Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Miry Lobaton | -- | 1246 | 2022-04-24 19:29:55 | | | |
| 2 | Dean Liu | -2 word(s) | 1244 | 2022-04-25 03:13:03 | | | | |
| 3 | Dean Liu | Meta information modification | 1244 | 2022-04-25 10:24:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lobaton, M.; Ferreira-Hermosillo, A.; García-Sáenz, M. Metformin in Differentiated Thyroid Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/22211 (accessed on 07 February 2026).
Lobaton M, Ferreira-Hermosillo A, García-Sáenz M. Metformin in Differentiated Thyroid Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/22211. Accessed February 07, 2026.
Lobaton, Miry, Aldo Ferreira-Hermosillo, Manuel García-Sáenz. "Metformin in Differentiated Thyroid Cancer" Encyclopedia, https://encyclopedia.pub/entry/22211 (accessed February 07, 2026).
Lobaton, M., Ferreira-Hermosillo, A., & García-Sáenz, M. (2022, April 24). Metformin in Differentiated Thyroid Cancer. In Encyclopedia. https://encyclopedia.pub/entry/22211
Lobaton, Miry, et al. "Metformin in Differentiated Thyroid Cancer." Encyclopedia. Web. 24 April, 2022.
Copy Citation
Metformin is a synthetic biguanide that improves insulin sensitivity and reduces hepatic gluconeogenesis. Aside being the first-line therapy for Type 2 Diabetes (T2D), many pleiotropic effects have been discovered in recent years, such as its capacity to reduce cancer risk and tumorigenesis.
metformin
thyroid cancer
pharmacological mechanisms of action
1. Introduction
Metformin is a synthetic biguanide derived from the French lilac (Galega officinalis) which has guanidine as an active component. It improves insulin sensitivity and reduces hepatic gluconeogenesis [1]. Metformin is not metabolized by cytochromes P450 in the liver, so drug transporters have the major role of its pharmacokinetics. Due to its hydrophilicity, metformin cannot simply diffuse through cell membranes, so is transported into the cell via uptake transporters as the organic cation transporter 1 (OCT1), which is primarily expressed in the hepatocytes [2]. It is excreted via active tubular secretion in the kidneys, with a half-life of ~5 h.
The uptake from circulation to the renal epithelium is facilitated by organic cation transporter 2 (OCT2). Meanwhile, its excretion to the lumen is mediated through human multidrug and toxin extrusion (MATE)-1 and MATE2-K, which are expressed in the apical membrane of the renal proximal tubule cells [3]. Its maximum recommended dose for treatment of type 2 diabetes (T2D) is 2.5 g per day (35 mg/kg body weight). Based on experiments in animal models and positron emission tomography (PET) in humans, it is estimated that the metformin concentration in the human liver is about 50–100 μM.
Aside being the first-line therapy for most of the patients with T2D, many pleiotropic effects have been discovered in recent years, such as its capacity to reduce cancer risk and tumorigenesis [2]. The interest on metformin for cancer prevention and treatment is based on clinical studies that showed that it is associated with significantly lower cancer incidence in patients with diabetes [4][5][6][7][8]. It has been proposed that metformin inhibits cellular proliferation, has anti-inflammatory and anti-angiogenic effects, which inhibits stem-cells, and has immunomodulatory effects on tumoral cells, which inhibits DNA damage. Many clinical trials have proved its positive effects on lung, liver, pancreas, endometrium, colorectal, breast, prostate, and bladder cancer [1][2]. Nevertheless, information regarding its effect on endocrine cancers is limited [9].
The incidence of endocrine tumors has increased worldwide. Thyroid cancer is the most common endocrine malignancy and, its prevalence and incidence have risen; meanwhile, its mortality has remained low. That increase could be associated with insulin resistance (IR) [9][10]. According to worldwide Globocan 2020 statistics, thyroid cancer reached ninth place with 586,202 new cases (incidence of 6.6/100,000) and represents the 24th place in the mortality rate (0.43/100,000). This cancer was more common in Asia, in the female population, and in those aged among 35–64 years [11][12].
Thyroid cancer is classified in two categories based on its origin: epithelial-derived and neuroendocrine C-cell-derived medullary thyroid cancer (MTC). Furthermore, epithelial-derived cancer is subclassified into differentiated cancer (DTC) that includes: papillary (PTC), follicular (FTC), and Hürthle cell thyroid cancer (HCTC); poorly differentiated thyroid cancer (PDTC); and anaplastic thyroid cancer (ATC) [9]. PTC is the most common histologic subtype, accounting for 90% of new cases and has the best prognosis. Some of the risk factors to develop thyroid cancer are exposure to ionizing radiation in the head and neck, family history, female sex, advanced age, iodine deficiency or excess, and tobacco and alcohol use. Obesity, insulin resistance (IR), hyperinsulinemia, diabetes mellitus, and other metabolic disturbances have been associated with a higher incidence of thyroid carcinoma [13][14].
2. Molecular Insights of Metformin in Thyroid Cancer
Although widely studied, the effect of metformin on thyroid cancer remains controversial. Potential mechanisms for its growth inhibitory effects have been elucidated in various preclinical studies [9]. Experimental studies have shown that metformin can influence the growth and progression of thyroid cancer cells. Those studies demonstrated that metformin has different molecular targets that are involved in the control of several metabolic and inflammatory pathways, which can be divided into a) direct effects, related with its mechanism of action (as those related to adenosine mono-phosphate-activated protein kinase (AMPK), mammalian target of rapamycin (mTOR), mitochondrial glycerophosphate dehydrogenase (mGPDH), and the nuclear factor κB (NF-κB)) that induce energetic stress by mimicking a state of caloric deprivation; or b) indirect effects, which result from the interaction with other metabolic and hormonal systems (such as its effects on the thyroid-stimulating hormone (TSH)) [15][16][17]. Figure 1 summarizes the mechanisms described before.
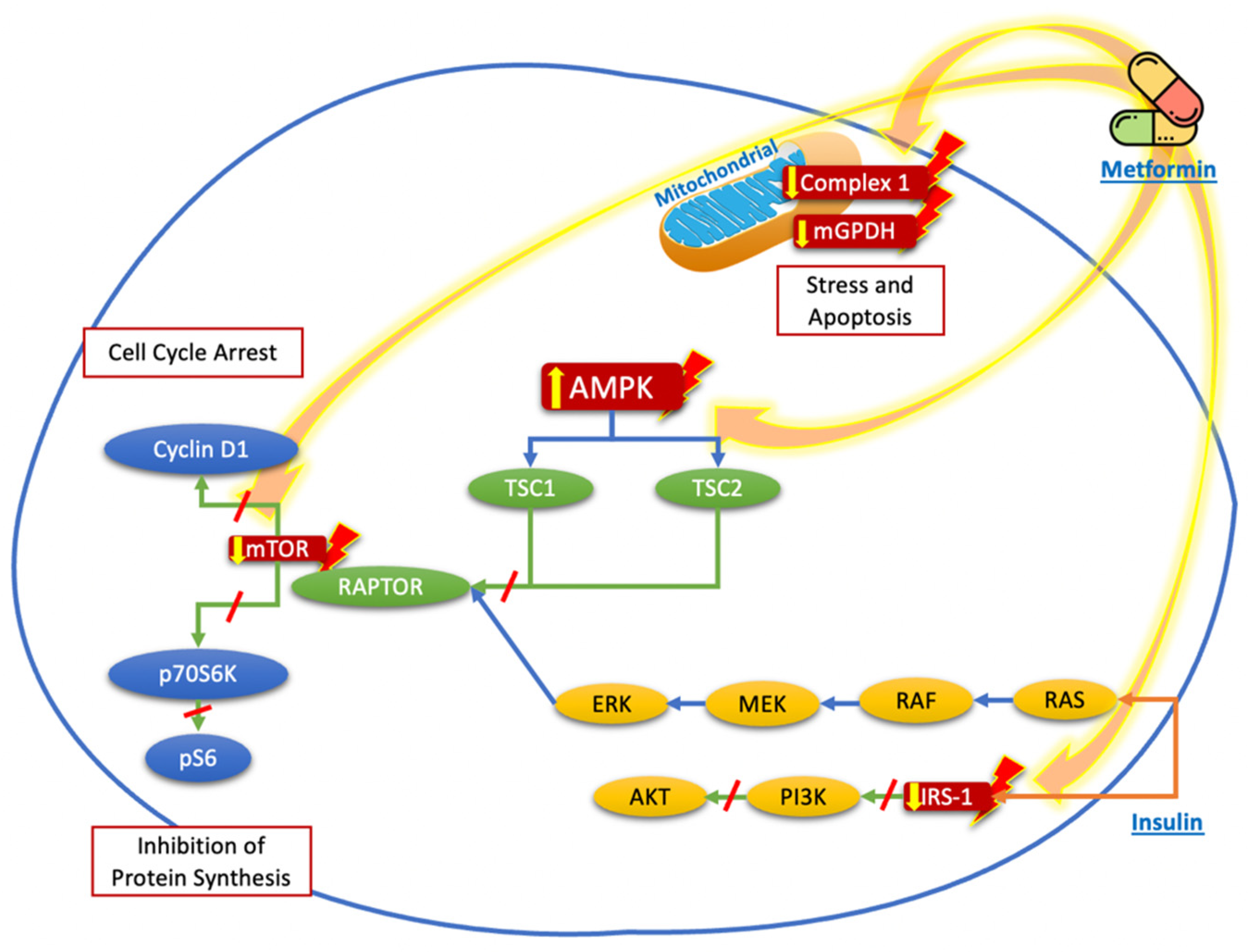
Figure 1. Proposed targets of the molecular mechanisms of metformin to reduce the proliferation and growth of thyroid cancer cells. Metformin acts mainly through nuclear stimulation of AMPK, which decreases the activation of TSC2 and the activation of mTOR, with the subsequent reduction in cyclin D1 and p70S6K/pS6 signaling, resulting in the inhibition of protein synthesis and cell cycle arrest. The blockade of IRS-1 phosphorylation decreases the signaling of the PI3K/AKT pathway, which also contributes to the reduction in mTOR pathway activation. Likewise, the direct effects of metformin on the mitochondria, by reducing Complex 1 or mGPDH, decreases energy production and cell stress and leads to cell apoptosis.
3. Clinical Evidence of Metformin Use in Thyroid Cancer
Within the different types of thyroid cancer, there is wider clinical evidence on the use of metformin on DTC. Meanwhile, in MTC and ATC, there are no clinical trials yet. As explained above, preclinical trials suggest that metformin can reduce thyroid cancer risk and tumorigenesis. The majority of thyroid cancers are detected as a small mass and have a good prognosis. This has led to changes in the treatment strategy, adopting less aggressive approaches. Researchers must consider that metformin is not as potent as a chemotherapeutic agent, but it has an important role in prevention, the reduction in progression, and as an adjuvant to chemotherapy treatment [18].
Table 1. Effects of metformin on thyroid cancer as reported in clinical studies.
| Reference | Study Design | Objective | Patients Characteristics | Metformin Dose | Duration of Treatment or Follow Up | Conclusions |
|---|---|---|---|---|---|---|
| Tseng et al. [19] | Clinical observational trial | To investigate the association between metformin use and thyroid cancer risk. | 795,321 metformin users and 619,402 non-metformin users, Taiwanese patients with T2D. | Cumulative dose of 263,000 mg. | 9 months | Metformin decreased thyroid cancer risk by 32%. |
| Cho et al. [18] | Retrospective cohort study | To investigate the association between metformin and thyroid cancer development. | Korean population: 128,453 metformin users and 128,453 non-users. | Mean cumulative dose of 868,169 (±563,221) mg. | 1633 (±915) days | Metformin reduced risk cancer by 31%. |
| Klubo-Gwiezdzinska et al. [20] | Single-center observational study | Whether the efficacy of conventional treatment of DTC is affected by therapy with metformin in patients with diabetes. | Patients with diabetes treated (n = 34) or not (n = 21) with metformin and control patients without diabetes (185). | 500–2000 mg/day. | 4.4 (±3) years | Age, locoregional metastases, distant metastases, and lack of treatment with metformin were associated with increased risk for shortened progression-free survival. Metformin-treated individuals had smaller tumor size and better remission rates. |
| Jang et al. [21] | Retrospective study | To evaluate the clinical outcome of patients with diabetes and DTC according to metformin treatment. | 60 patients with diabetes and 201 control patients with DTC after total thyroidectomy. | Mean dose of 979 mg. | 7.4 (±4.8) years | Metformin treatment was associated with longer disease-free survival. |
T2D: Type 2 Diabetes Mellitus, IR: Insulin resistance, DTC: Differentiated Thyroid Cancer.
4. Conclusions
Metformin inhibits the growth and migration of thyroid cancer cells directly by mechanisms related to AMPK and mitochondrial respiration, and indirectly through effects on TSH levels and metabolic parameters associated with a less favorable environment for cell proliferation, as well as potentiating the effect of certain chemotherapeutic drugs. This suggests that metformin has an important supportive effect in patients with thyroid cancer and metabolic diseases such as insulin resistance or diabetes and may be an adjuvant treatment for differentiated or poorly differentiated thyroid cancer.
References
- Mallik, R.; Chowdhury, T.A. Metformin in cancer. Diabetes Res. Clin. Pract. 2018, 143, 409–419.
- Vancura, A.; Bu, P.; Bhagwat, M.; Zeng, J.; Vancurova, I. Metformin as an Anticancer Agent. Trends Pharmacol. Sci. 2018, 39, 867–878.
- Gong, L.; Goswami, S.; Giacomini, K.M.; Altman, R.B.; Klein, T.E. Metformin pathways: Pharmacokinetics and pharmacodynamics. Pharm. Genom. 2012, 22, 820–827.
- Evans, J.M.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. Br. Med. J. 2005, 330, 1304–1305.
- Malek, M.; Aghili, R.; Emami, Z.; Khamseh, M.E. Risk of Cancer in Diabetes: The Effect of Metformin. ISRN Endocrinol. 2013, 2013, 636927.
- Zingales, V.; Distefano, A.; Raffaele, M.; Zanghì, A.; Barbagallo, I.; Vanella, L. Metformin: A Bridge between Diabetes and Prostate Cancer. Front. Oncol. 2017, 7, 243.
- Zhou, X.-L.; Xue, W.-H.; Ding, X.-F.; Li, L.-F.; Dou, M.-M.; Zhang, W.-J.; Lv, Z.; Fan, Z.-R.; Zhao, J.; Wang, L.-X. Association between metformin and the risk of gastric cancer in patients with type 2 diabetes mellitus: A meta-analysis of cohort studies. Oncotarget 2017, 8, 55622–55631.
- Campbell, J.M.; Bellman, S.M.; Stephenson, M.D.; Lisy, K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Res. Rev. 2017, 40, 31–44.
- Thakur, S.; Daley, B.; Klubo-Gwiezdzinska, J. The role of an anti-diabetic drug metformin in the treatment of endocrine tumors. J. Mol. Endocrinol. 2019, 63, R17–R35.
- He, X.; Wu, D.; Hu, C.; Xu, T.; Liu, Y.; Liu, C.; Tang, W. Role of Metformin in the Treatment of Patients with Thyroid Nodules and Insulin Resistance: A Systematic Review and Meta-Analysis. Thyroid 2019, 29, 359–367.
- World Health Organization. Cancer Today–IARC. 2022. Available online: https://gco.iarc.fr (accessed on 14 February 2022).
- Li, M.; Maso, L.D.; Vaccarella, S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. 2020, 8, 468–470.
- Seib, C.D.; Sosa, J.A. Evolving Understanding of the Epidemiology of Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2018, 48, 23–35.
- Zhao, J.; Zhang, Q.; Yang, Y.; Yao, J.; Liao, L.; Dong, J. High prevalence of thyroid carcinoma in patients with insulin resistance: A meta-analysis of case-control studies. Aging 2021, 13, 22232–22241.
- Kheder, S.; Sisley, K.; Hadad, S.; Balasubramanian, S. Effects of prolonged exposure to low dose metformin in thyroid cancer cell lines. J. Cancer 2017, 8, 1053–1061.
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033.
- Rotondi, M.; Coperchini, F.; Pignatti, P.; Magri, F.; Chiovato, L. Metformin Reverts the Secretion of CXCL8 Induced by TNF-α in Primary Cultures of Human Thyroid Cells: An Additional Indirect Anti-Tumor Effect of the Drug. J. Clin. Endocrinol. Metab. 2015, 100, E427–E432.
- Cho, Y.Y.; Kang, M.J.; Kim, S.K.; Jung, J.H.; Hahm, J.R.; Kim, T.H.; Nam, J.Y.; Lee, B.-W.; Lee, Y.-H.; Chung, J.H.; et al. Protective Effect of Metformin Against Thyroid Cancer Development: A Population-Based Study in Korea. Thyroid 2018, 28, 864–870.
- Tseng, C.-H. Metformin Reduces Thyroid Cancer Risk in Taiwanese Patients with Type 2 Diabetes. PLoS ONE 2014, 9, e109852.
- Klubo-Gwiezdzinska, J.; Burman, K.D.; Van Nostrand, D.; Mete, M.; Jacqueline, J.; Wartofsky, L. Potential Use of Recombinant Human Thyrotropin in the Treatment of Distant Metastases in Patients with Differentiated Thyroid Cancer. Endocr. Pract. 2013, 19, 139–148.
- Jang, E.K.; Kim, W.G.; Kwon, H.; Choi, Y.M.; Jeon, M.J.; Kim, T.Y.; Shong, Y.K.; Kim, E.Y. Metformin Is Associated with a Favorable Outcome in Diabetic Patients with Cervical Lymph Node Metastasis of Differentiated Thyroid Cancer. Eur. Thyroid J. 2015, 4, 181–188.
More
Information
Subjects:
Medicine, General & Internal; Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
3 times
(View History)
Update Date:
25 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




