Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Verena Scheper | -- | 2646 | 2022-04-20 21:28:35 | | | |
| 2 | Amina Yu | + 35 word(s) | 2681 | 2022-04-21 04:39:49 | | | | |
| 3 | Amina Yu | -4 word(s) | 2677 | 2022-04-21 04:41:08 | | | | |
| 4 | Amina Yu | Meta information modification | 2677 | 2022-04-22 08:51:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Scheper, V.; Gao, Z.; Schwieger, J.; Matin, F.; , .; Lenarz, T. Dexamethasone for Inner Ear Therapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/22045 (accessed on 12 January 2026).
Scheper V, Gao Z, Schwieger J, Matin F, , Lenarz T. Dexamethasone for Inner Ear Therapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/22045. Accessed January 12, 2026.
Scheper, Verena, Ziwen Gao, Jana Schwieger, Farnaz Matin, , Thomas Lenarz. "Dexamethasone for Inner Ear Therapy" Encyclopedia, https://encyclopedia.pub/entry/22045 (accessed January 12, 2026).
Scheper, V., Gao, Z., Schwieger, J., Matin, F., , ., & Lenarz, T. (2022, April 20). Dexamethasone for Inner Ear Therapy. In Encyclopedia. https://encyclopedia.pub/entry/22045
Scheper, Verena, et al. "Dexamethasone for Inner Ear Therapy." Encyclopedia. Web. 20 April, 2022.
Copy Citation
Dexamethasone inhibits a tumor necrosis factor-α (TNFα) initiated inflammatory response of spiral ligament fibrocytes in vitro and reduces electrode impedances and fibrose tissue growth around the electrode array in an animal model of electrode insertion trauma. Additionally, dexamethasone protects hair cells of Organ of Corti explants that were challenged with an ototoxic level of TNF-α and protects the hearing ability in guinea pig models of cochlear implantation and in non-human primates.
cochlear implant
dexamethasone
LPS
fibrosis
anti-inflammatory
drug delivery
biocompatibility
1. Cell Viability for Biocompatibility
The orientation one was revealed that the tested concentrations of 3, 30, 60, 150, 300, and 600 µM were biocompatible (Figure 1) according to annex C of ISO 10993-5 for biocompatibility testing. Based on these results, the dexamethasone concentrations with the highest mean value per formulation were chosen for the bio-efficacy tests (Figure 1, red bars). For DEX-lab, a concentration of 300 µM (mean: 101.9% cell viability (CV), DEX-med 60 µM (mean: 88.53%), DPS-lab 150 µM (mean: 100.4%), and DPS-med 300 µM (means: 108.7%) were tested.
Since the results of the orientation showed that there was no toxic effect at 600 µM, the tested dexamethasone concentrations were increased to 10,000 µM to detect a toxicity limit. For better clarity, the results of the expanded MTT test are presented in two concentration groups for the various dexamethasone formulations: low concentrations ranging from 0.03 to 300 µM, and high concentrations ranging from 600 to 10,000 µM. The NC was set at the normal cell proliferation with CV% of 96.79 ± 7.718% and the included PC with DMSO treatment of the fibroblasts revealed a clear cytotoxic effect in the MTT test with a massive decreased CV% of 1.95 ± 1.30% in the performed experiments. The mean of all the low concentrations tested from 0.3 to 300 resulted in an average CV for DEX-lab of 108.9 ± 51.59%, for DEX-med of 113.3 ± 55.48% and for DPS-lab and DPS-med of 110.0 ± 49.67% and 96.15 ± 17.52%, respectively (Figure 1). A comparison within the tested low concentrations of each dexamethasone formulation showed no statistically significant difference (Figure 2; Table 1). Additionally, there was no significant difference detectable between the CVs of NC and the dexamethasone treated cells. None of the low concentrations of the dexamethasone formulations reduced the CV below 70%. According to annex C of ISO 10993-5 for the biocompatibility testing of medical devices, this suggests that all the named low concentrations tested were biocompatible.
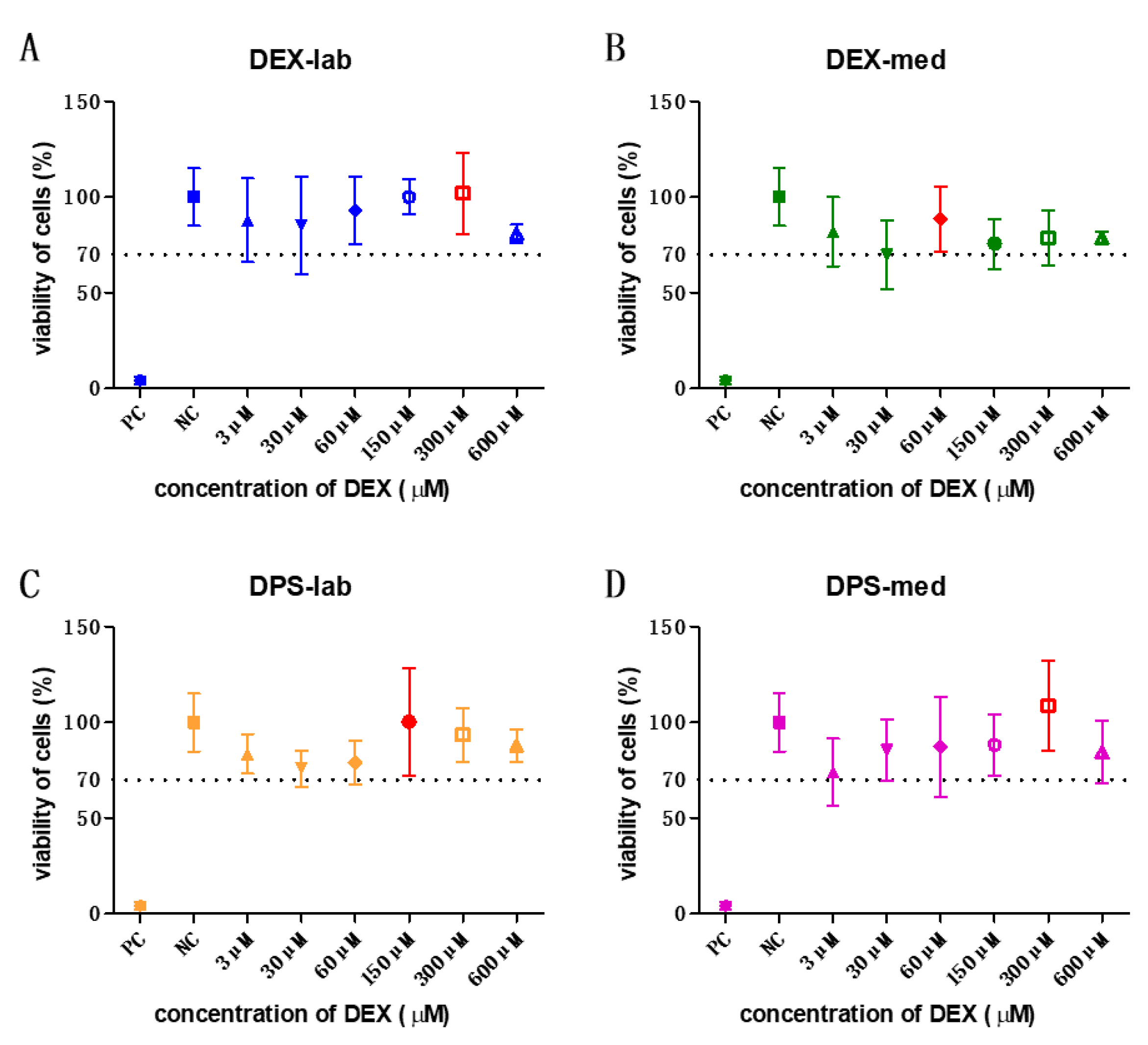
Figure 1. All tested concentrations of the orientation one were biocompatible. The results of these experiments were taken to decide the concentration to be used in the bio-efficacy tests. The highest mean viability of cells is labelled in red in each graph. The dotted line indicates the 70% viability rate.

Figure 2. Comparison of cell viability (CV in %) of fibroblasts treated with low concentrations (0.03–300 µM) of four different dexamethasone formulations. The CVs of the tested low concentrations within one tested dexamethasone formulation did not differ. No significant differences were detected between the NC and any low concentration DEX-treatment. The dashed line indicates 100% CV. The dotted line at 70% CV marks the toxicity level, based on the ISO guideline for biocompatibility testing of medical devices. The tested dexamethasone formulations and concentrations all resulted in cell vitality above the toxicity level. Data are given as mean ± SD in bar charts with single experimental results included as dots (N = 3, n = 3); ns = not significant.
Table 1. p value results of the statistical analysis of the CV data compared to negative control.
| Concentration (µM) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0.03–300 | 600 | 900 | 1000 | 2000 | 4000 | 8000 | 10,000 | |
| DEX-lab | n.s. | 0.9998 | 0.9546 | 0.0562 | 0.0990 | 0.0140 | 0.0024 | 0.0013 |
| DEX-med | n.s. | 0.9994 | 0.4153 | 0.6045 | 0.6919 | 0.0293 | 0.0053 | 0.0014 |
| DPS-lab | n.s. | 0.8198 | 0.0926 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| DPS-med | n.s. | 0.9161 | 0.9997 | 0.7436 | <0.0001 | <0.0001 | <0.0001 | - |
n.s. = not significant (p > 0.05) compare to the negative control; grey: highlighted statistically relevant differences compared to the negative control; DPS-med was not tested in 10,000 µM because the stock solution had a lower concentration.
The results of the statistical analysis of the CV of fibroblasts treated with higher dexamethasone concentrations (600 to 10,000 µM) compared to the NC are summarized in Table 1 and Figure 3. Initially, none of the dexamethasone formulations in concentrations of 600 and 900 µM has an effect on cell vitality. At higher concentrations, the various formulations became toxic. Dexamethasone dihydrogen phosphate disodium is already toxic at 1000 µM (DPS-lab, 51.73 ± 15.08%) and 2000 µM (DPS-med, 15.90 ± 4.36%). For pure dexamethasone, cell viability fell below the 70% CV limit at 4000 µM (DEX-lab and DEX-med, 67.12 ± 16.45% and 68.97 ± 13.71%). Overall, in the higher concentrations the toxicity is higher for DPS, with an average CV for DPS-lab and DPS-med of 7.26 ± 3.06% and 1.13 ± 0.79%, respectively, as compared for DEX, which had a CV for DEX-lab and DEX-med of 61.82 ± 13.55% and 60.16 ± 15.91%, respectively.
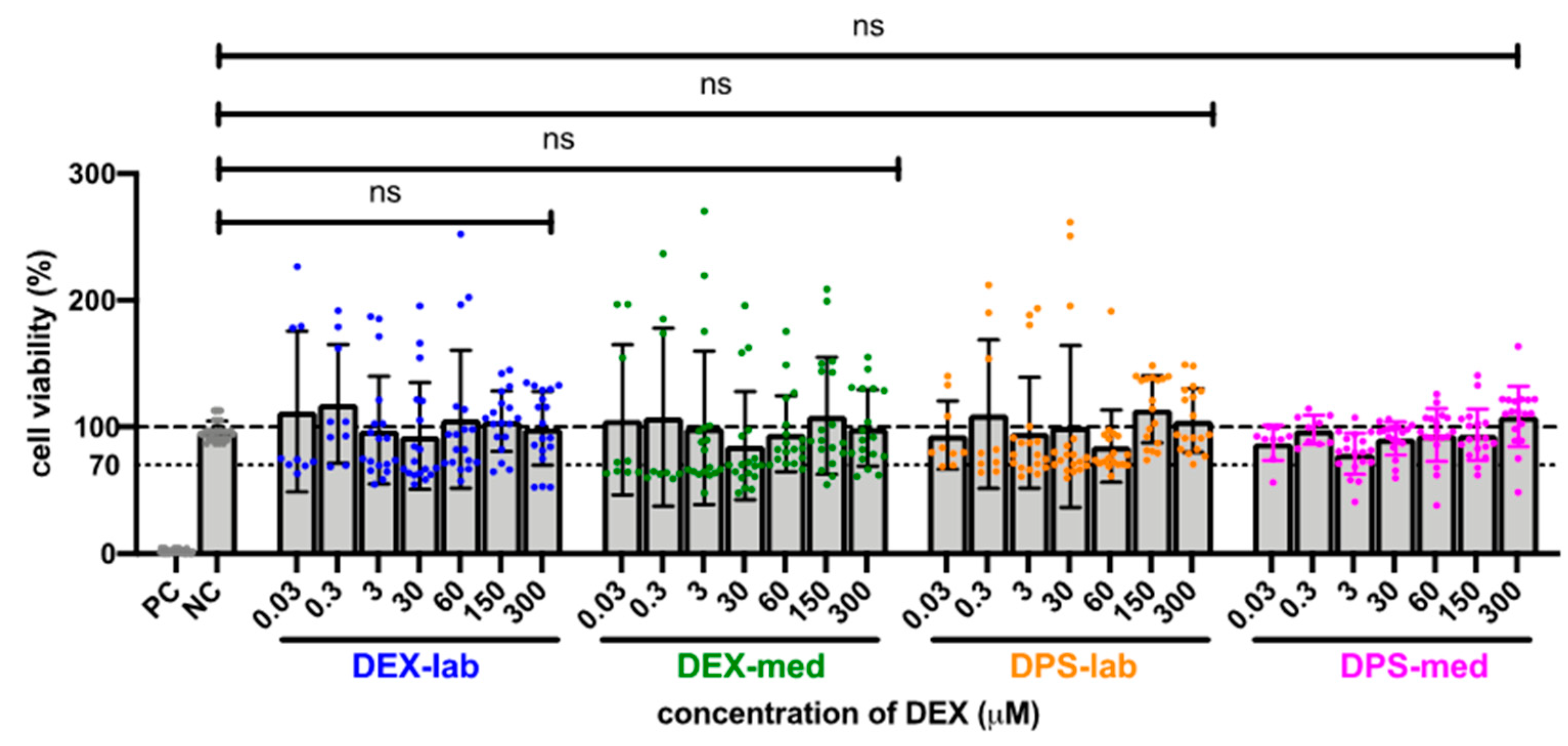
Figure 3. Influence of higher (600 to 10,000 µM) concentrations of dexamethasone formulations on cell viability (CV) detected by MTT assay. The dashed line indicates 100% CV. A reduction of the CV below the 70% level (dotted line) indicates a cytotoxic effect. At a concentration of 4000 µM and higher, the CV was significantly reduced in all treatment groups when compared to the NC. For DPS-lab this was already at a concentration of 1000 µM and for DPS-med this was already at 2000 µM. Data are given as mean ± SD in bar charts, with single experimental results included as dots (N = 3, n = 3). Significant differences to the NC are indicated by * (p < 0.05), ** (p < 0.01) and **** (p < 0.0001).
2. Bio-Efficiency Evaluation by TNF-α Detection
The potential of the different dexamethasone formulations to reduce an inflammatory response was analyzed by means of TNF-α production, measured by ELISA-detection after LPS-stress of the dendritic cells (DCs) (Figure 4). The dexamethasone concentrations with the best biocompatibility were added to the DCs: 300 µM DEX-lab, 60 µM DEX-med, 150 µM DPS-lab, and 300 µM DPS-med. The DCs cultured with pure medium (NC) produced a basic TNF-α level of 91.35 ± 18.73 pg/mL (mean ± SD). Adding 0.5 µg/mL LPS to induce cell stress (PC) increased the TNF-α amount in the supernatant to 3611 ± 2425 pg/mL and thus significantly compared to the NC (p = 0.001). Compared to the PC, all tested dexamethasone formulations reduced the measured TNF-α amount in the culture supernatants significantly (p = 0.0451 for DEX-lab, DEX-med and DPS-med; p = 0.0016 for DPS-lab). The TNF-α level was 1335 ± 1013 pg/mL for DEX-lab, 1272 ± 646.3 pg/mL for DEX-med, 1000 ± 552.4 pg/mL for DPS-lab, and 1169 ± 832.4 for DPS-med.

Figure 4. TNF-α amounts measured by ELISA in the supernatants of dendritic cells (DCs). TNF-α production is induced by addition of 0.5 µg/mL LPS to the culture medium. This results in a high release of TNF-α in the PC when compared with the basic TNF-α level of unstressed cells in the NC. All tested dexamethasone formulations reduced the TNF-α amount in culture. Data are given as mean ± SD and detected significances are marked with * (p < 0.05), ** (p < 0.01), and *** (p < 0.001).
3. Biocompatibility and Bio-Efficacy of Different Dexamethasone Formulations In Vitro
Glucocorticoids are considered to have great therapeutic potential for inner ear diseases. This class of steroidal hormones includes, prednisone, triamcinolone, cortisone, and dexamethasone, and dexamethasone is the most popular for use in the field of inner ear therapy. Although many have highlight the therapeutic effect of dexamethasone in the inner ear (for example [1][2][3]), factors such as the concentration to be applied or the delivery route affect its biological effects. Next to this the chemical compound composition may be one reason for the varying in vitro, in vivo and clinical results.
To the knowledge, no published ones has focused on investigating the biocompatibility of different types of dexamethasone in a direct comparison of a dilution series. This lack of information is intended to be remedied by the data presented here. It was tested the biocompatibility and bio-efficacy of the two dexamethasone formulations, dexamethasone and dexamethasone dihydrogen phosphate disodium, which have previously been used in hearing one (Table 2). Two products were selected for both formulations: one that was laboratory grade and one that can potentially be transferred as a therapeutic to the clinic as it is pharmaceutical grade. Concentrations from 0.03 µM to 10,000 µM were selected for biocompatibility testing.
Table 2. List using dexamethasone for inner ear therapy.
| Dexamethasone Formulation, Molecular Weight | Reference | Study Type | Delivery Method | Concentration (mg/mL) * |
Remarks |
|---|---|---|---|---|---|
| DEX-lab, 392.46 g/mol |
Connolly et al., 2011 [1] | In vivo | i.v. prior to CI | 0.0002; 0.002 | Lower dose failed to maintain ABR thresholds. High-dose treatment resulted in a reduction of ABR threshold shift. |
| Kuthubutheen et al., 2014 [4] | In vivo | i.p. | 0.002 | Spiral ganglion neuron (SGN) density was increased compared to traumatized controls. | |
| Jia et al., 2016 [5] | In vitro; in vivo | explants; pump based delivery (1 µL/h, 7 days); |
0.00117; 0.0117; 0.117; 0.117 |
In vitro: 0.00117 and 0.0117 mg/mL start to have toxic effects on outer hair cells, 0.117 mg/mL is toxic for inner and outer hair cells; in vivo: 0.117 mg/mL is toxic for SGN but improves ABR thresholds at selected frequencies. |
|
| Takeda et al., 2021 [6] | In vivo | i.p. | 0.002 | No effect. | |
| DEX-med, 392.46 g/mol |
Serrano Cardona et al., 2013 [7] | Clinical | DEX in PLGA polymer | 0.7 | Mean hearing threshold improved. |
| Bas et al., 2016 [2] | In vivo | DEX in CI silicone; 0.1% = 13 ng/day, 1.0% = 60 ng/day and 10% = 161 ng/day | 1; 10; 100 | 10% and 1.0% protected against electrode insertion-induced HC loss, but increased ABR and CAP thresholds and impedance, fibrosis and loss of cochlear nerve elements. | |
| Wilk et al., 2016 [8] | In vivo | DEX in CI silicone (16 ng/day and 49 ng/day) |
10; 100 | Reduced impedances and fibrous tissue growth; increased hearing thresholds. | |
| Scheper et al., 2017 [9] | In vivo | DEX in CI silicone (16 ng/day and 49 ng/day; i.e., 0.66 ng/h and 2.04 ng/h) |
10; 100 | Normal SGN number and increased soma diameter. | |
| Ahmadi et al., 2019 [10] | In vivo | 6% DEX loaded hydrogel and DEX containing CI | 60 | Auditory nerve fiber protection. | |
| DPS-lab, 516.40 g/mol |
James et al., 2008 [11] | In vivo | i.t.; 5 µL of 2% | 20 | Residual hearing preservation. |
| Souter et al., 2009 [12] | In vivo | i.t., 20% in sponge | 200 | Hearing protection at lower concentrations. | |
| Hütten et al., 2014 [3] | In vitro, In vivo |
StarPEG-hydrogel filled reservoir, (50 µg DEX/µL hydrogel, 0.35 μg DEX/h) |
50; 50 |
Hearing protection, reduced fibrosis. | |
| Alexander et al., 2015 [13] | Clinical | DEX i.t., four injections in two weeks | 10; 24 | Recovery of hearing threshold after SSNHL. | |
| Scheper et al., 2017 [9] | In vivo | StarPEG-hydrogel filled reservoir, (50 µg DPS-lab/µL hydrogel, 0.35 μg DEX/h) |
50 | Biocompatible regarding SGN number and soma diameter. | |
| Lyu et al., 2018 [14] | In vivo | i.c., i.t. and i.p. | 5; 5; 0.01 | 5 but not 0.01 mg/mL preserved hearing in cochlear implanted animals. | |
| Ahmadi et al., 2018 [10] | Clinical | Temporarily implanted catheter (4 mg/mL/day) | 4 | No effect. | |
| DPS-med, 516.40 g/mol |
Coimbra et al., 2007 [15] | In vivo | i.p. every 8 h | 0.0007 | Not effective in preventing neuron loss in pneumococcal meningitis-induced hearing loss. |
| Berjis et al., 2016 [16] | Clinical | i.t. (4 mg/mL/day) | 4 | Hearing improvement. | |
| Scheper et al., 2017 [9] | In vivo | osmotic pump (25 pg/h) |
0.0001 | Biocompatible regarding SGN number, decreased soma diameter; with electrical stimulation: increased SGN number. |
*: most references do not state mg/mL. This information was calculated using the relevant information of the respective publication; i.v.: intravenous; i.p.: intraperitoneal; i.t.: intratympanic; i.c.: intracochlear.
All types of dexamethasone were biocompatible in lower concentrations (0.03–300 µM; Figure 3). However, with increasing concentrations of dexamethasone, the viability of the cells in each group decreased, albeit to a differing extent. DEX-lab and DEX-med were safe up to 2000 µM (784 µg/mL; 0.0784 mg/mL), while DPS treatment was only safe up to 900 µM (464 µg/mL; 0.464 mg/mL; DPS-lab) and 1000 µM (516 µg/mL; 0.516 mg/mL; DPS-med). To shed light on the results with regard to the current state of knowledge that was randomly reviewed presenting results for the usage of dexamethasone in cochlear pharmacotherapy (Table 2).
The concentrations listed in Table 2 cover the full range of concentrations that was tested in the biocompatibility MTT assay. Using dexamethasone concentrations below the concentrations, it was detected to be toxic in vitro (for example, <0.5 mg/mL for DPS and <1.6 mg/mL for DEX) did not report the biological effects of low concentrations on hearing ability (Conolly et al. 2011: 0.002 mg/mL DEX-lab i.v. prior to CI surgery; Taketa et al., 2021: 0.002 mg/mL DEX-lab; Lyu et al., 2018: 0.01 mg/mL DPS lab), but biocompatibility and a protective effect on SGN if applied in parallel with cochlear implant based electrical stimulation was reported (Scheper et al., 2017: 0.1 µg/mL pump based [9]). Concentrations which were cell compatible in the experiments (below 600 µM) were already classified as toxic in previous one (0.00117 mg/mL = 1.1177 µg/mL = 3 µM DEX-med in vitro [5]). In contrast, concentrations which significantly reduced the CV in the in vitro tests were already shown to have a beneficial effect on residual hearing in animal models, not being toxic in vivo. For example, 5 mg/mL (DPS-lab, in vivo, i.c, i.t., [14]) is much higher than the concentration detected to be toxi (DPS-lab: 900 µM = 0.464 mg/mL). The highest concentration was used by Alexander et al. (2015), where intratympanically injected 24 mg/mL DPS-lab resulted in pure tone hearing threshold recovery after SSNHL and was massively above the concentrations having a toxic effect for the different dexamethasone formulations.
Even though it was aimed to compare the listed concentrations (Table 2) to the results, it was admited that such a comparison is hardly possible since the reported dexamethasone amounts were mostly not the relevant concentrations in the inner ear but the drug load of a matrix or a concentration applied systemically. Therefore, the dexamethasone concentrations achieved in the scala tympani are mostly unknown.
The individual parameters of the different treatment protocols, like intravenous or intraperitoneal injection, systemic therapy, or drug release of a matrix such as the CI electrode array make it difficult to directly compare the outcomes, especially with respect to the actual dose of DEX delivered.
The best CV rate was detected for DEX-lab and DPS-med at 300 µM, for DEX-med at 60 µM, and for DPS-lab at 150 µM. Those optimal concentrations were compared regarding their anti-inflammatory effect. No differences were observed between the formulations but there was a slight tendency for DPS-lab to be more effective in reducing TNF-α production than the other formulations. This suggests that different dexamethasone formulations may achieve similar anti-inflammation effects in the inner ear. Future is focus on what should address this topic by investigating the anti-inflammatory potential of different dexamethasone formulations in a set up involving accelerating concentrations to be able to suggest the most promising dexamethasone formulation and respective concentration to reduce anti-inflammatory reactions in general, and in the inner ear in specific.
The choice of dexamethasone to be used and the concentration to be applied for inner ear pharmacotherapy was guided by different questions. What is the biological effect one is aiming for: hearing preservation, fibrosis reduction, SGN protection or a general reduction of inflammatory reactions? All dexamethasone formulations tested have an anti-inflammatory effect, as has been described in general for dexamethasone. Which concentrations have to be reached locally in the inner ear to receive the aimed effects was not previously known.
What will the delivery matrix be? Drug delivery systems are needed for the sustained treatment of inner ear diseases. Whereas a hydrogel-based delivery would favor DPS-lab because of its hydrophilicity [3][9] DEX-med is used for delivery through hydrophobic materials such as silicone [2][8] or PLGA [17].
Where is the matrix placed to release the DEX? If it is inserted into the inner ear, a direct release into the perilymph is possible, while with intratympanic application the drug has to pass the round window membrane, which affects the concentration to be reached in the inner ear [18].
Which concentration should be chosen? This is a trickier question as it is not known which concentration is needed in vivo to achieve a biological effect. As listed in Table 2, the biologically effective concentrations ranged from 0.00117 mg/mL [5] to 24 mg/mL [13]. Based on the results and in view of Table 2, it was concluded that the concentrations need to be chosen with respect to the DEX formulation used, since there are massive differences in cytotoxicity in vitro (900 µM (DPS-lab) versus 2000 µM (DEX-med and DEX-lab)). There is a large variability between concentrations being toxic in vivo and those having a beneficial effect. This could be attributed to the variations of the different treatment protocols. Individual parameters regarding the route of administration (intracochlear, intratympanic, intraperitoneal or intravenous), single shot injection or permanent infusion, delivery matrix, release kinetics, species and the trauma model used affect the DEX effect. With the data available until now, it was not possible to recommend one concentration for a specific DEX formulation to be used in clinical trials, since the in vitro and in vivo were too heterogeneous. Since animal trials need to be ethically justifiable, are time-consuming and costly, in silico trials are needed to harmonize the available data and to generate data sets which allow a decision for the most promising combination of DEX formulation and concentration, delivery route and therapy duration to induce relevant biological effects on the patients.
References
- Connolly, T.M.; Eastwood, H.; Kel, G.; Lisnichuk, H.; Richardson, R.; O’Leary, S. Pre-Operative Intravenous Dexamethasone Prevents Auditory Threshold Shift in a Guinea Pig Model of Cochlear Implantation. Audiol. Neurotol. 2011, 16, 137–144.
- Bas, E.; Bohorquez, J.; Goncalves, S.; Perez, E.; Dinh, C.T.; Garnham, C.; Hessler, R.; Eshraghi, A.A.; Van De Water, T.R. Electrode array-eluted dexamethasone protects against electrode insertion trauma induced hearing and hair cell losses, damage to neural elements, increases in impedance and fibrosis: A dose response study. Hear. Res. 2016, 337, 12–24.
- Hütten, M.; Dhanasingh, A.; Hessler, R.; Stöver, T.; Esser, K.-H.; Möller, M.; Lenarz, T.; Jolly, C.; Groll, J.; Scheper, V. In Vitro and In Vivo Evaluation of a Hydrogel Reservoir as a Continuous Drug Delivery System for Inner Ear Treatment. PLoS ONE 2014, 9, e104564.
- Kuthubutheen, J.; Coates, H.; Rowsell, C.; Nedzelski, J.; Chen, J.M.; Lin, V. The role of extended preoperative steroids in hearing preservation cochlear implantation. Hear. Res. 2015, 327, 257–264.
- Jia, H.; François, F.; Bourien, J.; Eybalin, M.; Lloyd, R.; Van De Water, T.; Puel, J.-L.; Venail, F. Prevention of trauma-induced cochlear fibrosis using intracochlear application of anti-inflammatory and antiproliferative drugs. Neuroscience 2016, 316, 261–278.
- Takeda, T.; Takeda, S.; Kakigi, A. Effects of Glucocorticoids on the Inner Ear. Front. Surg. 2021, 7, 157.
- Lien, K.-C.; Mooney, B.; DeLancey, J.O.L.; Ashton-Miller, J.A. Levator ani muscle stretch induced by simulated vaginal birth. Obstet. Gynecol. 2004, 103, 31–40.
- Wilk, M.; Hessler, R.; Mugridge, K.; Jolly, C.; Fehr, M.; Lenarz, T.; Scheper, V. Impedance Changes and Fibrous Tissue Growth after Cochlear Implantation Are Correlated and Can Be Reduced Using a Dexamethasone Eluting Electrode. PLoS ONE 2016, 11, e0147552.
- Scheper, V.; Hessler, R.; Hütten, M.; Wilk, M.; Jolly, C.; Lenarz, T.; Paasche, G. Local inner ear application of dexamethasone in cochlear implant models is safe for auditory neurons and increases the neuroprotective effect of chronic electrical stimulation. PLoS ONE 2017, 12, e812–e822.
- Ahmadi, N.; Gausterer, J.C.; Honeder, C.; Mötz, M.; Schöpper, H.; Zhu, C.; Saidov, N.; Gabor, F.; Arnoldner, C. Long-term effects and potential limits of intratympanic dexamethasone-loaded hydrogels combined with dexamethasone-eluting cochlear electrodes in a low-insertion trauma Guinea pig model. Hear. Res. 2019, 384, 107825.
- James, D.P.; Eastwood, H.; Richardson, R.; O’Leary, S.J. Effects of Round Window Dexamethasone on Residual Hearing in a Guinea Pig Model of Cochlear Implantation. Audiol. Neurotol. 2007, 13, 86–96.
- Souter, M.; Eastwood, H.; Marovic, P.; Kel, G.; Wongprasartsuk, S.; Ryan, A.F.; O’Leary, S.J. Systemic Immunity Influences Hearing Preservation in Cochlear Implantation. Otol. Neurotol. 2012, 33, 532–538.
- Alexander, T.H.; Harris, J.P.; Nguyen, Q.T.; Vorasubin, N. Dose Effect of Intratympanic Dexamethasone for Idiopathic Sudden Sensorineural Hearing Loss. Otol. Neurotol. 2015, 36, 1321–1327.
- Lyu, A.-R.; Kim, D.H.; Lee, S.H.; Shin, D.-S.; Shin, S.-A.; Park, Y.-H. Effects of dexamethasone on intracochlear inflammation and residual hearing after cochleostomy: A comparison of administration routes. PLoS ONE 2018, 13, e0195230.
- Coimbra, R.S.; Loquet, G.; Leib, S.L. Limited Efficacy of Adjuvant Therapy with Dexamethasone in Preventing Hearing Loss Due to Experimental Pneumococcal Meningitis in the Infant Rat. Pediatr. Res. 2007, 62, 291–294.
- Musavi, A.; Berjis, N.; Soheilipour, S.; Hashemi, S.M. Intratympanic dexamethasone injection vs methylprednisolone for the treatment of refractory sudden sensorineural hearing loss. Adv. Biomed. Res. 2016, 5, 111.
- Plontke, S.K.; Glien, A.; Rahne, T.; Mäder, K.; Salt, A. Controlled Release Dexamethasone Implants in the Round Window Niche for Salvage Treatment of Idiopathic Sudden Sensorineural Hearing Loss. Otol. Neurotol. 2014, 35, 1168–1171.
- Salt, A.N.; Plontke, S.K. Pharmacokinetic principles in the inner ear: Influence of drug properties on intratympanic applications. Hear. Res. 2018, 368, 28–40.
More
Information
Subjects:
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
729
Revisions:
4 times
(View History)
Update Date:
22 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




