Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tingting Jiang | -- | 5043 | 2022-04-22 10:01:45 | | | |
| 2 | Amina Yu | -18 word(s) | 5025 | 2022-04-22 10:42:26 | | | | |
| 3 | Amina Yu | Meta information modification | 5025 | 2022-04-25 10:00:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jiang, T.; Chen, G.; , . Enhanced Performance of Lithium-Ion Batteries. Encyclopedia. Available online: https://encyclopedia.pub/entry/22159 (accessed on 07 February 2026).
Jiang T, Chen G, . Enhanced Performance of Lithium-Ion Batteries. Encyclopedia. Available at: https://encyclopedia.pub/entry/22159. Accessed February 07, 2026.
Jiang, Tingting, George Chen, . "Enhanced Performance of Lithium-Ion Batteries" Encyclopedia, https://encyclopedia.pub/entry/22159 (accessed February 07, 2026).
Jiang, T., Chen, G., & , . (2022, April 22). Enhanced Performance of Lithium-Ion Batteries. In Encyclopedia. https://encyclopedia.pub/entry/22159
Jiang, Tingting, et al. "Enhanced Performance of Lithium-Ion Batteries." Encyclopedia. Web. 22 April, 2022.
Copy Citation
Lithium-ion batteries (LIBs) have been used in portable electric devices and electric vehicles (EVs) for years due to their high energy and power densities, satisfactory cycle life and the affordable materials and manufacturing costs. To meet the growing market demand for cheaper and more efficient energy storage technologies for EVs and power grids, higher energy storage density and efficiency, and a longer cycle life should be achieved in the next generation of LIBs. Silicon (Si) is considered as one of the most promising candidates for next generation negative electrode (negatrode) materials in LIBs due to its much higher theoretical specific charge capacity than the current commercial negatrode (carbon-based).
Silicon
composites
MXenes
negatrode materials
electrochemical performance
Lithium ion battery
Energy storage
Nanomaterials
Storage mechanism
Cycle life
1. Structures and Preparation Strategies of Si/MXene Composites as Negatrodes in lithium-ion Batteries (LIBs)
As a typical MXene material, Ti3C2Tx can be achieved by etching the layered ternary MAX phases and presents a 2D configuration with a series of terminal functional groups such as -F, -O, and -OH [1]. The Ti-C bond in the MXene helps to maintain the stable layered structure. In 2011, Gogotsi et al. published a report on 2D nanosheets, composed of a few Ti3C2 layers and conical scrolls constructed by in situ exfoliation of Ti3AlC2 in hydrofluoric acid [2]. These 2D nanosheets were then called MXene with an accordion-like structure. In their work, there are interspaces of about dozens to several hundred nanometers between the nanosheets. Then, in the following, researchers also prepared single and few-layered Ti3C2 nanosheets which are highly dispersible in water once Al layers are removed using the mixture of LiF and HCl. Therefore, Si/MXene composites can be synthesized with the above two kinds of MXenes. These composites can be categorized into several structures, such as self-standing film and layer-by-layer, three-dimensional (3D), and coating structures. These different structures of Si/MXene composites are illustrated in Figure 1. In this section, different composite structures, their advantages and relevant synthesis methods, and the interface control in these structures will be discussed.
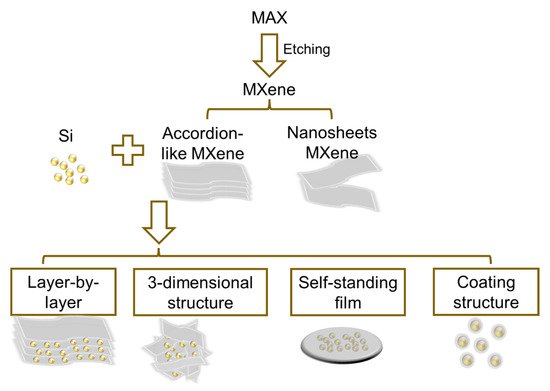
Figure 1. Schematic of the syntheses of Si/MXene composites with different structures.
1.1. Structures of Si/MXene Composites
1.1.1. Layer-by-Layer Structure
As mentioned above, the commercialization of silicon-based negatrodes is limited mainly by the inherently low electron and Li+ ion conductivities but more importantly by the huge volume change during repeated lithiation and delithiation processes, which may then lead to fractures in the active material, and a loss of contact with the electrode. Nanoparticles are often used to prevent pulverization and to increase the contacting area with electrolytes [3]. By combining silicon nanoparticles (SiNPs) with both accordion-like and thin nanosheets MXenes, layer-by-layer structured composites can be achieved, which is the most investigated structure so far [4][5].
After the exfoliation of the Al layer of the respective MAX phase material, the resulting MXene exhibits a loosely packed accordion-like structure composed of multiple layers with the interlayer space between the nanosheets being large enough for the intercalation of SiNPs. As for the thin MXene nanosheets, layer-by-layer self-assembly through electrostatic interactions between SiNPs and MXenes can be achieved in solution. This layer-by-layer structure mainly benefits from the following advantages. (1) The loose layered structure of MXene can accommodate SiNPs via the electrostatic effect due to the terminal functional groups of MXene, which can restrict volume expansion and prevent the aggregation of SiNPs during lithiation and delithiation. (2) The excellent electrical properties as well as the direct electronic transport pathway of layered MXene can effectively promote carrier transfer and hence improve the rate performance. (3) The satisfactory mechanical properties of MXene nanosheets can help to maintain the stability and integrity of the whole composite structure, while the large specific surface area can increase the reaction interface between active material and electrolyte.
The first Si/MXene composites with a layer-by-layer structure were reported in 2018 [6], after the simple mixing of SiNPs and the accordion-like MXenes.
Zhang et al. also prepared an MXene/Si@SiOx@C layer-by-layer assembling superstructure with accordion-like MXene [7]. The SiOx layer was first deposited on MXene sheets using the Stöber method and then magnesiothermic reduction to the Si/MXene composite was performed, followed by the carbonization process. The dispersed few-layered nanosheets of MXene can also be used to construct the layer-by-layer structure [8].
In addition, there were some attempts to surface-modify or add a third component to achieve a more controllable layer-by-layer structure [4][9][10]. The layer-by-layer Si/MXene structure integrates all the merits of both silicon and MXene, and is suitable and promising for the large-scale application of silicon-based negatrodes.
1.1.2. Self-Standing Film
Apart from the layer-by-layer structure, the thin few-layered MXene nanosheets can form free-standing films due to their unique bonding and mechanical properties. By mixing with MXene, SiNPs can be fixed inside the free-standing film supported by MXene nanosheets. The microstructure of this free-standing film prepared by vacuum filtration is quite similar to that of the layer-by-layer Si/MXene composites, but can be directly used as binder-free electrodes in LIBs [11][12].
This free-standing film of Si/MXene composites could provide a pathway to foster the large-scale application of silicon-based negatrodes in LIBs, benefiting from several advantages, as follows. (1) The 3D flexible and free-standing structure consisting of covalently anchored silicon on MXene nanosheets can effectively confine the volume change of silicon during lithiation/delithiation. (2) The highly conductive MXene can act as a matrix, the binder instead of inactive binders such as polyvinylidene fluoride (PVDF) and sodium carboxymethyl cellulose (CMC), and as well as the current collector, which can increase the active material ratio and facilitate carrier transport during dis-/charging.
At the same time, Zhang et al. composited Si@C particles with MXene, which could be used directly on electrodes for LIBs [12]. The free-standing film can also be prepared by combining silicon, MXene, and one-dimensional carbon nanotubes (CNTs). In 2020, Cao et al. [13] prepared flexible free-standing and resilient Ti3C2Tx-CNT/SiNPs films with a hierarchical porous structure by vacuum filtration by means of electrostatic interactions. In TEM images, the SiNPs, which were anchored tightly to the thin MXene plate, and the interconnected CNTs were distributed uniformly and formed a network, preventing the aggregation of silicon.
By eliminating the typical binder, conductive agent, and copper current collector, the Si/MXene free-standing film could provide a higher specific capacity and power density at a relatively lower cost. Preparation of the large-area uniform film is the key point of their application in mass production.
1.1.3. Three-Dimensional Structure
The three-dimensional (3D) porous structure has been investigated thoroughly in Si/graphene composites. With a similar 2D structure and morphology to graphene, MXene could also be constructed into a 3D matrix to allow SiNPs to form the porous structure. Both accordion-like and thin single- or few-layered MXene nanosheets can be designed and fabricated into a 3D structure with silicon [14][15].
Similarly to Si/MXene composites, the 3D porous Si/MXene attracted much attention because of the following reasons. (1) In the 3D Si/MXene porous structure, MXene nanosheets can interconnect into a skeleton-like frame, with SiNPs inside the skeleton by bonding and adherent to MXene plate. (2) The good mechanical strength of MXenes helps to alleviate the impact of volume expansion of Si during cycling and stabilize the 3D structure. (3) The porous structure could provide conductive pathways and a larger specific surface area for more active reaction sites. This 3D porous structure can be achieved by for example mechanical mixing and freeze-drying.
Wang et al. [16] performed a freeze-drying process in liquid nitrogen with the suspension mixture of Si and few-layered MXene nanosheets and prepared Si@Ti3C2Tx composite with a 3D structure. Since MXene could act as both the matrix of 3D structures and silicon loader, Li et al. [17] designed 3D hierarchical and porous structures in Si/MXene composites with dual MXene protection (SiNP@MXene1/MXene2). This dual MXene structure is shown schematically. Firstly, the few-layered MXene sheets interacted with CTAB (cetyltrimethylammonium bromide)-treated SiNPs via electrostatic self-assembly, leading to Si particles being riveted to MXene plates (SiNP@MXene1). Then, this SiNP@MXene1 precursor was embedded into another 3D MXene matrix by the hydrothermal process, followed by freeze-drying. The prepared composites showed a particle size of 1 to 2 μm, and the SEM micromorphology is displayed. The cross-linked MXene nanosheets provided a skeleton of a 3D porous structure in which SiNP@MXene1 composite nanoparticles were embedded. In this dual MXene structure, the internal MXene1 was present, which was coated on SiNPs to restrict volume expansion and prevent aggregation. The external MXene2 skeleton supported the 3D structure with abundant channels for electrolyte permeation and buffering volume changes. The MXene1 coating layer also helped to form a more stable SEI film.
Introducing another component, for example, carbon, into the 3D porous composites could further improve the structural stability and hence the cycling capacity. In 2019, Liu et al. [18] reported a dual-bond restricted MXene-Si-CNT composite as a negatrode material with enhanced electrochemical performance. By ball-milling MXene nanosheets, Si, and CNTs, the 3D porous homogeneous MXene/Si/CNTs composites were obtained. The energy from ball-milling reinforced the combination of the three reagents into a composite via the formation of the Ti−Si and C−Si bonds.
In addition to those mentioned above, other 3D Si-based composites were reported. For example, uniformly dispersed Si nanospheres [14] or Si/SiOx@TiO2 nano aggregates [19] were deposited on thin MXene nanosheets. The Si/MXene/CNTs composites were also prepared by electrospinning and pyrolysis into fibrous structures, which finally turned into 3D structures with crosslinked fibers [20]. Meng et al. [21] rolled up MXene nanosheets into scrolls and added SiNPs simultaneously. The Ti3C2Tx was rolled into hollow and fully curled scrolls with Si particles inside, and then the scrolls interconnected with each other into a 3D porous structure.
The 3D porous structure of Si/MXene composites is diverse. However, the connection and synergistic effect between the components are of great importance and should be thoroughly considered according to the synthetic process and electrochemical performances.
1.1.4. Coating Structure
Coating is one of the most frequently used strategies for surface modification and in the formation of silicon composites. The coating can be amorphous or crystalline, and may comprise amorphous carbon, metal oxide, metal, graphene and CNTs. As a flexible 2D material, single- or few-layered MXene nanosheets can be coated thinly on silicon, forming core–shell or overall coating structures.
Similar to the other three structures discussed above, a MXene coating could also function as a robust and flexible layer to accommodate for the volume expansion of silicon to prevent the pulverization. The bonds between silicon and Ti3C2Tx or other highly conductive MXene are beneficial to electron transport. In such structures, the most important point is that wrapping silicon with a homogeneous MXene layer can effectively prevent nanoparticles from directly contacting the electrolyte and thus enable the formation of a stable SEI film (in some cases, the artificial SEI film will form). Then, the interfacial stability and subsequently the cycling stability can be improved.
Besides the core–shell structure, Yan et al. prepared another coating structure, in which a bundle of SiNPs were encapsulated in robust micrometer-sized MXene frameworks
Coating silicon with MXene nanosheets has been proven to be a useful strategy by different researchers [22][23]. Both the core–shell and capsule structures could provide satisfactory interface modification for the formation of more stable SEI layers.
1.2. Preparation Methods
To obtain differently structured composites, various preparation theories and methods have been utilized, including mechanical mixing [6][18], vacuum-assisted filtration [24], the preparation of SiOx/MXene by hydrolysis followed by magnesiothermic reduction [7][19], solution treatment, freeze-drying [5][16], spray-drying [25], wet-processing [9][26], and electrospinning [20]. More specifically, free-standing Si/MXene composite films are usually fabricated by the filtration of the suspension of Si and MXene. Freeze-drying can lead to a porous 3D structure, and magnesiothermic reduction always maintains the microstructure and morphology of the precursor MXene/SiOx composites. A further discussion on Si/MXene preparation is provided below.
1.2.1. Mechanical Mixing
Mechanical mixing is one of the most facile, scalable, and widespread methods for the preparation of various silicon-based composites. Silicon and MXene in powder forms can be mechanically composited by simple mixing, ball-milling, or grinding. Energy derived from the mechanical impact could transfer to silicon and MXene, helping the components to connect. In particular, high-energy ball milling can both reduce the reaction activation energy and increase the adhesion of Si to MXene, forming the continuous electric network.
In 2018, Kong et al. first reported on the combination of Si and Ti3C2 MXene as a negatrode material in LIBs [6]. Generally, mechanical mixing is very attractive due to its simplicity, low energy consumption, affordable cost, environmental friendliness, and the potential for mass production. However, despite these advantages, several challenges should be considered, including the introduction of extra impurities and the poor diversity in the resulting structure.
1.2.2. Wet Processing Method
The wet processing method is another facile, easy-to-operate procedure for the synthesis of silicon-based composites. By dispersing the powders of silicon, MXenes, and other components in the same solvent, a uniform solution/slurry can be formed, which leads to efficient mixing and self-assembly. After solvent evaporation, centrifugation, or filtration, the composite powder can be obtained for further treatment.
In 2020, Cui et al. carried out a self-assembly process in solution and received a Si/MXene composite with a layer-by-layer structure [9]. SiNPs were firstly treated with 3-aminopropyltriethoxysilane (APTES), and a methanol solution containing NH2-SiNPs was added dropwise into the dispersion of thin MXene nanosheets.
Similar attempts were made by several groups, which all proved the wet processing method to be effective and reproducible. Zhang et al. [4] used accordion-like MXene nanosheets derived from etching Ti3AlC2 and amine functionalized SiNPs by dropwise hybridization to prepare the composite. The SiNPs were decorated on Ti3C2Tx nanosheets by electrostatic bonding. Recently, Jo et al. prepared a ternary composite of Si@N-doped C with 2D MXene nanosheets through wet processing [15]. Si@PDA (polydopamine) nanoparticles were first synthesized and then slowly added into a suspension of MXene nanosheets under stirring for 1 h. Strong chemical bonding formed to connect the Si@PDA particles and MXene nanosheets. The composites were then heated to carbonize the PDA layer, resulting in a 3D structure with the MXene framework and carbon coating.
As a remarkably simplified and contamination-free procedure, the wet processing method is suitable for commercial production. Meanwhile, the terminal surface groups of MXene make it easy to assemble with silicon into desired structures. The process should be controlled precisely to ensure the uniformity and desired structure of the synthesized products. Methods that require a lower energy input for a more efficient recovery of the solvent are also needed to mitigate any possible environmental impact.
1.2.3. Spray Drying Method
Spray drying is another frequently used and scalable preparation technique for Si/MXene powders [27]. Similar to the wet processing method, the starting materials are firstly mixed in solution for self-assembly or chemical interconnection. The composite materials can be dried and dispersed into dry, fine powders by quickly removing the solvent from the solution. The resulting dry particles are often uniform in size and morphology (usually spherical) and only require a one-step process.
Yan et al. fabricated Si@MXene with a coating structure by spray drying [25]. The single- or few-layer MXene nanosheets solution was obtained by etching the MAX phase, and SiNPs were dispersed in deionized water and then dropwise added into the MXene solution. After stirring for 8 h, the mixture suspension was spray-dried at 220 °C, leading to composites with a capsule structure.
Recently, they performed the following heat treatment on these capsule-structured composites [23]. After annealing at 1000 °C for 3 h under an Ar/5% H2 atmosphere, the Si@MXene composite transformed into an Si@TiO2-TiSi2 composite, which also exhibited high capacity retention after long cycling at large specific currents.
The spray drying method provides an environmentally friendly, high yield, easy, and continuous way by which to prepare powders with a modified micro-morphology and porous structure, showing a great prospect in large-scale production. However, in the reaction chamber, a large amount of hot air has no effect on drying of the powders, so the thermal efficiency is not high enough. Besides, it is difficult to construct 1D or 2D structures of Si/MXenes, which may better utilize the advantages of MXene.
1.2.4. Magnesiothermic Reduction Method
Magnesiothermic reduction is widely investigated as a promising process for making porous silicon with micro- or nanosized structures that promote the further formation of various silicon-based composites [28]. In a reducing ambient at relatively mild temperatures (600 to 700 °C), silica with various sizes, morphologies and crystalline structures are reduced by the magnesium (Mg) vapour via the following reaction [29].
SiO2(s) + 2Mg(g) → Si(s) + 2MgO(s)
In the process of Si/MXene composite synthesis, TEOS (Si(OC2H5)4), tetraethyl orthosilicate) is hydrolysed to produce SiO2 which can then be coated on the MXene template in the same reaction solution. After magnesiothermic reduction, the obtained silicon retains the microstructure of the SiO2 precursor.
Hui et al. synthesized a novel hierarchical porous Ti3C2/Si composite by a similar process [14]. After the etching and intercalation process, thin Ti3C2 nanosheets were obtained. By adding TEOS in the MXene suspension solution, the controlled hydrolysis process resulted in the heterogeneous nucleation and growth of SiO2 on the surfaces of individual MXene plates. Then, a low-temperature magnesiothermic reduction reaction (at about 200 °C) reduced silica into monodispersed SiNPs (d ≈ 40 nm) which were anchored on Ti3C2 nanosheets via Si-O-Ti bonds. In 2020, Jiang et al. [19] prepared a Ti3C2@Si/SiOx composite in the same hydrolysis and uncompleted magnesiothermic reduction process and then added TiO2 coating on the laminar composite to form a novel Ti3C2@Si/SiOx@TiO2 composite.
The magnesiothermic reduction method is a low-cost, simple, and convenient process, which can produce controllable morphology according to the precursor. However, obstacles exist for the magnesiothermic reduction process in terms of realizing large-scale production due to the low silicon yield, by-products, and the difficulty in precisely controlling the reaction.
1.2.5. Filtration Method
The filtration method is an effective and facile way by which to directly prepare free-standing film instead of powders [13]. Based on the wet processing method, silicon and MXene self-assemble in the mixing solution and form a thin free-standing film during the vacuum filtration procedure. By etching MAX, stirring, centrifugation, and hand-shaking, researchers obtained the MXene colloidal solution which was mainly composed of single- or few-layer Ti3C2Tx flakes [24]. After adding SiNPs to the colloidal solution and stirring, a flexible and freestanding paper can be prepared by vacuum filtration using a PVDF membrane. In the Si/MXene paper, Si nanospheres were dispersed randomly between parallel MXene sheets, and the mass loading of the active Si/MXene composite material was approximately 1.1 to 1.3 mg·cm−2.
Other components such as carbon can be added to the free-standing film. Cao et al. prepared the self-assembled Ti3C2Tx-CNT/SiNPs film by vacuum filtration [13]. The flexible and conductive CNTs and MXenes could act as the buffer matrix and carrier transportation channels simultaneously, leading to better electrochemical performance. Zhang et al. used Si@C nanoparticles as precursors and carried out vacuum filtration together with the single- or few-layer MXene nanosheets solution [12]. The achieved MXene-bonded Si@C film presented the porous structure and high conductivity for a higher rate performance.
The great virtue of this vacuum-assisted filtration method is the fact that one can directly obtain an electrode film in a facile process without slurry preparation and coating. The free-standing film is always flexible and conductive, without binder and other conductive additives, leading to a larger mass ratio of active material. However, it may not be easy for commercial development, and the thickness and mass loading should be controlled precisely during solution preparation and filtration.
1.2.6. Freeze-Drying Method
Another technique based on the wet process, freeze-drying, is a drying technique by freezing the product into solid-state by which the solvent (usual water) is removed by sublimation. Freeze-drying is a common method for the preparation of composite materials with hierarchical porous structures [30].
Numerous Si/graphene composites with aerogel structures have been prepared through the freeze-drying process [31]. Considering the structural similarity of MXene with graphene, Si/MXene 3D porous composites can be obtained by the same method.
Li et al. prepared 3D dual MXene/Si composites for LIBs negatrodes [17]. Firstly, they prepared SiNP@MXene1 by electrostatic self-assembly. Then, by mixing SiNP@MXene1 with MXene in a colloidal solution, they performed a hydrothermal reaction and freeze-drying to achieve the 3D hierarchically porous structure (SiNP@MXene1/MXene2).
The freeze-drying method is facile, environmentally friendly, controllable, and easy to use for the construction of porous frame structures which can accommodate the large volume change of silicon-based negatrodes with a high specific surface area. Nevertheless, freeze-drying often requires more time, and the yield is not high enough. For mass production, the constitution and the interaction between the components in the precursor solution should be designed carefully.
In the above methods, spray drying and magnesiothermic reduction are easily adopted for the construction of special microstructures, while mechanical mixing and wet processing are more promising for mass production. The filtration method can directly prepare the Si/MXene film without a binder.
1.3. Interface Modification of Si in Si/MXene Composites
In Si/MXene composites prepared via simple mixing, SiNPs are always chemically anchored by the Ti-Si bond. During lithiation, MXene could buffer the volume change of silicon to some extent and the whole composite can retain integrity without pulverization. However, there still exists the exfoliation phenomenon of SiNPs from MXene nanosheets due to insufficient bonding. Besides, the MXene framework may restrain the volume expansion of silicon and improve electron transportation but could not prevent the repeated formation of the SEI layer on the surface of silicon. Therefore, the modification of the interface between silicon and MXene nanosheets is necessary to strengthen their mutual interactions and control the surface reaction of silicon.
1.3.1. Si Surface Modification—Positive Charged
Many have been performed regarding the surface modification of silicon to decorate SiNPs with positive charges. The surface of Ti3C2Tx MXene nanosheets can be terminated with several functional groups including -O, -OH, and -F, and they are negatively polarized. The original SiNPs process negative surface charges due to the existence of a natural oxide layer outside silicon. By modifying with a surfactant such as CTAB, phthalic diglycol diacrylate (PDDA), and APTES, positively charged SiNPs could be obtained, which can then interact more tightly with MXene nanosheets, which in turn could improve charge transfer and structural stability.
In 2018, Zhang et al. [4] treated SiNPs by boiling an H2SO4/H2O2 (3:1 in volume) solution and subsequently a toluene solution containing 1 wt.% APTES to achieve the hydroxyl and amine functionalized SiNPs, respectively.
Li et al. [17] used CTAB, a cationic surfactant, to modify SiNPs (CTAB-SiNP) with positive charges. The surface terminal groups with negative charges on the single- or few-layer MXene nanosheets can capture the positively charged SiNPs with strong coulombic attraction, resulting in a wrapping structure of Si@MXene. Cao et al. prepared Ti3C2Tx-CNT/SiNPs composites [13]. The modified CNTs were attached with a large number of carboxyl groups, which helped form hydrogen bonds with MXene nanosheets. Then, the surface charges on SiNPs were adjusted from negative to positive by stirring in the CTAB solution. Due to the electrostatic interactions between positively charged SiNPs and negatively charged MXene/CNTs nanosheets, SiNPs tightly and homogeneously adhered on the surface of MXene nanosheets.
In addition, 3-aminopropyltrimethoxysilane (APS) and PDDA can provide surface modifications of silicon by a similar mechanism [22]. For example, the few-layer MXene nanosheets with negative charges electrostatically interact with positively charged Si@PDDA nanoparticles, leading to a self-assembly process [15].
Achieving tighter interactions between Si and MXene via electrostatic attraction is a facile, controllable, low-cost, and effective strategy for surface modification of silicon. Generally, the surfactant can be removed or carbonized during following treatments such as annealing.
1.3.2. Coating on the Surface of the Silicon
Another strategy for Si/MXene interface engineering is to deposit a coating layer on the surface of silicon. The single- or few-layer MXene nanosheets and other materials such as carbon and SiOx could wrap around SiNPs to form a fully enclosed structure. The coating can limit the volume change of silicon during lithiation and delithiation and prevent the direct contact of silicon with the electrolyte and help to form a more stable SEI layer. The MXene nanosheets working as coating layers have been discussed, other coating materials and their effects are introduced.
Zhang et al. prepared Si@C nanoparticles by self-polymerization and the carbonization of dopamine and then constructed a 3D conductive framework with few-layer MXene nanosheets with Si@C embedded inside by vacuum filtration [12]. The carbon coatings on SiNPs accounted for 24.8 wt.% of the total mass of Si@C nanocomposites according to a thermogravimetric analysis. The carbon coating could improve the bonding between Si and MXene, and prevent the fracture of silicon during the cycling. By mixing SiNPs with a dopamine hydrochloride solution, Jo et al. firstly obtained Si@PDA [15]. Upon mixing the suspensions of MXene and Si@PDA, the Si@PDA/MXene composite immediately precipitated due to the strong chemical bonding between PDA and MXene. Annealing the precipitate under an Ar atmosphere at 600 °C for 2 h transformed the PDA layer to N-doped carbon and finally the Si@NC/MXene composites were obtained. Zhang et al. [7] formed SiOx coating via the direct pyrolysis of the poly(methyl methacrylate) (PMMA) polymer on the surface of Si/MXene composites using urea as nitrogen source. During the pyrolysis process, the ester group in PMMA can be thermally decomposed to carbon with the simultaneous formation of SiOx, resulting in the MXene/Si@SiOx@C composites. From the TEM and HRTEM images, thin SiOx and carbon layers encapsulated Si/MXene, forming a micro 3D porous sandwich-like structure in the whole laminar composites. The lattice plane of SiNPs could be clearly observed and attributed to Si (111), indicating good crystallinity of silicon. Both the SiOx layer and carbon layer exhibited amorphous structures, which could tolerate the strain from the silicon lithiation process to a larger extent.
2. Electrochemical Performance of Si/MXene Composites as Negatrodes in LIBs
In 2014, Sun et al. [32] demonstrated the Li storage behavior of 2D Ti3C2 obtained by etching and intercalating with dimethyl sulfoxide via cyclic voltammetry (CV) and galvanostatic charging and discharging (GCD) cycling.
After compositing with silicon, due to the more obvious electrochemical reaction process and higher capacity of silicon, the lithiation and delithiation peaks of MXene are likely to be neglected. However, the SEI layer formed on surface Ti3C2 nanosheets is more stable and beneficial for Li+ ion transportation in the composites. Kong et al. [6] investigated the directly composited Si@MXene by CV and GCD.
Si + xLi+ + xe− ⇌ LixSi
Ti3C2 + xLi+ + xe− ⇌ LixTi3C2
Si and Ti3C2 would transform to LixSi and LixTixC2 during lithiation and back to initial Si and Ti3C2 after Li extraction.
The excellent mechanical stability of MXene nanosheets derived from the abundant covalent bonds (Ti-O-Ti) could help maintain the structure of Si/MXene negatrode. More importantly, the MXene coating prevented the contact between silicon to the electrolyte, leading to a different SEI layer from that on bare silicon. MXene nanosheets are full of fluorine terminations, resulting in the stable LiF-rich SEI layer on the MXene surface due to the following reaction [33].
–Ti–F + Li+ + e− ⇌ –T + LiF
The stable SEI layer could hinder the repeated reaction between silicon and electrolyte and improve the reversible cycling capacity. The Si@MXene composites revealed a reversible discharge capacity of 1155.4 mAh g−1 after 50 cycles at 0.5 A g−1, of much higher than that of bare silicon, which was only 74 mAh g−1 due to the fracture and extra Li+ ion consumption due to the repeated formation of the SEI layer. After 150 cycles, the capacity of Si@MXene negatrodes remained at 1004 mAh g−1, with a retention rate of 81%, indicating the positive effect of stable SEI film on the MXene coating layer. Benefiting from the high electroconductivity of MXene nanosheets, Si@MXene composites also exhibited better rate performance than bare silicon, with a reversible capacity of 1791, 1412, 1053, and 759 mAh g−1 at the specific current of 0.2, 0.5, 1, and 2 A g−1. Taking advantage of both silicon and MXene, this well-designed capsule structure could provide excellent electrochemical performance with a stable structure.
The above-mentioned 3D porous structure composites of Si@MXene1/MXene2 reported by Li et al. possess two benefits of the stabilized SEI layer by coating and 3D porous structures [17].
Introducing the third component into Si/MXene composites could improve the electrochemical performance of Si/MXene negatrodes, such as carbon and SiOx. Using N-doped carbon (NC) to wrap the SiNPs in Si/MXene composites, Jo et al. presented improved Li storage properties [15].
As can be seen, MXene/Si@SiOx@C-2 with approximately 16.9% MXene delivered a reversible capacity of 1547 mAh g−1 and a coulombic efficiency of nearly 100%, which is evidently higher than that of Si/C (238 mAh g−1) and the other two composites with MXene ratio of 10.7% (1049 mAh g−1) and 25.6% (1226 mAh g−1). They also carried out a much longer cycling test at a high rate of 10 C with the optimal composite and achieved a stable capacity of greater than 500 mAh g−1. The soft package full cell had also been prepared with NCM622 as the positive electrode to evaluate the applicability of MXene/Si@SiOx@C. The corresponding reaction equation is as follows [7]:
Li[Ni0.6Co0.2Mn0.2]O2 ⇌ Li1−x[Ni0.6Co0.2Mn0.2]O2 + Li+ + e−
[MXene/Si@SiOx@C] + Li+ + e− ⇌ Lix[MXene/Si@SiOx@C]
It presented high capacities of 181, 175, and 171 mAh g−1 at the first, 100th, and 200th with superior cyclic stability.
In the sandwiched structure on single layer MXene nanosheets, named as Ti3C2@Si/SiOx@TiO2, the co-introduction of amorphous SiOx and TiO2 layer on the surface of SiNPs could also assist in the formation of stable SEI film [19]. Both the amorphous SiOx and TiO2 layers should offer some capacity for lithium storage. Due to the lithiation characteristics of SiOx, the volume change would be smaller than that of silicon. Considering the higher flexibility of SiOx, it could alleviate the volume expansion of silicon. The high mechanical and chemical stability of TiO2 means that it could separate the silicon and electrolyte to construct a more stable SEI layer. The Ti3C2 nanosheets worked as high-conductive carriers and matrices to hold the SiNPs, which provide a large Li storage capacity.
References
- Luo, J.; Matios, E.; Wang, H.; Tao, X.; Li, W. Interfacial structure design of MXene-based nanomaterials for electrochemical energy storage and conversion. InfoMat 2020, 2, 1057–1076.
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253.
- Xiao, H.L.; Li, Z.; Shan, H.; Scott, X.M.; Ting, Z.; Jian, Y.H. Size-Dependent Fracture of Silicon Nanoparticles During Lithiation. ACS Nano 2012, 6, 1522–1531.
- Zhang, F.; Jia, Z.; Wang, C.; Feng, A.; Wang, K.; Hou, T.; Liu, J.; Zhang, Y.; Wu, G. Sandwich-like silicon/Ti3C2Tx MXene composite by electrostatic self-assembly for high performance lithium ion battery. Energy 2020, 195, 117047.
- Zhu, X.; Shen, J.; Chen, X.; Li, Y.; Peng, W.; Zhang, G.; Zhang, F.; Fan, X. Enhanced cycling performance of Si-MXene nanohybrids as anode for high performance lithium ion batteries. Chem. Eng. J. 2019, 378, 122212.
- Kong, F.; He, X.; Liu, Q.; Qi, X.; Sun, D.; Zheng, Y.; Wang, R.; Bai, Y. Enhanced reversible Li-ion storage in 3C2 MXene nanocomposite. Electrochem. Commun. 2018, 97, 16–21.
- Zhang, Y.; Mu, Z.; Lai, J.; Chao, Y.; Yang, Y.; Zhou, P.; Li, Y.; Yang, W.; Xia, Z.; Guo, S. MXene/x@C Layer-by-Layer Superstructure with Autoadjustable Function for Superior Stable Lithium Storage. ACS Nano 2019, 13, 2167–2175.
- Zhang, C.J.; Park, S.-H.; Seral-Ascaso, A.; Barwich, S.; McEvoy, N.; Boland, C.S.; Coleman, J.N.; Gogotsi, Y.; Nicolosi, V. High capacity silicon anodes enabled by MXene viscous aqueous ink. Nat. Commun. 2019, 10, 849.
- Cui, Y.; Wang, J.; Wang, X.; Qin, J.; Cao, M. A Hybrid Assembly of MXene with NH2 -Si Nanoparticles Boosting Lithium Storage Performance. Chem. Asian J. 2020, 15, 1376–1383.
- An, Y.; Tian, Y.; Zhang, Y.; Wei, C.; Tan, L.; Zhang, C.; Cui, N.; Xiong, S.; Feng, J.; Qian, Y. Two-Dimensional Silicon/Carbon from Commercial Alloy and CO2 for Lithium Storage and Flexible Ti3C2Tx MXene-Based Lithium-Metal Batteries. ACS Nano 2020, 14, 17574–17588.
- Li, H.; Lu, M.; Han, W.; Li, H.; Wu, Y.; Zhang, W.; Wang, J.; Zhang, B. Employing MXene as a matrix for loading amorphous Si generated upon lithiation towards enhanced lithium-ion storage. J. Energy Chem. 2019, 38, 50–54.
- Zhang, P.; Zhu, Q.; Guan, Z.; Zhao, Q.; Sun, N.; Xu, B. A Flexible Electrode with Excellent Stability Employing an MXene as a Multifunctional Binder for Lithium-Ion Batteries. ChemSusChem 2020, 13, 1621–1628.
- Cao, D.; Ren, M.; Xiong, J.; Pan, L.; Wang, Y.; Ji, X.; Qiu, T.; Yang, J.; Zhang, C. Self-assembly of hierarchical Ti3C2Tx-CNT/SiNPs resilient films for high performance lithium ion battery electrodes. Electrochim. Acta 2020, 348, 136211.
- Hui, X.; Zhao, R.; Zhang, P.; Li, C.; Wang, C.; Yin, L. Low-Temperature Reduction Strategy Synthesized Si/Ti3C2 MXene Composite Anodes for High-Performance Li-Ion Batteries. Adv. Energy Mater. 2019, 9, 1901065.
- Jo, D.Y.; Kim, J.K.; Oh, H.G.; Kang, Y.C.; Park, S.-K. Chemically Integrating MXene Nanosheets with N-Doped C-Coated Si Nanoparticles for Enhanced Li Storage Performance. Scr. Mater. 2021, 199, 113840.
- Wang, Z.; Cao, D.; Ren, M.; Zhang, H.; Pan, L.; John Zhang, C.; Yang, J. 3C2Tx with Si nanoparticles embedded in a 3D conductive network of crumpled Ti3C2Tx nanosheets for the anode of lithium-ion batteries with enhanced cycling performance. J. Alloys Compd. 2022, 892, 162037.
- Li, X.; Chen, Z.; Li, A.; Yu, Y.; Chen, X.; Song, H. Three-Dimensional Hierarchical Porous Structures Constructed by Two-Stage MXene-Wrapped Si Nanoparticles for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 48718–48728.
- Liu, S.; Zhang, X.; Yan, P.; Cheng, R.; Tang, Y.; Cui, M.; Wang, B.; Zhang, L.; Wang, X.; Jiang, Y.; et al. Dual Bond Enhanced Multidimensional Constructed Composite Silicon Anode for High-Performance Lithium Ion Batteries. ACS Nano 2019, 13, 8854–8864.
- Jiang, M.; Zhang, F.; Zhu, G.; Ma, Y.; Luo, W.; Zhou, T.; Yang, J. Interface-Amorphized Ti3C2@Si/SiOx@TiO2 Anodes with Sandwiched Structures and Stable Lithium Storage. ACS Appl. Mater. Interfaces 2020, 12, 24796–24805.
- Jiang, M.; Jiang, M.; Gao, H.; Chen, J.; Liu, W.; Ma, Y.; Luo, W.; Yang, J. Comparison of Additives in Anode: The Case of Graphene, MXene, CNTs Integration with Silicon Inside Carbon Nanofibers. Acta Metall. Sin. Engl. Lett. 2020, 34, 337–346.
- Meng, J.; Zhang, F.; Zhang, L.; Liu, L.; Chen, J.; Yang, B.; Yan, X. Rolling up MXene sheets into scrolls to promote their anode performance in lithium-ion batteries. J. Energy Chem. 2020, 46, 256–263.
- Zhou, H.; Cui, C.; Cheng, R.; Yang, J.; Wang, X. MXene Enables Stable Solid-Electrolyte Interphase for Composite with Enhanced Cycling Stability. ChemElectroChem 2021, 8, 3089–3094.
- Yan, Y.; He, Y.-S.; Zhao, X.; Zhao, W.; Ma, Z.-F.; Yang, X. Regulating adhesion of solid-electrolyte interphase to silicon via covalent bonding strategy towards high Coulombic-efficiency anodes. Nano Energy 2021, 84, 105935.
- Tian, Y.; An, Y.; Feng, J. Flexible and Freestanding Silicon/MXene Composite Papers for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 10004–10011.
- Yan, Y.; Zhao, X.; Dou, H.; Wei, J.; Sun, Z.; He, Y.S.; Dong, Q.; Xu, H.; Yang, X. MXene Frameworks Promote the Growth and Stability of LiF-Rich Solid-Electrolyte Interphases on Silicon Nanoparticle Bundles. ACS Appl. Mater. Interfaces 2020, 12, 18541–18550.
- Yang, Q.; Wang, Z.; Xia, Y.; Wu, G.; Chen, C.; Wang, J.; Rao, P.; Dong, A. Facile electrostatic assembly of superstructures for enhanced lithium-ion storage. J. Colloid Interface Sci. 2020, 580, 68–76.
- Mu, G.; Mu, D.; Wu, B.; Ma, C.; Bi, J.; Zhang, L.; Yang, H.; Wu, F. Microsphere-Like SiO2/MXene Hybrid Material Enabling High Performance Anode for Lithium Ion Batteries. Small 2020, 16, 1905430.
- Mei, S.; Liu, Y.; Fu, J.; Guo, S.; Deng, J.; Peng, X.; Zhang, X.; Gao, B.; Huo, K.; Chu, P.K. Waste-glass-derived silicon/CNTs composite with strong Si-C covalent bonding for advanced anode materials in lithium-ion batteries. Appl. Surf. Sci. 2021, 563, 150280.
- Tan, Y.; Jiang, T.; Chen, G.Z. Mechanisms and Product Options of Magnesiothermic Reduction of Silica to Silicon for Lithium-Ion Battery Applications. Front. Energy Res. 2021, 9, 651386.
- Liang, M.; Wang, W.; Jiang, Y.; Liao, C.; Long, Q.; Lai, X.; Liao, L. Fabrication of @G for flexible lithium-ion batteries. J. Alloys Compd. 2021, 878, 160357.
- Shao, J.; Yang, Y.; Zhang, X.; Shen, L.; Bao, N. 3D Yolk-Shell Structured Si/void/rGO Free-Standing Electrode for Lithium-Ion Battery. Materials 2021, 14, 2836.
- Sun, D.; Wang, M.; Li, Z.; Fan, G.; Fan, L.-Z.; Zhou, A. Two-dimensional Ti3C2 as anode material for Li-ion batteries. Electrochem. Commun. 2014, 47, 80–83.
- Das, P.; Wu, Z.-S. MXene for energy storage: Present status and future perspectives. J. Phys. Energy 2020, 2, 032004.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
944
Revisions:
3 times
(View History)
Update Date:
25 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




