
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hiroshi Kitoh | + 1534 word(s) | 1534 | 2020-09-07 08:03:41 | | | |
| 2 | Catherine Yang | Meta information modification | 1534 | 2020-09-28 11:50:42 | | |
Video Upload Options
Fibrodysplasia ossificans progressiva (FOP) is an extremely rare heritable disorder of connective tissues characterized by progressive heterotopic ossification in various skeletal sites.
1. Introduction
Fibrodysplasia ossificans progressiva (FOP) is a severely disabling heritable disorder of connective tissues characterized by progressive heterotopic ossification in the skeletal muscles, ligaments, tendons, fascia, and aponeuroses, and malformations of the great toes. Painful recurrent episodes of soft tissue swelling (flare-ups) precede to heterotopic ossification. Flare-ups usually begin in the first decade of life, and several patients with FOP are misdiagnosed as having soft tissue tumors or aggressive fibromatosis before the appearance of heterotopic ossification [1]. They sometimes undergo dangerous and unnecessary diagnostic procedures that provoke heterotopic ossification formation leading to permanent harm and lifelong disability [2]. Early clinical diagnosis and confirmatory genetic testing of FOP are extremely important to prevent additional iatrogenic harm or trauma [3].
Heterotopic ossification throughout the body is progressive, and patient’s disabilities are cumulative [4]. Currently, there are no definitive treatments for FOP; however, there has been substantial recent interest in clinical trials for novel treatments for this specific disease. In this review, we specifically describe various skeletal manifestations suggestive of FOP that can usually be seen before the appearance of heterotopic ossification, to make clinicians aware of these early signs and symptoms of FOP. We also discuss current therapeutic approaches for FOP based on molecular mechanisms of this disease, especially focusing on pharmacological drugs that are currently on-going clinical trials to evaluate their efficacy in FOP patients. Patients’ data presentation including photographs were approved by the ethical committee from the author’s institution.
2. Managements and Treatments
There is presently no definitive medical treatment to prevent, stop or reverse heterotopic ossification in FOP. Avoidance of trauma and prevention of injury remain the mainstays of therapy. Surgical removal of heterotopic ossification often leads to significant recurrence and expansion of ossification. Bracing for spinal deformity is ineffective [5]. Restriction of activity may be helpful to reduce trauma, but compromise of independence may be unacceptable to patients as well as their parents. Physical rehabilitation to maintain joint mobility may be harmful by provoking or exacerbating lesions and it should be focused on enhancing activity of daily living through approaches that avoid a passive range of motion exercises. Occupational therapy and vocational education consultations may be useful. Overstretching of the jaw and intramuscular injections of local anesthesia should not be attempted in dental care. A locked jaw sometimes necessitates surgery to avoid life threatening complications. Since conductive hearing loss is common, children should have audiology evaluations regularly. The management of FOP requires education of patients and caregivers, the use of medications to settle inflammation and flare-ups, instructions to ensure proper oral care, and other compensatory approaches that aid in rehabilitation [6].
The use of short-term high-dose corticosteroids is based on its potent anti-inflammatory effects [7]. It may help reduce the intense inflammation and tissue edema when they are used in an early stage of flare-ups. They can relieve but not completely resolve symptoms of flare-ups [8]. Corticosteroids are most effective if used within the first 24 h of a new flare-up. The dose of corticosteroids is dependent on body weight, and a recommended dose of prednisone for acute flare-ups is 2 mg/kg/day, administered as a single daily dose for no more than 4 days. Corticosteroids should be used for treatment of flare-ups that affect major joints, the jaw, or the submandibular area, and should not be used for flare-ups that involve the back, neck, or trunk due to the long duration and recurring nature of these flare-ups. Corticosteroids should not be used for long-term, and when prednisone is discontinued, non-steroidal anti-inflammatory drugs (NSAIDs) or selective cyclooxygenase-2 (COX2) inhibitors may be used for the duration of flare-ups, although there is no evidence that chronic treatment with these drugs prevent flare-ups in FOP. Bisphosphonates have been used for the symptomatic management of flare-ups in FOP, although concrete clinical data for these treatments are sparse [9]. Mast cells could provide an important role for the pathology of heterotopic ossification in FOP [10]. Imatinib, a tyrosine kinase inhibitor initially developed for chronic myeloid leukemia, has anti-proliferative and immunomodulatory effects in mast cells. The administration of imatinib demonstrated positive effects on decrease in the intensity of flare-ups in seven FOP patients who did not respond the standard medications such as corticosteroids, NSAIDs, or intravenous bisphosphonates [11].
3. On-Going Clinical Trials for FOP (Phase 2 or Phase 3)
Several researches to develop therapeutic drugs have focused on target inhibition of the ACVR1 receptor, ACVR1 ligand, BMP intracellular signaling, and inflammatory triggers of disease activity. Exciting advances in new therapeutic approaches for FOP have developed recently [12][13][14]. We highlight novel treatment drugs that are currently on-going phase 2 or phase 3 clinical trials (Figure 1).
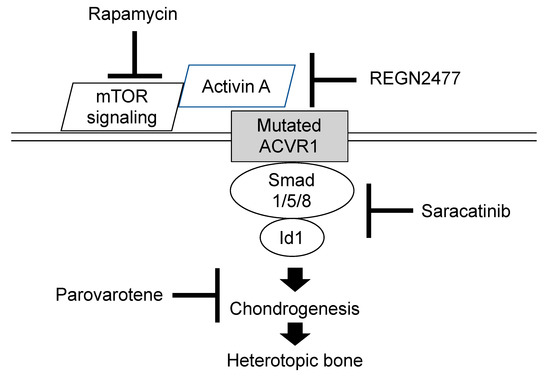
Figure 1. Molecular targeting of therapeutic drugs on on-going phase 2 or 3 clinical trials.
Retinoid signaling is normally attenuated during chondrogenesis and exogenous retinoid agonists can block chondrogenesis effectively and rapidly [15]. Agonists for retinoic acid receptors (PARα or RARγ) experimentally inhibited chondrogenesis of heterotopic ossification in transgenic mice model of FOP, and the RARγ agonists were far more effective [16]. One of the RARγ class drugs is palovarotene, a highly specific RARγ agonist that has already been evaluated in another clinical trial for α-1-antitrypsin-induced emphysema, and its safety profile has been well-characterized. Palovarotene inhibits heterotopic ossification and maintains limb mobility and growth in mice model of FOP [17]. Palovarotene is also evaluated in another phase 2 trials for treatment of hereditary multiple exostoses to suppress the formation of osteochondromas. Phase 2 clinical trials were initiated in 2014 by Clementia Pharmaceuticals to evaluate the safety and efficacy of palovarotene for treatment of FOP (Clinicaltrials.gov registration NCT02190747). The primary outcome was to compare the volume of heterotopic ossification formation between treated patients and untreated patients. Palovarotene decreased the percentage of FOP patients who develop heterotopic ossification, the time to flare-ups resolution, and patient-reported pain. Phase 3 trial is currently in progress (Clinicaltrials.gov registration NCT03312634). Palovarotene is a known teratogen that causes limb malformations in the developing fetus and may decline growth in children [18]. Other potential risks of palovarotene include pancreatitis, hearing and vision impairment, mouth ulcer, sensitivity to sunlight, and dry skin. These adverse events are being monitored closely during the trials.
The R206H mutation causes the ACVR1 receptor to misinterpret activin A and generate a signal as if BMP ligands are present [19]. The ACVR1 mutant mice developed more heterotopic ossification throughout the skeleton when activin A was injected, and those treated with a blocking antibody of activin A did not develop heterotopic ossification [15]. Activin A is, thus, an obligatory secreted factor that is required for the initiation of heterotopic ossification in FOP, and the blocking of activin A could prevent the formation of heterotopic bone. As a result of preclinical studies, REGN 2477 (garetosmab)—an antibody that binds to activin A and blocks its activity—is now in a clinical trial to examine safety, tolerability, and efficacy on abnormal bone formation in adult patients with FOP (Clinicaltrials.gov registration NCT03188666).
Activin A enhances the chondrogenesis of induced mesenchymal stromal cells derived from FOP patients-derived induced pluripotent stem cells (FOP-iPSCs) via the aberrant activation of BMP signaling in vitro, and induced endochondral ossification of FOP-iPSCs in vivo [20]. By using a high-throughput screening system of small molecules to suppress activin A induced chondrogenesis, Hino et al. demonstrated that mTOR signaling is a critical pathway for the aberrant chondrogenesis of mesenchymal stromal cells derived from FOP-iPSCs and inhibited the heterotopic ossification of multiple model mice, including FOP-ACVR1 transgenic mice and a heterotopic ossification model utilizing FOP-iPSCs [21]. Rapamycin is a commonly-used immunosuppressant that exerts its biological effect by inhibiting mTOR1 kinase activity. Heterotopic ossification was decreased after treatment with rapamycin in mice model of FOP as well as FOP-iPSC-based heterotopic ossification model mice [22]. A phase 2 clinical trial for a 6-month randomized placebo-controlled study and subsequent open label extension study is now opening in Japan (UMIN000028429). Primary endpoint for evaluating the efficacy of rapamycin is based on objective physical function assessment using the Japanese version of Health Assessment Questionnaire or Childhood Health Assessment Questionnaire.
Saracatinib, also known as AZD0530, is an investigational drug that was initially developed as a potential treatment for patients with cancer. Saracatinib inhibits the serum activation of Id1, which is a transcriptional factor mediated by Smad 1/5/8 phosphorylation, by direct inhibition of BMPR-I kinase activity [23]. Other research also demonstrated that Saracatinib was effective at suppressing the enhanced chondrogenesis of FOP-iPSCs and suppressed the heterotopic ossification or bone formation in multiple FOP animal models [24]. A phase 2A proof of concept study including a 6-month randomized placebo-controlled study and 12-month open label extension study using historical data is proposed in the Netherlands, the United Kingdom, and Germany (Clinicaltrials.gov registration NCT04307953).
4. Conclusions
Clinicians should become aware of early detectable skeletal malformations, including great toe deformities, shortened thumb, neck stiffness associated with hypertrophy of the posterior elements of the cervical spine, multiple ossification centers in the calcaneus, and osteochondroma-like lesions of the long bones, to make an early diagnosis and prevent iatrogenic harm or trauma. Although there is presently no definitive medical treatment to prevent, stop or reverse heterotopic ossification in FOP, exciting advances in novel therapeutic approaches using pharmacological drugs, including palovarotene, REGN 2477, rapamycin, and saracatinib, have been developed and are currently in clinical trials.
References
- Kitterman, J.A.; Kantanie, S.; Rocke, D.M.; Kaplan, F.S. Iatrogenic harm caused by diagnostic errors in fibrodysplasia ossificans progressiva. Pediatrics 2005, 116, e654–e661.
- Zaghloul, K.A.; Heuer, G.G.; Guttenberg, M.D.; Shore, E.M.; Kaplan, F.S.; Storm, P.B. Lumbar puncture and surgical intervention in a child with undiagnosed fibrodysplasia ossificans progressiva. J. Neursurg. Pediatr. 2008, 1, 91–94.
- Kaplan, F.S.; Xu, M.; Glaser, D.L.; Collins, F.; Connor, M.; Kitterman, J.; Sillence, D.; Zackai, E.; Ravitsky, V.; Zasloff, M.; et al. Early diagnosis of fibrodysplasia ossificans progressive. Pediatrics 2008, 151, e1295–e1300.
- Pignolo, R.J.; Shore, E.M.; Kaplan, F.S. Fibrodysplasia ossificans progressiva: Diagnosis, management, and therapeutic horizons. Pediatr. Endocrinol. Rev. 2013, 10, 437–448.
- Shah, P.B.; Zasloff, M.A.; Drummond, D.; Kaplan, F.S. Spinal deformity in patients who have fibrodysplasia ossificans progressive. J. Bone Joint Surg. Am. 1994, 76, 705–712.
- Haga, N.; Nakashima, Y.; Kitoh, H.; Kamizono, J.; Katagiri, T.; Saijo, H.; Tsukamoto, S.; Shinoda, Y.; Sawada, R.; Nakahara, Y. Fibrodysplasia ossificans progressive: Review and research activities in Japan. Pediatr. Int. 2020, 62, 3–13.
- Rhen, T.; Cidlowski, J.A. Anti-inflammatory action of glucocorticoids—New mechanisms for old drugs. N. Engl. J. Med. 2005, 353, 1711–1723.
- Pignolo, R.J.; Bedford-Gay, C.; Liljesthrom, M.; Durbin-Johnson, B.P.; Shore, E.M.; Rocke, D.M.; Kaplan, F.S. The natural history of flare-ups in fibrodysplasia ossificans progressiva: A comprehensive global assessment. J. Bone Miner. Res. 2016, 31, 650–656.
- Brantus, J.F.; Meunier, P.J. Effects of intravenous etidronate and oral corticosteroids in fibrodysplasia ossificans progressiva. Clin. Orthop. 1998, 346, 117–120.
- Gannon, F.H.; Glaser, D.; Caron, R.; Thompson, L.D.R.; Shore, E.M.; Kaplan, F.S. Mast cell involvement in fibrodysplasia ossificans progressiva. Hum. Pathol. 2001, 32, 842–848.
- Kaplan, F.S.; Andolina, J.R.; Adamson, P.C.; Teachey, D.T.; Finklestein, J.Z.; Ebb, D.H.; Whitehead, B.; Jacobs, B.; Siegel, D.M.; Keen, R.; et al. Early clinical observations on the use of imatinib mesylate in FOP: A report of seven cases. Bone 2018, 109, 276–280.
- Cappato, S.; Giacopelli, F.; Ravazzolo, R.; Bocciardi, R. The horizon of a therapy for rare genetic diseases: A “druggable” future for fibrodysplasia ossificans progressiva. Int. J. Mol. Sci. 2018, 19, 989.
- Katagiri, T.; Tsukamoto, S.; Nakachi, Y.; Kuratani, M. Recent topics in fibrodysplasia ossificans progressiva. Endocrinol. Metab. 2018, 33, 331–338.
- Wentworth, K.L.; Masharani, U.; Hsiao, E.C. Therapeutic advanced for blocking heterotopic ossification in fibrodysplasia ossificans progressiva. Br. J. Clin. Pharmacol. 2019, 85, 1180–1187. [Google Scholar] [CrossRef]
- Pacifici, M.; Cossu, G.; Molinaro, M.; Tato, F. Vitamin A inhibits chondrogenesis but not myogenesis. Exp. Cell Res. 1980, 129, 469–474.
- Shimono, K.; Tung, W.-E.; Macolino, C.; Chi, A.H.T.; Didizian, J.H.; Mundy, C.; Chandraratna, R.A.; Mishina, Y.; Enomoto-Iwamoto, M.; Pacifici, M.; et al. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-g agonists. Nat. Med. 2011, 17, 454–460.
- Chakkalakal, S.A.; Uchibe, K.; Convente, M.R.; Zhang, D.; Economides, A.N.; Kaplan, F.S.; Pacifici, M.; Iwamoto, M.; Shore, E.M. Palovarotene inhibits heterotopic ossification and maintains limb mobility and growth in mice with the human ACVR1R206H fibrodysplasia ossificans progressiva (FOP) Mutation. J. Bone Miner. Res. 2016, 31, 1666–1675.
- Lee-Shepard, J.B.; Nicholas, S.A.; Stoessel, S.J.; Devarakonda, P.M.; Schneider, M.J.; Yamamoto, M.; Goldhamer, D.J. Palovarotene reduced heterotopic ossification in juvenile FOP mice but exhibits pronounced skeletal toxicity. eLife 2018, 7, e40814.
- Hatsell, S.J.; Idone, V.; Wolken, D.M.; Huang, L.; Kim, H.J.; Wang, L.; Wen, X.; Nannuru, K.C.; Jimenez, J.; Xie, L.; et al. Economides AN. ACVR1(R206H) receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci. Transl. Med. 2015, 7, 303ra137.
- Hino, K.; Ikeya, M.; Horigome, K.; Matsumoto, Y.; Ebise, H.; Nishio, M.; Sekiguchi, K.; Shibata, M.; Nagata, S.; Matsuda, S.; et al. Neofunction of ACVR1 in fibrodysplasia ossificans progressiva. Proc. Natl. Acad. Sci. USA 2015, 112, 15438–15443.
- Hino, K.; Zhao, C.; Horigome, K.; Nishio, M.; Okanishi, Y.; Nagata, S.; Komura, S.; Yamada, Y.; Toguchida, J.; Ohta, A.; et al. An mTOR signaling modulator suppressed heterotopic ossification of fibrodysplasia ossificans progressiva. Stem Cell Rep. 2018, 11, 1106–1119.
- Hino, K.; Horigome, K.; Nishio, M.; Komura, S.; Nagata, S.; Zhao, C.; Jin, Y.; Kawakami, K.; Yamada, Y.; Ohta, A.; et al. Activin-A enhances mTOR signaling to promote aberrant chondrogenesis in fibrodysplasia ossificans progressive. J. Clin. Investig. 2017, 127, 3339–3352.
- Lewis, T.C.; Prywes, R. Serum regulation of Id1 expression by a BMP pathway and BMP responsive element. Biochim. Biophys. Acta 2013, 1829, 1147–1159.
- Shimono, K.; Tung, W.-E.; Macolino, C.; Chi, A.H.T.; Didizian, J.H.; Mundy, C.; Chandraratna, R.A.; Mishina, Y.; Enomoto-Iwamoto, M.; Pacifici, M.; et al. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-g agonists. Nat. Med. 2011, 17, 454–460.




