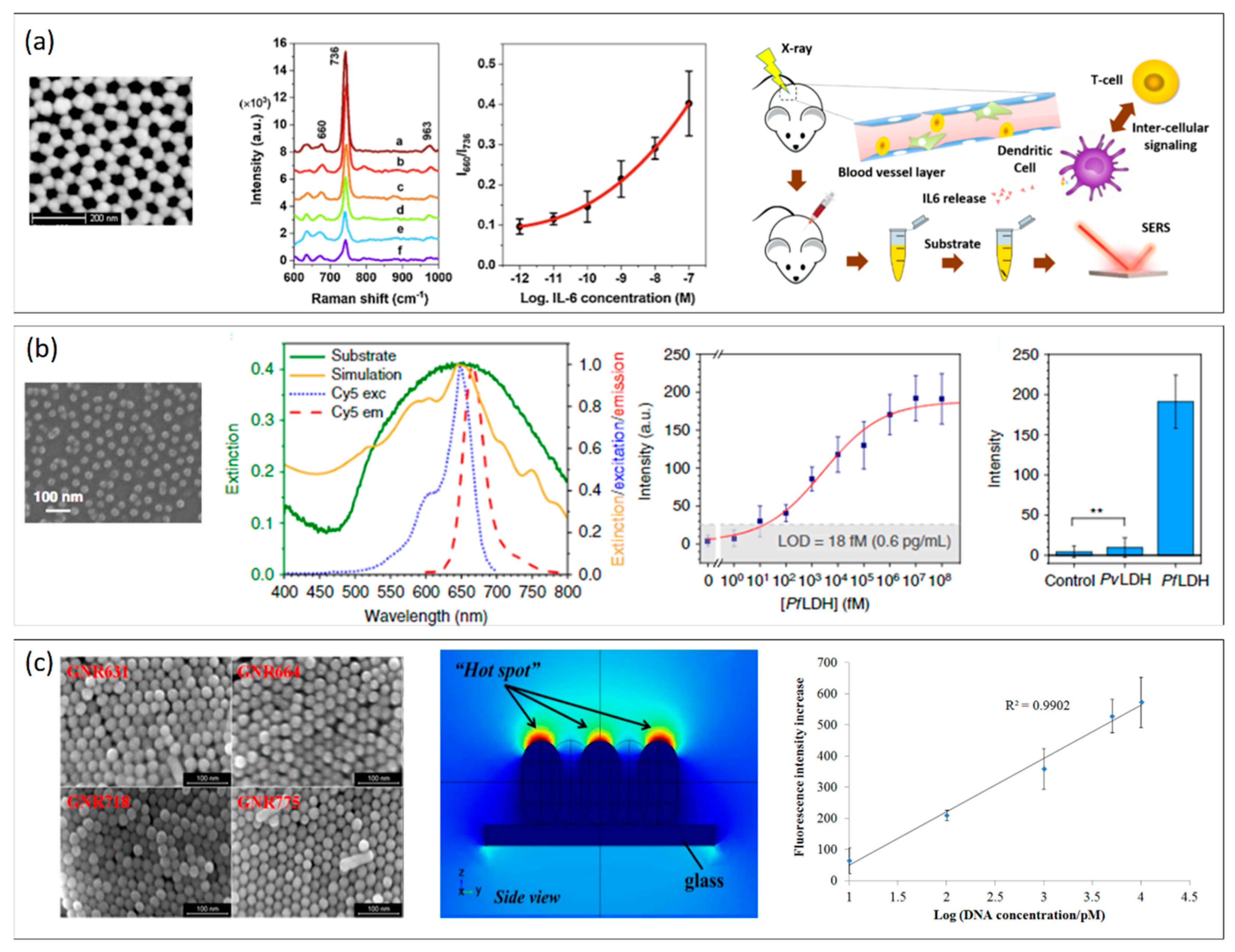
Figure 4. (a) From left to right: SEM image of a gold NP nanoarray for SERS detection of IL. The SERS signals prove the recognition of the target IL-6 in serum via corresponding changes of the output Raman intensity ratio (I660/I736) in the concentration range 10−12–10−7 M. The proposed nanoarray is reported to give results comparable to ELISA tests in serum. Adapted with permission from Ref. [88]. Copyright (2021), Elsevier. (b) From left to right: SEM image of the AuNP hexagonal array self-assembled on the top of a glass coverslip; experimental extinction spectrum of the substrate (green continuous line) and theoretical simulation of the optical response provided by the substrate morphology as measured by SEM (gold continuous line); on the same graph, the excitation and emission peaks of Cy5 (blue and red dashed lines) are reported; calibration curve (fluorescence intensity vs. PfLDH concentration in spiked human blood) of the immunoassay for PfLDH concentration in the dynamic range from 35 fg/mL to 3.5 μg/mL. The MEF substrate is reported to be highly specific, as confirmed by specificity tests (** p-value < 0.001). Adapted with permission from Ref. [90], Copyrigh (2020), Springer Nature. (c) From left to right: SEM image of gold nanorods (GNRs) of different sizes; simulation of the electromagnetic field distribution of the gold nanorods array (side view). Calibration curve of GNR nanoarray-based DNA chip as a function of the increasing target ssDNA concentration. Adapted with permission from Ref. [91]. Copyright (2017), American Chemical Society.
References
- Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localized Surface Plasmon Resonance Biosensing: Current Challenges and Approaches. Sensors 2015, 15, 15684–15716.
- Kermanshahian, K.; Yadegar, A.; Ghourchian, H. Gold nanorods etching as a powerful signaling process for plasmonic multicolorimetric chemo-/biosensors: Strategies and applications. Coord. Chem. Rev. 2021, 442, 213934.
- Orendorff, C.J.; Sau, T.K.; Murphy, C.J. Shape-Dependent Plasmon-Resonant Gold Nanoparticles. Small 2006, 2, 636–639.
- Nehl, C.L.; Hafner, J.H. Shape-dependent plasmon resonances of gold nanoparticles. J. Mater. Chem. 2008, 18, 2415–2419.
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857.
- Mock, J.J.; Smith, D.R.; Schultz, S. Local Refractive Index Dependence of Plasmon Resonance Spectra from Individual Nanoparticles. Nano Lett. 2003, 3, 485–491.
- Sun, Y.; Xia, Y. Increased Sensitivity of Surface Plasmon Resonance of Gold Nanoshells Compared to That of Gold Solid Colloids in Response to Environmental Changes. Anal. Chem. 2002, 74, 5297–5305.
- Link, S.; El-Sayed, M.A. Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217.
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape- and Size-Dependent Refractive Index Sensitivity of Gold Nanoparticles. Langmuir 2008, 24, 5233–5237.
- Sherry, L.J.; Chang, S.-H.; Schatz, G.C.; Van Duyne, R.P.; Wiley, B.J.; Xia, Y. Localized Surface Plasmon Resonance Spectroscopy of Single Silver Nanocubes. Nano Lett. 2005, 5, 2034–2038.
- Mahani, M.; Alimohamadi, F.; Torkzadeh-Mahani, M.; Hassani, Z.; Khakbaz, F.; Divsar, F.; Yoosefian, M. LSPR biosensing for the early-stage prostate cancer detection using hydrogen bonds between PSA and antibody: Molecular dynamic and experimental study. J. Mol. Liq. 2021, 324, 114736.
- Besselink, G.A.J.; Kooyman, R.P.H.; Van Os, P.J.H.J.; Engbers, G.H.M.; Schasfoort, R.B.M. Signal amplification on planar and gel-type sensor surfaces in surface plasmon resonance-based detection of prostate-specific antigen. Anal. Biochem. 2004, 333, 165–173.
- Iarossi, M.; Schiattarella, C.; Rea, I.; De Stefano, L.; Fittipaldi, R.; Vecchione, A.; Velotta, R.; Ventura, B. Della Colorimetric Immunosensor by Aggregation of Photochemically Functionalized Gold Nanoparticles. ACS Omega 2018, 3, 3805–3812.
- Della Ventura, B.; Schiavo, L.; Altucci, C.; Esposito, R.; Velotta, R. Light assisted antibody immobilization for bio-sensing. Biomed. Opt. Express 2011, 2, 3223–3231.
- Funari, R.; Della Ventura, B.; Altucci, C.; Offenhäusser, A.; Mayer, D.; Velotta, R. Single Molecule Characterization of UV-Activated Antibodies on Gold by Atomic Force Microscopy. Langmuir 2016, 32, 8084–8091.
- Gao, Y.; Wu, Y.; Di, J. Colorimetric detection of glucose based on gold nanoparticles coupled with silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 207–212.
- Politi, J.; De Stefano, L.; Rea, I.; Gravagnuolo, A.M.; Giardina, P.; Methivier, C.; Casale, S.; Spadavecchia, J. One-pot synthesis of a gold nanoparticle-Vmh2 hydrophobin nanobiocomplex for glucose monitoring. Nanotechnology 2016, 27, 195701.
- Spadavecchia, J.; Perumal, R.; Casale, S.; Krafft, J.-M.; Methivier, C.; Pradier, C.-M. Polyethylene glycol gold-nanoparticles: Facile nanostructuration of doxorubicin and its complex with DNA molecules for SERS detection. Chem. Phys. Lett. 2016, 648, 182–188.
- Politi, J.; De Stefano, L.; Longobardi, S.; Giardina, P.; Rea, I.; Methivier, C.; Pradier, C.-M.; Casale, S.; Spadavecchia, J. The amphiphilic hydrophobin Vmh2 plays a key role in one step synthesis of hybrid protein–gold nanoparticles. Colloids Surf. B Biointerfaces 2015, 136, 214–221.
- Lee, J.-H.; Cho, H.-Y.; Choi, H.K.; Lee, J.-Y.; Choi, J.-W. Application of Gold Nanoparticle to Plasmonic Biosensors. Int. J. Mol. Sci. 2018, 19, 2021.
- Guo, Y.; Wu, J.; Li, J.; Ju, H. A plasmonic colorimetric strategy for biosensing through enzyme guided growth of silver nanoparticles on gold nanostars. Biosens. Bioelectron. 2016, 78, 267–273.
- Faridfar, G.; Zeinoddini, M.; Akbarzedehkolahi, S.; Faridfar, S.; Nemati, A.S. Immunodiagnostic of Vibrio cholerae O1 using localized surface plasmon resonance (LSPR) biosensor. Int. Microbiol. 2021, 24, 115–122.
- Tramontano, C.; Miranda, B.; Chianese, G.; De Stefano, L.; Forestiere, C.; Pirozzi, M.; Rea, I. Design of gelatin-capped plasmonic-diatomite nanoparticles with enhanced galunisertib loading capacity for drug delivery applications. Int. J. Mol. Sci. 2021, 22, 10755.
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-Functional Plasmonic Photothermal Biosensors for Highly Accurate Severe Acute Respiratory Syndrome Coronavirus 2 Detection. ACS Nano 2020, 14, 5268–5277.
- Lee, T.; Kim, G.H.; Kim, S.M.; Hong, K.; Kim, Y.; Park, C.; Sohn, H.; Min, J. Label-free localized surface plasmon resonance biosensor composed of multi-functional DNA 3 way junction on hollow Au spike-like nanoparticles (HAuSN) for avian influenza virus detection. Colloids Surf. B Biointerfaces 2019, 182, 110341.
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W.; et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020, 92, 1518–1524.
- Moitra, P.; Alafeef, M.; Dighe, K.; Frieman, M.B.; Pan, D. Selective Naked-Eye Detection of SARS-CoV-2 Mediated by N Gene Targeted Antisense Oligonucleotide Capped Plasmonic Nanoparticles. ACS Nano 2020, 14, 7617–7627.
- Karakuş, E.; Erdemir, E.; Demirbilek, N.; Liv, L. Colorimetric and electrochemical detection of SARS-CoV-2 spike antigen with a gold nanoparticle-based biosensor. Anal. Chim. Acta 2021, 1182, 338939.
- Sheth, S.; Barnard, E.; Hyatt, B.; Rathinam, M.; Zustiak, S.P. Predicting Drug Release From Degradable Hydrogels Using Fluorescence Correlation Spectroscopy and Mathematical Modeling. Front. Bioeng. Biotechnol. 2019, 7, 410.
- Zhang, C.; Rodriguez, E.; Bi, C.; Zheng, X.; Suresh, D.; Suh, K.; Li, Z.; Elsebaei, F.; Hage, D.S. High performance affinity chromatography and related separation methods for the analysis of biological and pharmaceutical agents. Analyst 2018, 143, 374–391.
- Zheng, F.; Xiong, W.; Sun, S.; Zhang, P.; Zhu, J.J. Recent advances in drug release monitoring. Nanophotonics 2019, 8, 391–413.
- Okabe, K.; Inada, N.; Gota, C.; Harada, Y.; Funatsu, T.; Uchiyama, S. Intracellular temperature mapping with a fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy. Nat. Commun. 2012, 3, 705.
- Qiu, F.; Wang, D.; Zhu, Q.; Zhu, L.; Tong, G.; Lu, Y.; Yan, D.; Zhu, X. Real-time monitoring of anticancer drug release with highly fluorescent star-conjugated copolymer as a drug carrier. Biomacromolecules 2014, 15, 1355–1364.
- Tian, F.; Conde, J.; Bao, C.; Chen, Y.; Curtin, J.; Cui, D. Gold nanostars for efficient in vitro and in vivo real-time SERS detection and drug delivery via plasmonic-tunable Raman/FTIR imaging. Biomaterials 2016, 106, 87–97.
- Montgomery, J.M.; Imre, A.; Welp, U.; Vlasko-Vlasov, V.; Gray, S.K.; Ghaemi, H.F.; Thio, T.; Grupp, D.E.; Ebbesen, T.W. SERS enhancements via periodic arrays of gold nanoparticles on silver film structures. Opt. Express 2009, 17, 8669–8675.
- Terracciano, M.; Napolitano, M.; De Stefano, L.; De Luca, A.C.; Rea, I. Gold decorated porous biosilica nanodevices for advanced medicine. Nanotechnology 2018, 29, 235601.
- Lai, C.H.; Wang, G.A.; Ling, T.K.; Wang, T.J.; Chiu, P.K.; Chou Chau, Y.F.; Huang, C.C.; Chiang, H.P. Near infrared surface-enhanced Raman scattering based on star-shaped gold/silver nanoparticles and hyperbolic metamaterial. Sci. Rep. 2017, 7, 5446.
- Managò, S.; Tramontano, C.; Cave, D.D.; Chianese, G.; Zito, G.; De Stefano, L.; Terracciano, M.; Lonardo, E.; De Luca, A.C.; Rea, I.; et al. SERS quantification of Galunisertib delivery in colorectal cancer cells by plasmonic-assisted diatomite nanoparticles. Small 2021, 17, 2101711.
- Reifarth, M.; Hoeppener, S.; Schubert, U.S. Uptake and Intracellular Fate of Engineered Nanoparticles in Mammalian Cells: Capabilities and Limitations of Transmission Electron Microscopy—Polymer-Based Nanoparticles. Adv. Mater. 2018, 30, 1703704.
- Li, L.; Tang, B.; Li, X.; Duan, X.; Yang, P. Accurate in situ monitoring of mitochondrial H2O2 by robust SERS nanoprobes with a Au−Se interface. Anal. Chem. 2021, 93, 4059–4065.
- Tahir, M.A.; Dina, N.E.; Cheng, H.; Valev, V.K.; Zhang, L. Surface-enhanced Raman spectroscopy for bioanalysis and diagnosis. Nanoscale 2021, 13, 11593–11634.
- Yücel, G.; Zhao, Z.; El-Battrawy, I.; Lan, H.; Lang, S.; Li, X.; Buljubasic, F.; Zimmermann, W.H.; Cyganek, L.; Utikal, J.; et al. Lipopolysaccharides induced inflammatory responses and electrophysiological dysfunctions in human-induced pluripotent stem cell derived cardiomyocytes. Sci. Rep. 2017, 7, 2935.
- Li, Y.; Italiani, P.; Casals, E.; Tran, N.; Puntes, V.F.; Boraschi, D. Optimising the use of commercial LAL assays for the analysis of endotoxin contamination in metal colloids and metal oxide nanoparticles. Nanotoxicology 2015, 9, 462–473.
- Verde, A.; Mangini, M.; Managò, S.; Tramontano, C.; Rea, I.; Boraschi, D.; Italiani, P.; De Luca, A.C. SERS Sensing of Bacterial Endotoxin on Gold Nanoparticles. Front. Immunol. 2021, 12, 758410.
- Hu, W.; Xia, L.; Hu, Y.; Li, G. Recent progress on three-dimensional substrates for surface-enhanced Raman spectroscopic analysis. Microchem. J. 2022, 172, 106908.
- Han, Y.; Wu, S.R.; Tian, X.D.; Zhang, Y. Optimizing the SERS Performance of 3D Substrates through Tunable 3D Plasmonic Coupling toward Label-Free Liver Cancer Cell Classification. ACS Appl. Mater. Interfaces 2020, 12, 28965–28974.
- Kolle, M. Photonic Structures Inspired by Nature; Springer: Berlin/Heidelberg, Germany, 2011.
- Pannico, M.; Rea, I.; Chandrasekaran, S.; Musto, P.; Voelcker, N.H.; De Stefano, L. Electroless Gold-Modified Diatoms as Surface-Enhanced Raman Scattering Supports. Nanoscale Res. Lett. 2016, 11, 315.
- Managò, S.; Zito, G.; Rogato, A.; Casalino, M.; Esposito, E.; De Luca, A.C.; De Tommasi, E. Bioderived Three-Dimensional Hierarchical Nanostructures as Efficient Surface-Enhanced Raman Scattering Substrates for Cell Membrane Probing. ACS Appl. Mater. Interfaces 2018, 10, 12406–12416.
- De Angelis, F.; Malerba, M.; Patrini, M.; Miele, E.; Das, G.; Toma, A.; Zaccaria, R.P.; Di Fabrizio, E. 3D hollow nanostructures as building blocks for multifunctional plasmonics. Nano Lett. 2013, 13, 3553–3558.
- Andreou, C.; Neuschmelting, V.; Tschaharganeh, D.F.; Huang, C.H.; Oseledchyk, A.; Iacono, P.; Karabeber, H.; Colen, R.R.; Mannelli, L.; Lowe, S.W.; et al. Imaging of Liver Tumors Using Surface-Enhanced Raman Scattering Nanoparticles. ACS Nano 2016, 10, 5015–5026.
- Mir-Simon, B.; Reche-Perez, I.; Guerrini, L.; Pazos-Perez, N.; Alvarez-Puebla, R.A. Universal one-pot and scalable synthesis of SERS encoded nanoparticles. Chem. Mater. 2015, 27, 950–958.
- Oseledchyk, A.; Andreou, C.; Wall, M.A.; Kircher, M.F. Folate-Targeted Surface-Enhanced Resonance Raman Scattering Nanoprobe Ratiometry for Detection of Microscopic Ovarian Cancer. ACS Nano 2017, 11, 1488–1497.
- Pazos-Perez, N.; Fitzgerald, J.M.; Giannini, V.; Guerrini, L.; Alvarez-Puebla, R.A. Modular assembly of plasmonic core–satellite structures as highly brilliant SERS-encoded nanoparticles. Nanoscale Adv. 2019, 1, 122–131.
- Mallia, R.J.; McVeigh, P.Z.; Fisher, C.J.; Veilleux, I.; Wilson, B.C. Wide-field multiplexed imaging of EGFR-targeted cancers using topical application of NIR SERS nanoprobes. Nanomedicine 2014, 10, 89–101.
- Nicolson, F.; Andreiuk, B.; Andreou, C.; Hsu, H.T.; Rudder, S.; Kircher, M.F. Non-invasive in vivo imaging of cancer using Surface-Enhanced spatially offset raman spectroscopy (SESORS). Theranostics 2019, 9, 5899–5913.
- Wen, Y.; Truong, V.X.; Li, M. Real-Time Intraoperative Surface-Enhanced Raman Spectroscopy-Guided Thermosurgical Eradication of Residual Microtumors in Orthotopic Breast Cancer. Nano Lett. 2021, 21, 3066–3074.
- Wang, Q.; Wang, L. Lab-on-fiber: Plasmonic nano-arrays for sensing. Nanoscale 2020, 12, 7485–7499.
- Levin, A.D.; Ringaci, A.; Alenichev, M.K.; Drozhzhennikova, E.B.; Shevchenko, K.G.; Cherkasov, V.R.; Nikitin, M.P.; Nikitin, P.I. Dynamic light scattering biosensing based on analyte-induced inhibition of nanoparticle aggregation. Anal. Bioanal. Chem. 2020, 412, 3423–3431.
- Hu, S.; Huang, P.-J.J.; Wang, J.; Liu, J. Dissecting the Effect of Salt for More Sensitive Label-Free Colorimetric Detection of DNA Using Gold Nanoparticles. Anal. Chem. 2020, 92, 13354–13360.
- Aryal, S.; Remant, R.B.; Bhattarai, N.; Kim, C.K.; Kim, H.Y. Study of electrolyte induced aggregation of gold nanoparticles capped by amino acids. J. Colloid Interface Sci. 2006, 299, 191–197.
- Guo, R.; Hakala, T.K.; Törmä, P. Geometry dependence of surface lattice resonances in plasmonic nanoparticle arrays. Phys. Rev. B 2017, 95, 155423.
- Teo, B.K.; Sun, X.H. From top-down to bottom-up to hybrid nanotechnologies: Road to nanodevices. J. Clust. Sci. 2006, 17, 529–540.
- Iqbal, P.; Preece, J.A.; Mendes, P.M. Nanotechnology: The “Top-Down” and “Bottom-Up” Approaches. In Supramolecular Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2012.
- Bhalla, N.; Sathish, S.; Sinha, A.; Shen, A.Q. Biosensors: Large-Scale Nanophotonic Structures for Long-Term Monitoring of Cell Proliferation (Adv. Biosys. 4/2018). Adv. Biosyst. 2018, 2, 1870031.
- Im, H.; Lee, S.H.; Wittenberg, N.J.; Johnson, T.W.; Lindquist, N.C.; Nagpal, P.; Norris, D.J.; Oh, S.H. Template-stripped smooth Ag nanohole arrays with silica shells for surface plasmon resonance biosensing. ACS Nano 2011, 5, 6244–6253.
- Jeon, H.C.; Heo, C.J.; Lee, S.Y.; Yang, S.M. Hierarchically Ordered Arrays of Noncircular Silicon Nanowires Featured by Holographic Lithography Toward a High-Fidelity Sensing Platform. Adv. Funct. Mater. 2012, 22, 4268–4274.
- Menezes, J.W.; Ferreira, J.; Santos, M.J.L.; Cescato, L.; Brolo, A.G. Large-Area Fabrication of Periodic Arrays of Nanoholes in Metal Films and Their Application in Biosensing and Plasmonic-Enhanced Photovoltaics. Adv. Funct. Mater. 2010, 20, 3918–3924.
- Miranda, B.; Chu, K.-Y.; Maffettone, P.L.; Shen, A.Q.; Funari, R. Metal-Enhanced Fluorescence Immunosensor Based on Plasmonic Arrays of Gold Nanoislands on an Etched Glass Substrate. ACS Appl. Nano Mater. 2020, 10, 10470–10478.
- Kim, D.M.; Park, J.S.; Jung, S.W.; Yeom, J.; Yoo, S.M. Biosensing Applications Using Nanostructure-Based Localized Surface Plasmon Resonance Sensors. Sensors 2021, 21, 3191.
- Asgari, S.; Sun, L.; Lin, J.; Weng, Z.; Wu, G.; Zhang, Y.; Lin, M. Nanofibrillar cellulose/ nanoparticle nanocomposite as a SERS substrate for detection of paraquat and thiram in lettuce. Microchim. Acta 2020, 187, 390.
- Bhalla, N.; Jamshaid, A.; Leung, M.H.M.; Ishizu, N.; Shen, A.Q. Electrical contact of metals at the nanoscale overcomes the oxidative susceptibility of silver-based nanobiosensors. ACS Appl. Nano Mater. 2019, 2, 2064–2075.
- Ren, S.; Zhang, Z.; Xu, C.; Guo, L.; Lu, R.; Sun, Y.; Guo, J.; Qin, R.; Qin, W.; Gu, J. Distribution of IgG galactosylation as a promising biomarker for cancer screening in multiple cancer types. Cell Res. 2016, 26, 963–966.
- Vestri, A.; Rippa, M.; Marchesano, V.; Sagnelli, D.; Margheri, G.; Zhou, J.; Petti, L. LSPR immuno-sensing based on iso-Y nanopillars for highly sensitive and specific imidacloprid detection. J. Mater. Chem. B 2021, 9, 9153–9161.
- Chen, J.S.; Chen, P.F.; Lin, H.T.H.; Huang, N.T. A Localized surface plasmon resonance (LSPR) sensor integrated automated microfluidic system for multiplex inflammatory biomarker detection. Analyst 2020, 145, 7654–7661.
- Khan, Y.; Li, A.; Chang, L.; Li, L.; Guo, L. Gold nano disks arrays for localized surface plasmon resonance based detection of PSA cancer marker. Sens. Actuators B Chem. 2018, 255, 1298–1307.
- Terracciano, M.; Rea, I.; Borbone, N.; Moretta, R.; Oliviero, G.; Piccialli, G.; De Stefano, L. Porous silicon-based aptasensors: The next generation of label-free devices for health monitoring. Molecules 2019, 24, 2216.
- Taghavi, A.; Rahbarizadeh, F.; Abbasian, S.; Moshaii, A. Label-Free LSPR Prostate-Specific Antigen Immune-Sensor Based on GLAD-Fabricated Silver Nano-columns. Plasmonics 2020, 15, 753–760.
- Vieth, R. Vitamin D Toxicity, Policy, and Science. J. Bone Miner. Res. 2007, 22, V64–V68.
- Jo, S.; Lee, W.; Park, J.; Park, H.; Kim, M.; Kim, W.; Hong, J.; Park, J. Wide-range direct detection of 25-hydroxyvitamin D3 using polyethylene-glycol-free gold nanorod based on LSPR aptasensor. Biosens. Bioelectron. 2021, 181, 956–5663.
- Kim, J.; Oh, S.Y.; Shukla, S.; Hong, S.B.; Heo, N.S.; Bajpai, V.K.; Chun, H.S.; Jo, C.-H.; Choi, B.G.; Huh, Y.S.; et al. Heteroassembled gold nanoparticles with sandwich-immunoassay LSPR chip format for rapid and sensitive detection of hepatitis B virus surface antigen (HBsAg). Biosens. Bioelectron. 2018, 107, 118–122.
- Bastús, N.G.; Comenge, J.; Puntes, V. Kinetically controlled seeded growth synthesis of citrate-stabilized gold nanoparticles of up to 200 nm: Size focusing versus ostwald ripening. Langmuir 2011, 27, 11098–11105.
- Funari, R.; Chu, K.Y.; Shen, A.Q. Detection of antibodies against SARS-CoV-2 spike protein by gold nanospikes in an opto-microfluidic chip. Biosens. Bioelectron. 2020, 169, 112578.
- Focsan, M.; Campu, A.; Craciun, A.M.; Potara, M.; Leordean, C.; Maniu, D.; Astilean, S. A simple and efficient design to improve the detection of biotin-streptavidin interaction with plasmonic nanobiosensors. Biosens. Bioelectron. 2016, 86, 728–735.
- Jeong, Y.; Kook, Y.M.; Lee, K.; Koh, W.G. Metal enhanced fluorescence (MEF) for biosensors: General approaches and a review of recent developments. Biosens. Bioelectron. 2018, 111, 102–116.
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57.
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117.
- Muhammad, M.; Shao, C.-S.; Huang, Q. Aptamer-functionalized Au nanoparticles array as the effective SERS biosensor for label-free detection of interleukin-6 in serum. Sens. Actuators B Chem. 2021, 334, 129607.
- Kang, T.; Zhu, J.; Luo, X.; Jia, W.; Wu, P.; Cai, C. Controlled Self-Assembly of a Close-Packed Gold Octahedra Array for SERS Sensing Exosomal MicroRNAs. Anal. Chem. 2021, 93, 2519–2526.
- Minopoli, A.; Della Ventura, B.; Lenyk, B.; Gentile, F.; Tanner, J.A.; Offenhäusser, A.; Mayer, D.; Velotta, R. Ultrasensitive antibody-aptamer plasmonic biosensor for malaria biomarker detection in whole blood. Nat. Commun. 2020, 11, 1–10.
- Mei, Z.; Tang, L. Surface-Plasmon-Coupled Fluorescence Enhancement Based on Ordered Gold Nanorod Array Biochip for Ultrasensitive DNA Analysis. Anal. Chem. 2017, 89, 633–639.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
956
Revisions:
2 times
(View History)
Update Date:
26 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




