
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohammed Ghiboub | + 242 word(s) | 242 | 2020-09-23 05:54:08 | | | |
| 2 | Nora Tang | Meta information modification | 242 | 2020-09-28 08:37:43 | | | | |
| 3 | Mohammed Ghiboub | + 623 word(s) | 865 | 2020-10-12 10:28:07 | | | | |
| 4 | Nora Tang | -6 word(s) | 859 | 2020-11-09 11:51:43 | | |
Video Upload Options
Tryptophan (Trp) is an essential aromatic amino acid that is acquired exclusively through dietary intake in humans (since Trp is not produced by animal cells). Common sources of dietary Trp are fish, poultry, cereals, and dairy foods. The World Health Organization advises an intake of 4 mg of Trp/kg/day. Trp is a precursor of several microbial and host metabolites, including several molecules such as serotonin, melatonin, nicotinamide and vitamin B3. The intestine is a prime location of Trp metabolism. Three main metabolic pathways can process dietary Trp: (i) the kynurenine pathway (KP) via indoleamine 2,3-dioxygenase (IDO)1, mainly occurring in both immune and epithelial cells, (ii) the serotonin (5-hydroxytryptamine [5-HT]) pathway via Trp hydroxylase 1 (TpH1) in enterochromaffin cells, and finally (iii) the direct conversion of Trp by the intestinal microbiota into several.
1. Kynurenine Pathway (KP)
Intestinal Trp metabolism through the KP is initially mediated by the enzyme Indoleamine 2,3-dioxygenase (IDO)1 and leads to the production of kynurenine pathway metabolites (KYNs); such as kynurenine (Kyn), quinolinic acid (QUIN), niacin, nicotinamide adenine dinucleotide and kynurenic acid (KYNA). Trp 2,3-dioxygenase (TDO) and IDO2 also participate in Trp metabolism to form Kyn. However, these enzymes are not expressed in the gut. Over 95% of Trp has been reported to be metabolized via the KP in mammals.The gut microbiota has a critical role in stimulating IDO1 activity. This was demonstrated in germ-free and antibiotic treated mice. Depletion of Trp by IDO1, IDO2 and TDO can have fundamental consequences on cellular function and survival. Several intestinal bacteria produce homologous enzymes and are thus also capable of Kyn synthesis and downstream neurotoxic metabolites such as 3-hydroxyanthranilic acid. It has been reported that KYNs are implicated in host biological processes such as cell differentiation, neurotransmission, inflammation, immune response, as well as its affinity to AhR.
2. Serotonin Pathway
The neurotransmitter serotonin, also known as 5-HT, is a monoamine neurotransmitter that is mostly produced by a specialized subtype of intestinal epithelial cells called enterochromaffin in the gastrointestinal tract. Trp is initially processed by TpH1 to 5-HTP metabolite, then further metabolized to serotonin. Serotonin produced in the enterochromaffin cells is eventually released to the blood. Peripheral serotonin participates in several intestinal functions and is involved in several human physiological functions by activating the serotonin receptors. Serotonin is an important gastrointestinal signaling substance that transmits signals from the intestine to the neuronal network and influences intestinal motility, secretion, vasodilatation, and the absorption of nutrients. The intestinal microbiota is a key player for the production of intestinal serotonin. Germ-free mice have demonstrated impaired production of serotonin in the colon and low concentration of circulating serotonin. While the mechanism modulating the production of serotonin by the intestinal microbiota remains poorly understood, SCFAs have been suggested to have a role in the stimulation of TpH1 expression. In addition, some secondary bile acids, such as deoxycholate synthesized by microbial biotransformation of cholate, can also stimulate serotonin biosynthesis. Serotonin can also be produced in the brain through TpH2 enzyme in serotonergic neurons, where it regulates major physiological properties such as mood, appetite, and sleep.
3. Indole Pathway
Through its metabolic activity, the intestinal microbiota orchestrates the direct Trp degradation into indole derivatives, including indole-3-acid-acetic (IAA), indole-3-aldehyde (IAId), indole-3-propionic acid (IPA), indole-3-lactic acid (ILA), indole-3-acetaldehyde (IAAId) and indole-acrylic acid. Indole is an aromatic organic compound produced by a variety of bacteria. These ligands are able to affect bacterial physiology and to modulate antibiotic resistance, sporulation, and biofilm formation. Although the role of the microbiota in this process is critical, only a few commensal species such as Bifidobacterium spp., Peptostreptococcus russellii and Lactobacillus spp. have been well characterized to contribute to Trp metabolism. In these species, the oxidative/reductive pathways lead to the production of IAA and IPA, two Trp metabolites known to improve the intestinal barrier and boost host immunity. The metabolism of Trp into AhR ligands by Lactobacilli has been shown to control intestinal colonization by pathogenic Candida albicans via AhR dependent IL22 production. Lactobacillus reuteri and Lactobacillus johnsonii have been identified to produce the indole derivative IAId, generated through the indole pyruvate pathway and catalyzed by the enzyme aromatic amino acid aminotransferase.Tryptophanase, an enzyme expressed in E.coli and lactobacilli, was described to be involved in Trp conversion into indole. Indole is transported in and out of the bacteria by passive diffusion through the membrane or actively with AcrEF-TolC and Mtr transporters. Exogenous diet-derived indole molecules, such as Trp and glucobrassicin, are a major source of endogenous AhR ligand precursors.
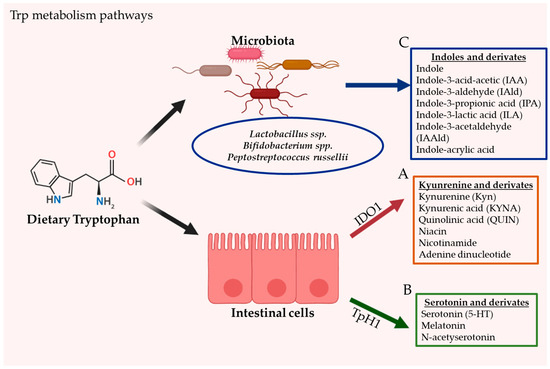
Figure. The gastrointestinal tract is the principal location of tryptophan (Trp) metabolism. In humans, Trp is acquired exclusively through dietary intake. Dietary Trp can be processed by three main metabolism routes: (A) the kynurenine pathway (KP), which mainly occurs in both immune and epithelial cells via IDO1, leading to several kynurenine metabolites (KYNs) including ligands for AhR (B) the serotonin production pathway, taking place in enterochromaffin cells (subtype of intestinal epithelial cells) and via TpH1, and finally (C) the direct conversion of Trp by the gut microbiota into several Indoles and derivates, including ligands of the AhR.




