Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marco Mouanness | -- | 1795 | 2022-04-21 15:57:25 | | | |
| 2 | Camila Xu | -89 word(s) | 1706 | 2022-04-22 05:36:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mouanness, M.; , . Impact of Dietary AGEs on Female Reproduction. Encyclopedia. Available online: https://encyclopedia.pub/entry/22123 (accessed on 08 February 2026).
Mouanness M, . Impact of Dietary AGEs on Female Reproduction. Encyclopedia. Available at: https://encyclopedia.pub/entry/22123. Accessed February 08, 2026.
Mouanness, Marco, . "Impact of Dietary AGEs on Female Reproduction" Encyclopedia, https://encyclopedia.pub/entry/22123 (accessed February 08, 2026).
Mouanness, M., & , . (2022, April 21). Impact of Dietary AGEs on Female Reproduction. In Encyclopedia. https://encyclopedia.pub/entry/22123
Mouanness, Marco and . "Impact of Dietary AGEs on Female Reproduction." Encyclopedia. Web. 21 April, 2022.
Copy Citation
Advanced glycation end products (AGEs), a heterogenous group of products formed by the reaction between protein and reducing sugars, can form endogenously due to non-enzymatic reactions or by exogenous sources such as diet where considerable increase in AGEs is observed due to the modification of food mainly by thermal processing. Recent studies have suggested that AGEs could impact, via inducing inflammation and oxidative stress, the reproductive health and fertility in both males and females.
advanced glycation end product
infertility
PCOS
1. Introduction
The Maillard reaction was first reported in 1912 by French scientist Louis Camille Maillard [1][2] and is defined as the chemical reaction in which the carbonyl group of carbohydrates reacts non-enzymatically with primary amino groups of proteins [3][4]. This reaction leads to the formation of advanced glycation end-products (AGEs). The early stages of the Maillard reaction lead to the formation of chemically reversible glycosylation products with proteins called Schiff bases and Amadori adducts [5]. The late stages of this glycation reaction forms complex glycation products which are the AGEs [6] (Figure 1). Since the 1980s, AGEs have been shown in several studies to be implicated in many health complications such as diabetes and aging [7], as well as many inflammatory diseases, obesity, cardiovascular diseases (CVD), metabolic syndrome and neurodegenerative disorders [8][9][10][11][12]. In the last decade, several studies have shown a potentially significant impact of AGEs on reproductive health in both males and females. This research will summarize the different types of AGEs and their receptors as well as the effect of dietary AGEs on female reproduction, in particular ovarian function, polycystic ovary syndrome (PCOS), and perinatally in utero on the female offspring reproduction. It also addresses the possible mechanistic pathways by which dietary AGEs alter female reproductive health.
1.1. What Are AGEs? How Do They Form?
AGEs are stable non-enzymatically catalyzed compounds which are formed by condensation of the amino groups of protein, lipid, amino acid and nucleic acid with the aldehyde group of reducing carbohydrate [13]. This nonenzymatic modification of proteins, lipids, and nucleic acids by glucose is one of the most important post-translational modifications in the formation of AGEs [14][15]. Once formed, the products of advanced glycation result in an irreversible cross-linking of proteins, loss of protein structure and function, followed by apoptosis and damage to cellular structures [14][15]. AGEs constitute a heterogeneous group of compounds of more than 20 members such as N-carboxymethyl-lysine (CML), pentosidine, 1,2-dicarbonyl precursor compounds glyoxal, and methylglyoxal. Pentosidine and CML are the most commonly studied AGEs [16][17] and have been used as markers of dietary AGE’s accumulation in various tissues [16][18][19][20][21][22]. Some of these compounds are fluorescent crosslinking (such as pentosidine [23]) products while others are non-fluorescent and/or non-crosslinking (such as CML [24][25]).
AGEs can be formed either endogenously by the body or from exogenous sources [1]. Endogenous AGEs are normally formed by glycosylation in different tissues of the body, occur slowly, increase progressively with aging and even faster with abnormal medical health conditions such as hyperglycemia and several chronic degenerative diseases [26]. Exogenous AGEs are obtained from food consumption and they are in very high levels in unhealthy food that is cooked at high temperature, such frying [27] and from smoking [28]. Contemporary methods of cooking (precooked fast-food meals), food high in protein and fat such as meat, cheese, and egg yolk dramatically increase serum AGEs’ concentration [27][29]. In addition to serum level, tissue AGEs can be influenced by diet as well [16][27]. Even though it is beyond the scope of the article, smoking has been identified as an exogenous source of AGEs [30]. Glycation products are present in tobacco and smoke in a form that can rapidly react with proteins to form AGEs [30].
1.2. How Do Dietary AGEs Act?
Dietary AGEs bind to several types of receptors (Figure 2). First, AGEs can act by binding to a receptor called RAGE (Receptor for Advanced Glycation End product) which is member of the immunoglobulin superfamily [31]. The expression of the RAGE protein is detected in human trophoblasts in chorionic villi early in fetal life (such as in endothelial cells of embryonic vessels and alveolar capillaries) and gradually increases after birth and in adulthood [32][33].
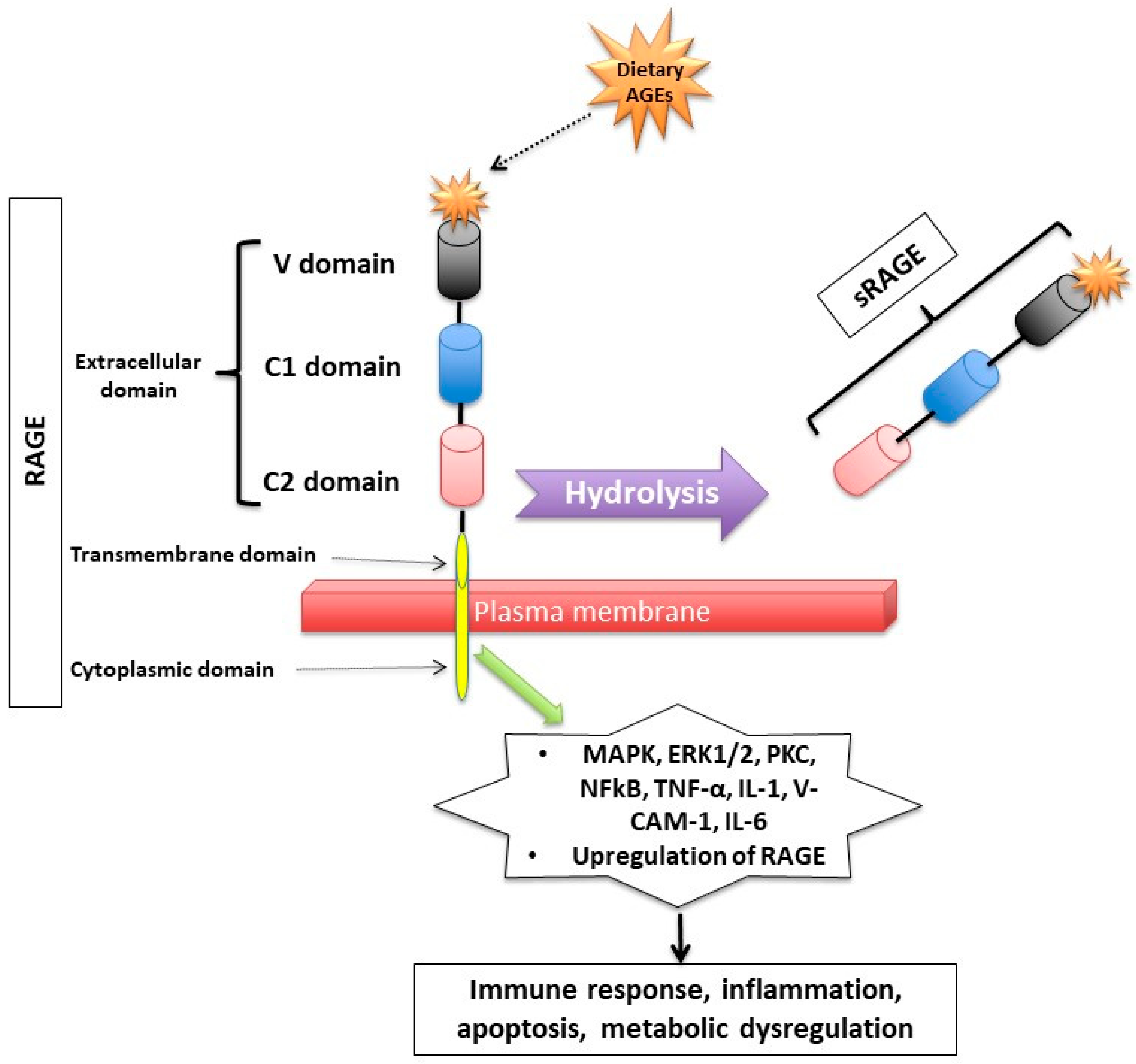 Figure 2. Receptor for Advanced Glycation End (RAGE) products and its mechanism of action. RAGE consists of transmembrane, cytosolic and extracellular domains. The extracellular domain consists of V, C1, and C2 domains. The soluble fragment of RAGE (sRAGE) is produced by hydrolysis of the RAGE receptor and contains the RAGE’s extracellular domain only. The binding of AGEs to RAGE induces a series of inflammatory and apoptotic responses intracellularly and contributes to metabolic dysfunction [32][33][34][35][36][37][38][39][40]. Mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase1/2 (ERK1/2), protein kinase C (PKC) and nuclear factor kappa B (NF-κB), lysyl oxidase (LOX), tumor necrosis factor (TNF-α), interleukin-1 (IL-1), vascular adhesion molecule-1 (VCAM-1), and interleukin-6 (IL-6).
Figure 2. Receptor for Advanced Glycation End (RAGE) products and its mechanism of action. RAGE consists of transmembrane, cytosolic and extracellular domains. The extracellular domain consists of V, C1, and C2 domains. The soluble fragment of RAGE (sRAGE) is produced by hydrolysis of the RAGE receptor and contains the RAGE’s extracellular domain only. The binding of AGEs to RAGE induces a series of inflammatory and apoptotic responses intracellularly and contributes to metabolic dysfunction [32][33][34][35][36][37][38][39][40]. Mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase1/2 (ERK1/2), protein kinase C (PKC) and nuclear factor kappa B (NF-κB), lysyl oxidase (LOX), tumor necrosis factor (TNF-α), interleukin-1 (IL-1), vascular adhesion molecule-1 (VCAM-1), and interleukin-6 (IL-6).RAGE has a triple domain: transmembrane, a cytosolic and an extracellular [34], is expressed in cell membranes of several tissues such as heart, lung, skeletal muscle, the vessel wall and the reproductive system [10][34][41] and can be activated by many other ligands including amyloid β peptide, high-mobility group protein B1 (HMGB1) and the S100 group of protein [42].
After binding to RAGE, there is an activation of several intracellular inflammatory signaling pathways that include mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase1/2 (ERK1/2), protein kinase C (PKC) and nuclear factor kappa B (NF-κB) [43][44]. The activation of those pathways can lead to inflammatory state, cellular oxidative stress, and cellular damage [31] via upregulating markers of reactive oxygen species (ROS), and inflammatory molecules such as tumor necrosis factor (TNF-α), interleukin-1 (IL-1), vascular adhesion molecule-1 (VCAM-1), and interleukin-6 (IL-6) [45]. Interestingly, the binding of AGEs to RAGE upregulates RAGE expression itself, causing inflammation to get worse [31][46][47][48].
Second, RAGEs have been found to have multiple soluble forms detected mainly in body fluids and blood. The two most common forms are: sRAGE (soluble fragment of RAGE) and esRAGE (endogenous secretory RAGE). The sRAGE is produced by hydrolysis (MMPs and ADAM-10 induced proteolytic cleavage mechanisms [35][49]) of the RAGE receptor at the level of the cell surface and can be detected in the blood and bodily fluids [36][48]. Unlike RAGE, sRAGE contains only the extracellular domain of RAGE [36][48], and unlike RAGE, it has an “anti-inflammatory” action since it holds on to the circulating AGEs, thus inhibiting them from exercising their pro-inflammatory effect by binding to RAGE [37][38][39]. Unlike sRAGE, which is derived from the full-length form of RAGE [49], esRAGE is only derived from a part of the RAGE, specifically from pre-mRNA alternative splicing [40]. The esRAGE, also called variant RAGE-v1, usually comprises 20% of the total soluble RAGE receptors [40].
1.3. How Are Dietary AGEs Cleared from the Body?
Dietary AGEs are orally absorbed [50], with approximately 10% of them being absorbed in the GI tract and delivered to the liver and to other organs including but not limited to the reproductive system [51]. Dietary AGEs are mainly cleared by the urinary tract system (kidneys): nearly a third of dietary AGEs are excreted in the urine, with approximately 50% of the AGEs remaining quantified in the urine until approximately a few days following its consumption [52], and accumulating in the body leading to inflammation and oxidative stress [51]. The beginning of AGEs’ degradation occurs mainly intracellularly, therefore they first need to be inserted into the cell. Some of the AGE-receptors that are involved in the detoxification process are the AGE-R1/OST-48, AGE-R3/galectin-3 and scavenger-receptors [53]. These receptors compete with RAGE and try to bind the circulating dietary AGEs, thus they inhibit the toxic RAGE-mediated signaling pathways. The uptake of AGEs takes place through the activation of membrane receptors via phosphorylation or ubiquitinylation of the cytoplasmic side of the receptor, thus inducing its endocytosis [54].
2. Polycystic Ovary Syndrome (PCOS) and Dietary AGEs
PCOS is arguably the most common endocrinopathy in reproductive-aged women [55][56]. It is associated with significant metabolic changes and reproductive alterations, making it the most common cause (up to 70%) of anovulation [56]. Most women with PCOS display some type of metabolic dysfunction [57]. Studies have shown that women with PCOS have elevated circulating AGEs, which is exacerbated by exogenous absorption of AGEs from western heat processed diets [58]. AGEs contribute to the pathogenesis of PCOS as well as the consequential metabolic and reproductive system effects as proven by several in vitro experiments, animal models, and human studies [9][48][59][60][61][62].
When quantified at the ovarian tissue level by immunohistochemistry, RAGE and AGE-modified proteins are expressed in women with or without PCOS, though at much different concentrations [9]. There are alterations in the AGE system that have been shown to be related to reproductive impairment in women with PCOS [63]. It was first demonstrated in 2005 that overweight women with PCOS, compared to those without PCOS and independently of the hyperglycemia level (well known to be correlated to an increase in AGEs level), have increased AGEs’ levels and the upregulation of monocyte RAGE expression [48]. Then, in 2008, it was shown that lean women with PCOS without insulin resistance (another factor that is well known to be correlated with elevated body AGEs) also have elevated serum AGE levels compared to women with components of PCOS only (such as hyperandrogenemia with or without PCO-ovarian morphology) [59]. These findings suggest that these harmful molecules and the pro-inflammatory multi-ligand receptor RAGE have a pathological significance in reproductive abnormalities, in particular in ovarian dysfunction, in PCOS. Additionally, several studies in women who underwent IVF, assessed the relationship between sRAGE and PCOS and showed that compared to women without PCOS, those with PCOS had significantly lower sRAGE levels in the follicular fluid [64][65][66][67]. These findings suggest that there are alterations even in the anti-inflammatory sRAGE receptors in women with PCOS.
Other studies have demonstrated that women with PCOS given isocaloric diets high in AGEs for 2 months had significantly higher testosterone, free androgen index, and androstendione levels compared to women with PCOS on two-months low-AGE isocaloric diet [29]. Animal studies in animals confirmed the same findings, where rats put on a high-AGE diet for six months showed elevated AGE deposition in the reproductive system (theca cells), increased RAGE staining in granulosa cells, and higher plasma testosterone levels compared to low-AGE diet rats [16]. In another study, high-AGE diet showed increased plasma testosterone and decreased plasma estradiol and progesterone in female rats compared to their female rats counterparts on low-AGE diets [68]. This underscores an irrefutable correlation between dietary AGEs and hyperandrogenemia, solidifying the hypothesis that lowering dietary AGEs in PCOS could reduce some of the symptomatology of hyperandrogenemia.
References
- Moschonas, D.P.; Piperi, C.; Korkolopoulou, P.; Levidou, G.; Kavantzas, N.; Trigka, E.-A.; Vlachos, I.; Arapostathi, C.; Perrea, D.; Mitropoulos, D.; et al. Impact of diet-induced obesity in male mouse reproductive system: The role of advanced glycation end product–receptor for advanced glycation end product axis. Exp. Biol. Med. 2014, 239, 937–947.
- Maillard, L.C. Action des acides aminés sur les sucres; formation des méla-noidines par voie methodique. Comptes Rendus Acad. Sci. 1912, 154, 66–68.
- Unoki, H.; Yamagishi, S. Advanced glycation end products and insulin resistance. Curr. Pharm. Des. 2008, 14, 987–989.
- Brownlee, M.; Cerami, A.; Vlassara, H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N. Engl. J. Med. 1988, 318, 1315–1321.
- Brownlee, M. Glycosylation products as toxic mediators of diabetic complications. Annu. Rev. Med. 1991, 42, 159–166.
- Bucala, R.; Cerami, A. Advanced glycosylation: Chemistry, biology, and implications for diabetes and aging. Stud. Surf. Sci. Catal. 1992, 23, 1–34.
- Monnier, V.M.; Stevens, V.J.; Cerami, A. Maillard reactions involving proteins and carbohydrates in vivo: Relevance to diabetes mellitus and aging. Prog. Food Nutr. Sci. 1981, 5, 315–327.
- Yamagishi, S.-I.; Nakamura, K.; Imaizumi, T. Advanced glycation end products (AGEs) and diabetic vascular complications. Curr. Diabetes Rev. 2005, 1, 93–106.
- Diamanti-Kandarakis, E.; Piperi, C.; Patsouris, E.; Korkolopoulou, P.; Panidis, D.; Pawelczyk, L.; Papavassiliou, A.G.; Duleba, A.J. Immunohistochemical localization of advanced glycation end-products (AGEs) and their receptor (RAGE) in polycystic and normal ovaries. Histochem. Cell Biol. 2007, 127, 581–589.
- Tatone, C.; Amicarelli, F. The aging ovary—the poor granulosa cells. Fertil. Steril. 2013, 99, 12–17.
- Pertynska-Marczewska, M.; Merhi, Z. Relationship of advanced glycation end products with cardiovascular disease in menopausal women. Reprod. Sci. 2015, 22, 774–782.
- Merhi, Z. Advanced glycation end-products: Pathway of potentially significant pathophysiological and therapeutic relevance for metabolic syndrome in menopausal women. J. Clin. Endocrinol. Metab. 2014, 99, 1146–1148.
- Khalifah, R.G.; Baynes, J.W.; Hudson, B. Amadorins: Novel post-amadori inhibitors of advanced glycation reactions. Biochem. Biophys. Res. Commun. 1999, 257, 251–258.
- Inagi, R. Inhibitors of advanced glycation and endoplasmic reticulum stress. Methods Enzymol. 2011, 491, 361–380.
- Piperi, C.; Adamopoulos, C.; Dalagiorgou, G.; Diamanti-Kandarakis, E.; Papavassiliou, A.G. Crosstalk between advanced glycation and endoplasmic reticulum stress: Emerging therapeutic targeting for metabolic diseases. J. Clin. Endocrinol. Metab. 2012, 97, 2231–2242.
- Diamanti-Kandarakis, E.; Piperi, C.; Korkolopoulou, P.; Kandaraki, E.; Levidou, G.; Papalois, A.; Patsouris, E.; Papavassiliou, A.G. Accumulation of dietary glycotoxins in the reproductive system of normal female rats. Klin. Wochenschr. 2007, 85, 1413–1420.
- Takahashi, M.; Oikawa, M.; Nagano, A. Effect of age and menopause on serum concentrations of pentosidine, an advanced glycation end product. J. Gerontol. Ser. A 2000, 55, M137–M140.
- Merhi, Z. Advanced glycation end products and their relevance in female reproduction. Hum. Reprod. 2014, 29, 135–145.
- Desai, K.; Wu, L. Methylglyoxal and advanced glycation endproducts: New therapeutic horizons? Recent patents on cardiovascular drug discovery. Recent Pat. Cardiovasc. Drug Discov. (Discontin.) 2007, 2, 89–99.
- Kerkeni, M.; Saïdi, A.; Bouzidi, H.; Ben Yahya, S.; Hammami, M. Elevated serum levels of AGEs, sRAGE, and pentosidine in Tunisian patients with severity of diabetic retinopathy. Microvasc. Res. 2012, 84, 378–383.
- Saito, M.; Marumo, K. Bone quality. Nihon Rinsho 2015, 73, 1665–1672.
- Saeki, C.; Saito, M.; Kanai, T.; Nakano, M.; Oikawa, T.; Torisu, Y.; Saruta, M.; Tsubota, A. Plasma pentosidine levels are associated with prevalent fractures in patients with chronic liver disease. PLoS ONE 2021, 16, e0249728.
- Ghanem, A.A.; Elewa, A.; Arafa, L.F. Pentosidine and N-Carboxymethyl-Lysine: Biomarkers for Type 2 diabetic retinopathy. Eur. J. Ophthalmol. 2011, 21, 48–54.
- Brouwers, O.; Niessen, P.M.; Ferreira, I.; Miyata, T.; Scheffer, P.G.; Teerlink, T.; Schrauwen, P.; Brownlee, M.; Stehouwer, C.D.; Schalkwijk, C.G. Overexpression of glyoxalase-i reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J. Biol. Chem. 2011, 286, 1374–1380.
- Liu, X.; Zheng, L.; Zhang, R.; Liu, G.; Xiao, S.; Qiao, X.; Wu, Y.; Gong, Z. Toxicological evaluation of advanced glycation end product Nε-(carboxymethyl)lysine: Acute and subacute oral toxicity studies. Regul. Toxicol. Pharmacol. 2016, 77, 65–74.
- Jud, P.; Sourij, H. Therapeutic options to reduce advanced glycation end products in patients with diabetes mellitus: A review. Diabetes Res. Clin. Pract. 2019, 148, 54–63.
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291.
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.-J. Advanced glycation end products (AGEs) may be a striking link between modern diet and health. Biomolecules 2019, 9, 888.
- Tantalaki, E.; Piperi, C.; Livadas, S.; Kollias, A.; Adamopoulos, C.; Koulouri, A.; Christakou, C.; Diamanti-Kandarakis, E. Impact of dietary modification of advanced glycation end products (AGEs) on the hormonal and metabolic profile of women with polycystic ovary syndrome (PCOS). Hormones 2014, 13, 65–73.
- Cerami, C.; Founds, H.; Nicholl, I.; Mitsuhashi, T.; Giordano, D.; Vanpatten, S.; Lee, A.; Al-Abed, Y.; Vlassara, H.; Bucala, R.; et al. Tobacco smoke is a source of toxic reactive glycation products. Proc. Natl. Acad. Sci. USA 1997, 94, 13915–13920.
- Kalea, A.Z.; Schmidt, A.M.; Hudson, B.I. RAGE: A novel biological and genetic marker for vascular disease. Clin. Sci. 2009, 116, 621–637.
- Reynolds, P.R.; Kasteler, S.D.; Cosio, M.G.; Sturrock, A.; Huecksteadt, T.; Hoidal, J.R. RAGE: Developmental expression and positive feedback regulation by Egr-1 during cigarette smoke exposure in pulmonary epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2008, 294, L1094–L1101.
- Konishi, H.; Nakatsuka, M.; Chekir, C.; Noguchi, S.; Kamada, Y.; Sasaki, A.; Hiramatsu, Y. Advanced glycation end products induce secretion of chemokines and apoptosis in human first trimester trophoblasts. Hum. Reprod. 2004, 19, 2156–2162.
- Basta, G. Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis 2008, 196, 9–21.
- Zhang, L.; Bukulin, M.; Kojro, E.; Roth, A.; Metz, V.V.; Fahrenholz, F.; Nawroth, P.P.; Bierhaus, A.; Postina, R. Receptor for advanced glycation end products is subjected to protein ectodomain shedding by metalloproteinases. J. Biol. Chem. 2008, 283, 35507–35516.
- Diamanti-Kandarakis, E. Insulin resistance in PCOS. Endocrine 2006, 30, 13–17.
- Asadipooya, K.; Uy, E.M. Advanced Glycation End Products (AGEs), receptor for AGEs, diabetes, and bone: Review of the literature. J. Endocr. Soc. 2019, 3, 1799–1818.
- Schmidt, A.M. Soluble RAGEs—Prospects for treating & tracking metabolic and inflammatory disease. Vasc. Pharmacol. 2015, 72, 1–8.
- Selvin, E.; Halushka, M.K.; Rawlings, A.M.; Hoogeveen, R.C.; Ballantyne, C.M.; Coresh, J.; Astor, B.C. sRAGE and risk of diabetes, cardiovascular disease, and death. Diabetes 2013, 62, 2116–2121.
- Yonekura, H.; Yamamoto, Y.; Sakurai, S.; Petrova, R.G.; Abedin, J.; Li, H.; Yasui, K.; Takeuchi, M.; Makita, Z.; Takasawa, S.; et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem. J. 2003, 370, 1097–1109.
- Fujii, E.Y.; Nakayama, M. The measurements of RAGE, VEGF, and AGEs in the plasma and follicular fluid of reproductive women: The influence of aging. Fertil. Steril. 2010, 94, 694–700.
- Palanissami, G.; Paul, S.F.D. RAGE and Its Ligands: Molecular interplay between glycation, inflammation, and hallmarks of cancer—A review. Horm. Cancer 2018, 9, 295–325.
- Kandaraki, E.A.; Chatzigeorgiou, A.; Papageorgiou, E.; Piperi, C.; Adamopoulos, C.; Papavassiliou, A.G.; Koutsilieris, M.; Diamanti-Kandarakis, E. Advanced glycation end products interfere in luteinizing hormone and follicle stimulating hormone signaling in human granulosa KGN cells. Exp. Biol. Med. 2017, 243, 29–33.
- Verma, N.; Manna, S.K. Advanced Glycation End Products (AGE) potently induce autophagy through activation of RAF protein kinase and nuclear factor κB (NF-κB). J. Biol. Chem. 2016, 291, 1481–1491.
- Guimarães, E.L.; Empsen, C.; Geerts, A.; van Grunsven, L.A. Advanced glycation end products induce production of reactive oxygen species via the activation of NADPH oxidase in murine hepatic stellate cells. J. Hepatol. 2010, 52, 389–397.
- Dunaif, A.; Segal, K.R.; Futterweit, W.; Dobrjansky, A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989, 38, 1165–1174.
- Ramasamy, R.; Yan, S.F.; D’Agati, V.; Schmidt, A.M. Receptor for Advanced Glycation Endproducts (RAGE): A formidable force in the pathogenesis of the cardiovascular complications of diabetes & aging. Curr. Mol. Med. 2007, 7, 699–710.
- Diamanti-Kandarakis, E.; Piperi, C.; Kalofoutis, A.; Creatsas, G. Increased levels of serum advanced glycation end-products in women with polycystic ovary syndrome. Clin. Endocrinol. 2005, 62, 37–43.
- Raucci, A.; Cugusi, S.; Antonelli, A.; Barabino, S.M.; Monti, L.; Bierhaus, A.; Reiss, K.; Saftig, P.; Bianchi, M.E. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J. 2008, 22, 3716–3727.
- He, C.; Sabol, J.; Mitsuhashi, T.; Vlassara, H. Dietary glycotoxins: Inhibition of reactive products by aminoguanidine facilitates renal clearance and reduces tissue sequestration. Diabetes 1999, 48, 1308–1315.
- Semba, R.D.; Ang, A.; Talegawkar, S.A.; Crasto, C.; Dalal, M.; Jardack, P.; Traber, M.; Ferrucci, L.; Arab, L. Dietary intake associated with serum versus urinary carboxymethyl-lysine, a major advanced glycation end product, in adults: The Energetics Study. Eur. J. Clin. Nutr. 2011, 66, 3–9.
- Förster, A.; Kühne, Y.; Henle, T. Studies on absorption and elimination of dietary maillard reaction products. Ann. N. Y. Acad. Sci. 2005, 1043, 474–481.
- Vlassara, H.; Palace, M. Diabetes and advanced glycation endproducts. J. Intern. Med. 2002, 251, 87–101.
- Araki, N.; Higashi, T.; Mori, T.; Shibayama, R.; Kawabe, Y.; Kodama, T.; Takahashi, K.; Shichiri, M.; Horiuchi, S. Macrophage scavenger receptor mediates the endocytic uptake and degradation of advanced glycation end products of the Maillard reaction. Eur. J. Biochem. 1995, 230, 408–415.
- Diamanti-Kandarakis, E.; Kouli, C.R.; Bergiele, A.T.; Filandra, F.A.; Tsianateli, T.C.; Spina, G.G.; Zapanti, E.D.; Bartzis, M.I. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: Hormonal and metabolic profile. J. Clin. Endocrinol. Metab. 1999, 84, 4006–4011.
- Carmina, E.; Lobo, R.A. Polycystic ovary syndrome (PCOS): Arguably the most common endocrinopathy is associated with significant morbidity in women. J. Clin. Endocrinol. Metab. 1999, 84, 1897–1899.
- Cussons, A.J.; Stuckey, B.G.; Watts, G.F. Cardiovascular disease in the polycystic ovary syndrome: New insights and perspectives. Atherosclerosis 2006, 185, 227–239.
- Garg, D.; Merhi, Z. Advanced glycation end products: Link between diet and ovulatory dysfunction in PCOS? Nutrients 2015, 7, 10129–10144.
- Diamanti-Kandarakis, E.; Katsikis, I.; Piperi, C.; Kandaraki, E.; Piouka, A.; Papavassiliou, A.G.; Panidis, D. Increased serum advanced glycation end-products is a distinct finding in lean women with polycystic ovary syndrome (PCOS). Clin. Endocrinol. 2008, 69, 634–641.
- Rutkowska, A.Z.; Diamanti-Kandarakis, E. Do Advanced Glycation End Products (AGEs) contribute to the comorbidities of Polycystic Ovary Syndrome (PCOS)? Curr. Pharm. Des. 2016, 22, 5558–5571.
- Pertynska-Marczewska, M.; Diamanti-Kandarakis, E.; Zhang, J.; Merhi, Z. Advanced glycation end products: A link between metabolic and endothelial dysfunction in polycystic ovary syndrome? Metabolism 2015, 64, 1564–1573.
- Merhi, Z. Crosstalk between advanced glycation end products and vitamin D: A compelling paradigm for the treatment of ovarian dysfunction in PCOS. Mol. Cell. Endocrinol. 2019, 479, 20–26.
- Merhi, Z.; Kandaraki, E.A.; Diamanti-Kandarakis, E. Implications and future perspectives of AGEs in PCOS pathophysiology. Trends Endocrinol. Metab. 2019, 30, 150–162.
- Wang, B.; Li, J.; Yang, Q.; Zhang, F.; Hao, M.; Guo, Y. Decreased levels of sRAGE in follicular fluid from patients with PCOS. Reproduction 2017, 153, 285–292.
- Garg, D.; Grazi, R.; Lambert-Messerlian, G.M.; Merhi, Z. Correlation between follicular fluid levels of sRAGE and vitamin D in women with PCOS. J. Assist. Reprod. Genet. 2017, 34, 1507–1513.
- Irani, M.; Minkoff, H.; Seifer, D.; Merhi, Z. Vitamin D increases serum levels of the soluble receptor for advanced glycation end products in women with PCOS. J. Clin. Endocrinol. Metab. 2014, 99, E886–E890.
- Merhi, Z.; Irani, M.; Doswell, A.D.; Ambroggio, J. Follicular fluid soluble receptor for advanced glycation end-products (sRAGE): A potential indicator of ovarian reserve. J. Clin. Endocrinol. Metab. 2014, 99, E226–E233.
- Chatzigeorgiou, A.; Kandaraki, E.; Piperi, C.; Livadas, S.; Papavassiliou, A.G.; Koutsilieris, M.; Papalois, A.; Diamanti-Kandarakis, E. Dietary glycotoxins affect scavenger receptor expression and the hormonal profile of female rats. J. Endocrinol. 2013, 218, 331–337.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
756
Revisions:
2 times
(View History)
Update Date:
22 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No


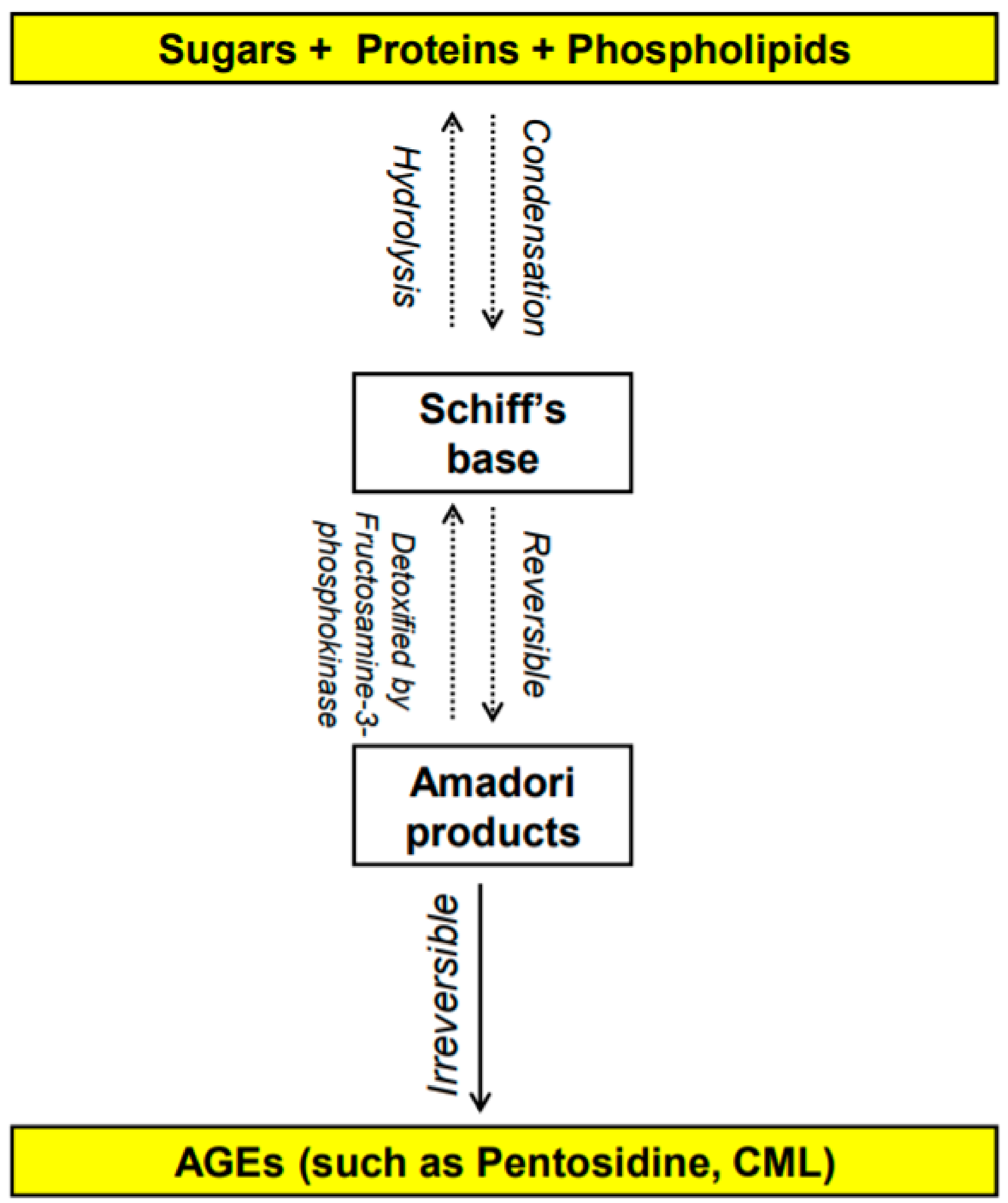 Figure 1. Overview of the formation of advanced glycation end products (AGEs) [
Figure 1. Overview of the formation of advanced glycation end products (AGEs) [

