
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna Christofides | -- | 1750 | 2022-04-21 16:36:33 | | | |
| 2 | Beatrix Zheng | + 14 word(s) | 1764 | 2022-04-22 08:34:26 | | |
Video Upload Options
The advent of anti-HER2 targeted therapies has dramatically improved the outcome of HER2-positive breast cancer; however, resistance to treatment in the metastatic setting remains a challenge, highlighting the need for novel therapies. The arrival of new treatment options and clinical trials examining the efficacy of novel agents may improve outcomes in the metastatic setting, including in patients with brain metastases. In the first-line setting, the researchers can potentially cure a selected number of patients treated with pertuzumab + trastuzumab + taxane. In the second-line setting, clinical trials show that trastuzumab deruxtecan (T-DXd) is a highly effective option, resulting in a shift from trastuzumab emtansine (T-DM1) as the previous standard of care. Moreover, the researchers now have data for patients with brain metastases to show that tucatinib + trastuzumab + capecitabine can improve survival in this higher-risk group and be an effective regimen for all patients in the third-line setting. Finally, the researchers have a number of effective anti-HER2 therapies that can be used in subsequent lines of therapy to improve patient outcomes.
1. Canadian Perspective and Recommendations
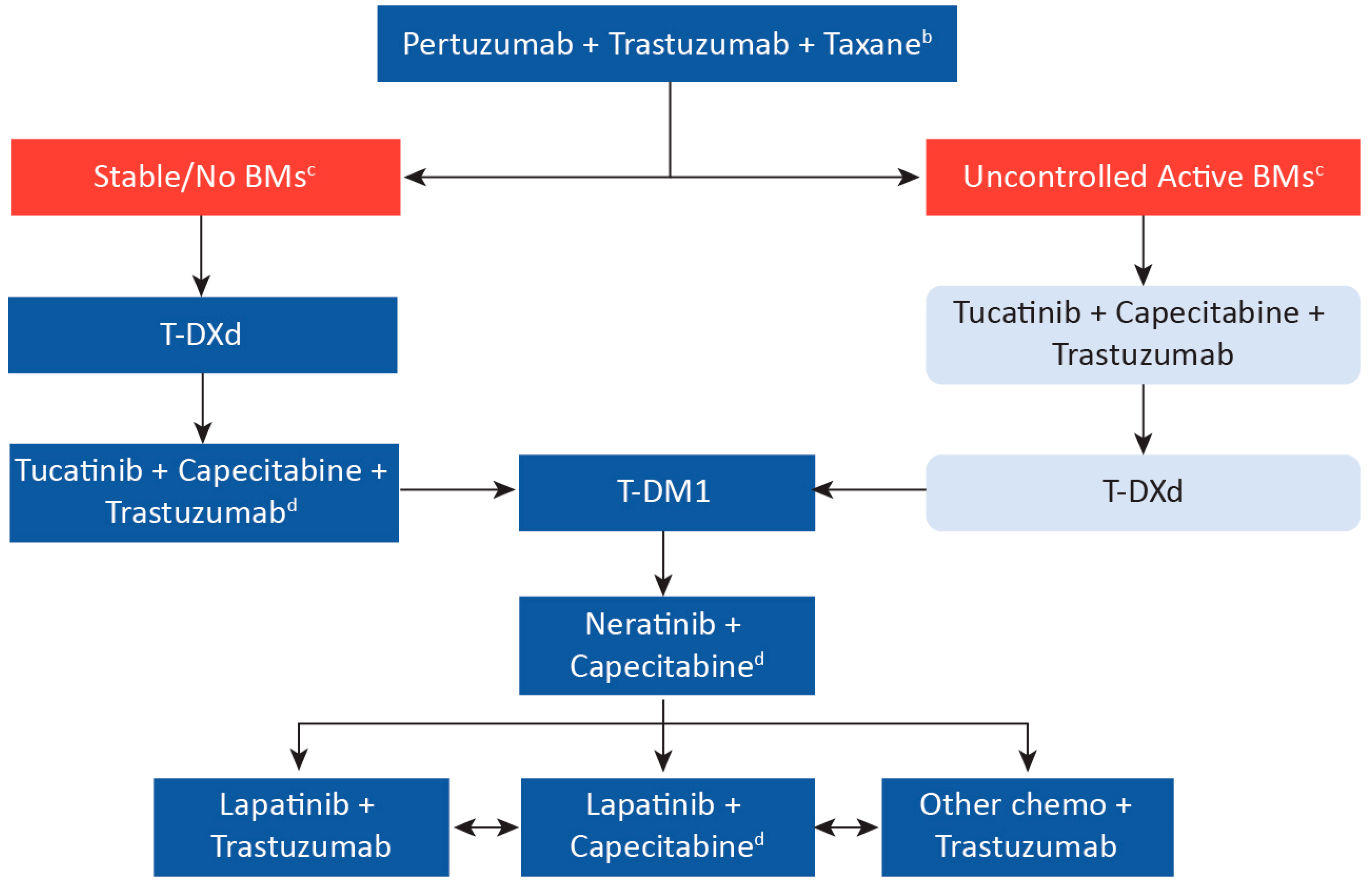
2. First-Line Treatment
3. Second-Line Treatment
Patients with Brain Metastases
4. Third-Line Treatment
Patients with Brain Metastases
5. Subsequent Treatments
6. Summary
References
- Simmons, C.; Rayson, D.; Joy, A.A.; Henning, J.W.; Lemieux, J.; McArthur, H.; Card, P.B.; Dent, R.; Brezden-Masley, C. Current and future landscape of targeted therapy in HER2-positive advanced breast cancer: Redrawing the lines. Ther. Adv. Med. Oncol. 2022, 14, 17588359211066677.
- Aversa, C.; Rossi, V.; Geuna, E.; Martinello, R.; Milani, A.; Redana, S.; Valabrega, G.; Aglietta, M.; Montemurro, F. Metastatic breast cancer subtypes and central nervous system metastases. Breast 2014, 23, 623–628.
- Kim, J.S.; Kim, I.A. Evolving treatment strategies of brain metastases from breast cancer: Current status and future direction. Ther. Adv. Med. Oncol. 2020, 12, 1758835920936117.
- Venur, V.A.; Leone, J.P. Targeted Therapies for Brain Metastases from Breast Cancer. Int. J. Mol. Sci. 2016, 17, 1543.
- Devanaboyina, M.; Bailey, M.M.; Hamouda, D.M. A retrospective study of characteristics and survival in patients with breast cancer brain metastases classified by subtype using NCI SEER registry. J. Clin. Oncol. 2021, 39, 1031.
- Ramakrishna, N.; Temin, S.; Chandarlapaty, S.; Crews, J.R.; Davidson, N.E.; Esteva, F.J.; Giordano, S.H.; Kirshner, J.J.; Krop, I.E.; Levinson, J.; et al. Recommendations on Disease Management for Patients with Advanced Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 2804–2807.
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2019, 382, 597–609.
- Lin, N.U.; Borges, V.; Anders, C.; Murthy, R.K.; Paplomata, E.; Hamilton, E.; Hurvitz, S.; Loi, S.; Okines, A.; Abramson, V.; et al. Intracranial Efficacy and Survival With Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2-Positive Breast Cancer With Brain Metastases in the HER2CLIMB Trial. J. Clin. Oncol. 2020, 38, 2610–2619.
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; De Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495.
- Hoffmann-La Roche. PrPERJETA®; Product Monograph; Hoffmann-La Roche: Mississauga, ON, Canada, 2021.
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791.
- Hoffmann-La Roche Limited. PrKADCYLA®; Product Monograph; Hoffmann-La Roche: Mississauga, ON, Canada, 2021.
- von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2018, 380, 617–628.
- Stemmler, H.J.; Schmitt, M.; Willems, A.; Bernhard, H.; Harbeck, N.; Heinemann, V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anti-Cancer Drugs 2007, 18, 23–28.
- Stringer-Reasor, E.M.; O’Brien, B.J.; Topletz-Erickson, A.; White, J.B.; Lobbous, M.; Riley, K.; Childress, J.; LaMaster, K.; Melisko, M.E.; Morikawa, A.; et al. Pharmacokinetic (PK) analyses in CSF and plasma from TBCRC049, an ongoing trial to assess the safety and efficacy of the combination of tucatinib, trastuzumab and capecitabine for the treatment of leptomeningeal metastasis (LM) in HER2 positive breast cancer. J. Clin. Oncol. 2021, 39, 1044.
- von Minckwitz, G.; du Bois, A.; Schmidt, M.; Maass, N.; Cufer, T.; de Jongh, F.E.; Maartense, E.; Zielinski, C.; Kaufmann, M.; Bauer, W.; et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: A german breast group 26/breast international group 03-05 study. J. Clin. Oncol. 2009, 27, 1999–2006.
- Blackwell, K.L.; Burstein, H.J.; Storniolo, A.M.; Rugo, H.; Sledge, G.; Koehler, M.; Ellis, C.; Casey, M.; Vukelja, S.; Bischoff, J.; et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J. Clin. Oncol. 2010, 28, 1124–1130.
- Smart & Biggar. Biosimilars Approved in Canada. Available online: https://www.smartbiggar.ca/insights/biosimilars (accessed on 23 February 2022).
- Krop, I.E.; Kim, S.B.; Martin, A.G.; LoRusso, P.M.; Ferrero, J.M.; Badovinac-Crnjevic, T.; Hoersch, S.; Smitt, M.; Wildiers, H. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): Final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017, 18, 743–754.
- Krop, I.E.; Kim, S.B.; González-Martín, A.; LoRusso, P.M.; Ferrero, J.M.; Smitt, M.; Yu, R.; Leung, A.C.; Wildiers, H. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 689–699.
- Saura, C.; Oliveira, M.; Feng, Y.H.; Dai, M.S.; Chen, S.W.; Hurvitz, S.A.; Kim, S.B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. J. Clin. Oncol. 2020, 38, 3138–3149.
- Rugo, H.S.; Im, S.A.; Cardoso, F.; Cortés, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Wright, G.S.; Saura, C.; Escrivá-de-Romaní, S.; et al. Efficacy of Margetuximab vs Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 573–584.
- Burstein, H.J.; Keshaviah, A.; Baron, A.D.; Hart, R.D.; Lambert-Falls, R.; Marcom, P.K.; Gelman, R.; Winer, E.P. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: The trastuzumab and vinorelbine or taxane study. Cancer 2007, 110, 965–972.
- Oh, D.-Y.; Chung, H.C.; Im, Y.H.; Yen, C.J.; Chao, Y.; Li, Z.; Wang, X.; Wang, J.; Li, H.; Kang, Y.-K. ZW25, an anti-HER2 bispecific antibody, plus chemotherapy with/without tislelizumab as first-line treatment for patients with advanced HER2-positive breast cancer or gastric/gastroesophageal junction adenocarcinoma: A phase 1B/2 trial-in-progress. J. Clin. Oncol. 2020, 38, TPS3145.
- Hamblett, K.; Barnscher, S.; Davies, R.; Hammond, P.; Hernandez, A.; Wickman, G.; Fung, V.; Ding, T.; Garnett, G.; Galey, A.; et al. Abstract P6-17-13: ZW49, a HER2 targeted biparatopic antibody drug conjugate for the treatment of HER2 expressing cancers. Cancer Res. 2019, 79, P6-17-13.
- Tolaney, S.M.; Wardley, A.M.; Zambelli, S.; Hilton, J.F.; Troso-Sandoval, T.A.; Ricci, F.; Im, S.A.; Kim, S.B.; Johnston, S.R.; Chan, A.; et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): A randomised, open-label, phase 2 trial. Lancet Oncol. 2020, 21, 763–775.





