Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Audrey Ann Grossen | -- | 1646 | 2022-04-20 16:46:30 | | | |
| 2 | Peter Tang | Meta information modification | 1646 | 2022-04-21 04:03:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Grossen, A.; Smith, K.; Coulibaly, N.; , .; Wilhelm, S.; Dunn, I.; Towner, R.A.; Wu, D.; Battiste, J. Physical Forces in Glioblastoma Migration. Encyclopedia. Available online: https://encyclopedia.pub/entry/22037 (accessed on 07 February 2026).
Grossen A, Smith K, Coulibaly N, , Wilhelm S, Dunn I, et al. Physical Forces in Glioblastoma Migration. Encyclopedia. Available at: https://encyclopedia.pub/entry/22037. Accessed February 07, 2026.
Grossen, Audrey, Kyle Smith, Nangorgo Coulibaly, , Stefan Wilhelm, Ian Dunn, Rheal A. Towner, Dee Wu, James Battiste. "Physical Forces in Glioblastoma Migration" Encyclopedia, https://encyclopedia.pub/entry/22037 (accessed February 07, 2026).
Grossen, A., Smith, K., Coulibaly, N., , ., Wilhelm, S., Dunn, I., Towner, R.A., Wu, D., & Battiste, J. (2022, April 20). Physical Forces in Glioblastoma Migration. In Encyclopedia. https://encyclopedia.pub/entry/22037
Grossen, Audrey, et al. "Physical Forces in Glioblastoma Migration." Encyclopedia. Web. 20 April, 2022.
Copy Citation
The invasive capabilities of glioblastoma (GBM) define the cancer’s aggressiveness, treatment resistance, and overall mortality. The tumor microenvironment influences the molecular behavior of cells, both epigenetically and genetically. Current forces being studied include properties of the extracellular matrix (ECM), such as stiffness and “sensing” capabilities. There is currently limited data on the physical forces in GBM—both relating to how they influence their environment and how their environment influences them.
glioblastoma
chemoresistance
physical forces
tumor microenvironment
1. Introduction
The mechanobiology of brain tumors is a vast and essential part of understanding their growth, progression, and chemoresistance [1]. Over the last two decades, there has been continuous study and development of the molecular biological underpinnings of glioblastoma (GBM), but with little focus on the relationship between physical forces and migration. In GBM, it is known that certain molecular aberrations exhibit more aggressive migratory patterns. Classic glioma biomarkers include isocitrate dehydrogenase (IDH) mutation, 6-methylguanine–DNA methyltransferase (MGMT) modification, and the deletion of 1p19q, which are hallmarks of the aforementioned molecular profiling that lead to the backbone of GBM classification, nomenclature, and scientific research, but they are more associated with DNA repair than invasive characteristics. Emerging research focuses on the complex contributions of the physical forces to cancer aggression, invasion, and migration.
Emerging data indicates that tissue invasion increases GBM aggressiveness, chemoresistance, and overall mortality [2]. Brain invasion is associated both with poor prognosis and a median survival of under one year for a majority of patients. This invasion is often accompanied by neurologic dysfunction leading to reduced quality of life. A myriad of potential targets are emerging for further research into the invasion and migration of GBM. The tumor microenvironment influences the molecular behavior of cells, inducing mutations. Current forces being studied include the properties of the extracellular matrix (ECM), such as stiffness and “sensing” capabilities [3].
The brain presents unique challenges when it is affected directly by cancer, as it is confined within the rigid skull. This poses questions with regard to how increased edema, producing elevated intracranial pressure (ICP), compression, tension, and other mechanical forces, affects GBM. Additionally, the brain also uniquely has the blood–brain barrier (BBB) and is an “immunologically privileged” anatomical location. The effect that the input of these physical forces has on the hallmark macrophage/microglial infiltration in GBM is not well understood. However, it is known that these tumors disrupt the BBB integrity and have the potential to alter the ECM [4]. However, it is also known that in diffuse GBM, the BBB remains essentially intact, which has reduced some therapeutic advances [5].
2. External/Applied/Mechanical Forces
Physical forces have varying impacts depending on many factors, including the rigidity of the object, the composition of the object, and the geometry of the object. At the molecular and cellular level, the applied physics cause cellular responses depending on the nature of the force and the intensity of its application. The brain has compensatory tools available, with the ability to adjust to changes when physical forces are applied. Blood cells traversing through capillaries have been thoroughly studied, with their unique geometry and internal structure aiding in the delivery of oxygen to tissues while maintaining structural integrity for up to 120 days. Some cellular responses to external forces are based on the physical characteristics of the cell, such as the blood cell. Other responses to external stimuli can produce a biomechanical response, such as mechanoreceptors opening their ion channels to pressure on peripheral neurons, or a regulatory response, such as the upregulation of angiogenesis recruitment chemicals, such as VEGF [6]. In tissues, the physiologic response is a directed transmission of applied forces to invoke a downstream function.
3. Tumor Microenvironment (TME)
3.1. Intracranial Pressure
Increased intracranial pressure is defined as an elevated pressure within the skull. This is a common clinical problem encountered in patients with brain tumors. However, the effects of increased pressure on tumor cell migration are not fully understood. The increased pressure is due to compounded forces that are applied to the brain and can be caused by additional fluid, or the growth of a brain tumor that applies more physical forces than normal on the brain all within the rigid space contained by the skull (Figure 1). Normal supine intracranial pressure is between 7 and 15 mmHg. One case report of a woman diagnosed with GBM in the postpartum period without signs of myelopathy presented with ~20.6 mmHg opening pressure on a lumbar puncture. It was assumed that the GBM primarily grew in the cervical cord and metastasized into the intracranial subarachnoid space [7].
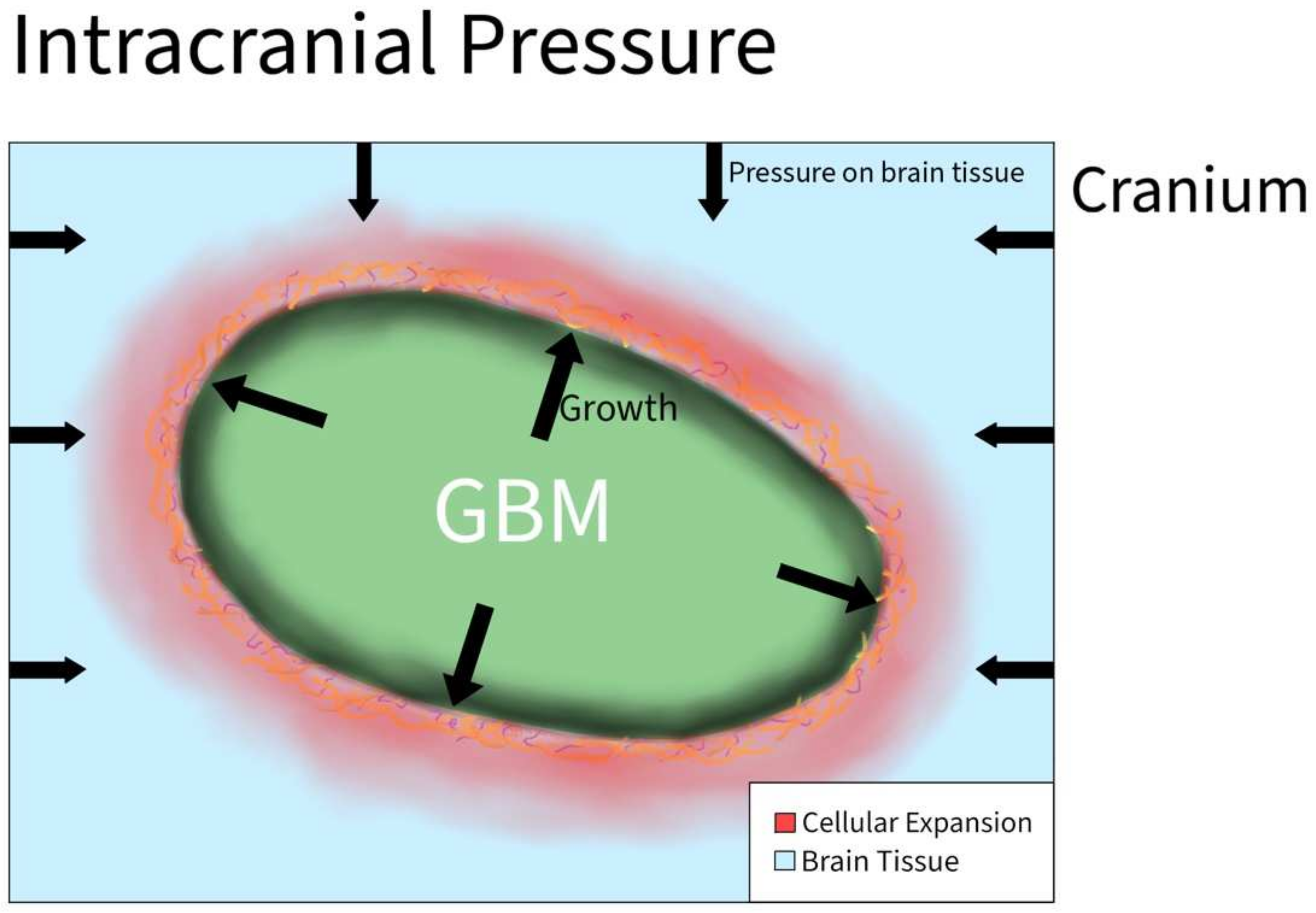
Figure 1. Intracranial Pressure in GBM. As the tumor grows rapidly inside the brain, the overall size of the brain increases and causes tissue to start pressing against the cranium. As a result, the cranium exerts compressive forces back on the brain that result in an increase in intracranial pressure.
In another study, 171 patients with intramedullary spinal cord tumors underwent surgical resection. Twenty patients had a malignant tumor, in which thirteen out of twenty cases were complicated by increased intracranial pressure and ventriculomegaly. Of the remaining 151 patients, an addition 12 developed systematic hydrocephalus. Increasing intracranial pressure generates a holocephalic compressive force that causes the compression of neoplastic and normal brain tissue, simultaneously creating a global effect on all tissues in the brain.
Hyaluronic acid (HA) is a component of brain outflow pathways that has been shown to regulate fluid movement [8]. The increased production of HA can potentially lead to increased ICP. Yoo et al. [9] described how HA production was increased in GBM cells following radiation. This mechanism included the upregulation of HA synthase-2 (HAS2) by NF-ĸB. Notably, NF-ĸB was persistently activated by an IL-1α-feedback loop, making HA abundant in the tumor microenvironment after radiation
3.2. Cellular Volume
Cellular volume is defined by the amount of fluid (primarily water) contained within the cell. Because of osmosis, the cellular volume is usually determined by the cellular environment: hypertonic, isotonic, or hypotonic. GBM cells express abundant Cl channels whose activity supports cell volume and membrane potential changes (Figure 2). Similar to non-tumor tissues, Cl channels are modulated by hypoxia in GBM. Acute hypoxia increased the cell volume by 20%. However, when GBM cells are in a 30% hypertonic environment, they showed partial inhibition of the hypoxia-activated Cl current. ICl,swell was observed to mediate the regulatory volume decrease in GBM, and increase the hypoxia-induced necrotic death in GBM. As a result, cellular volume through Cl channels plays a role in the survival of GBM cells [10].
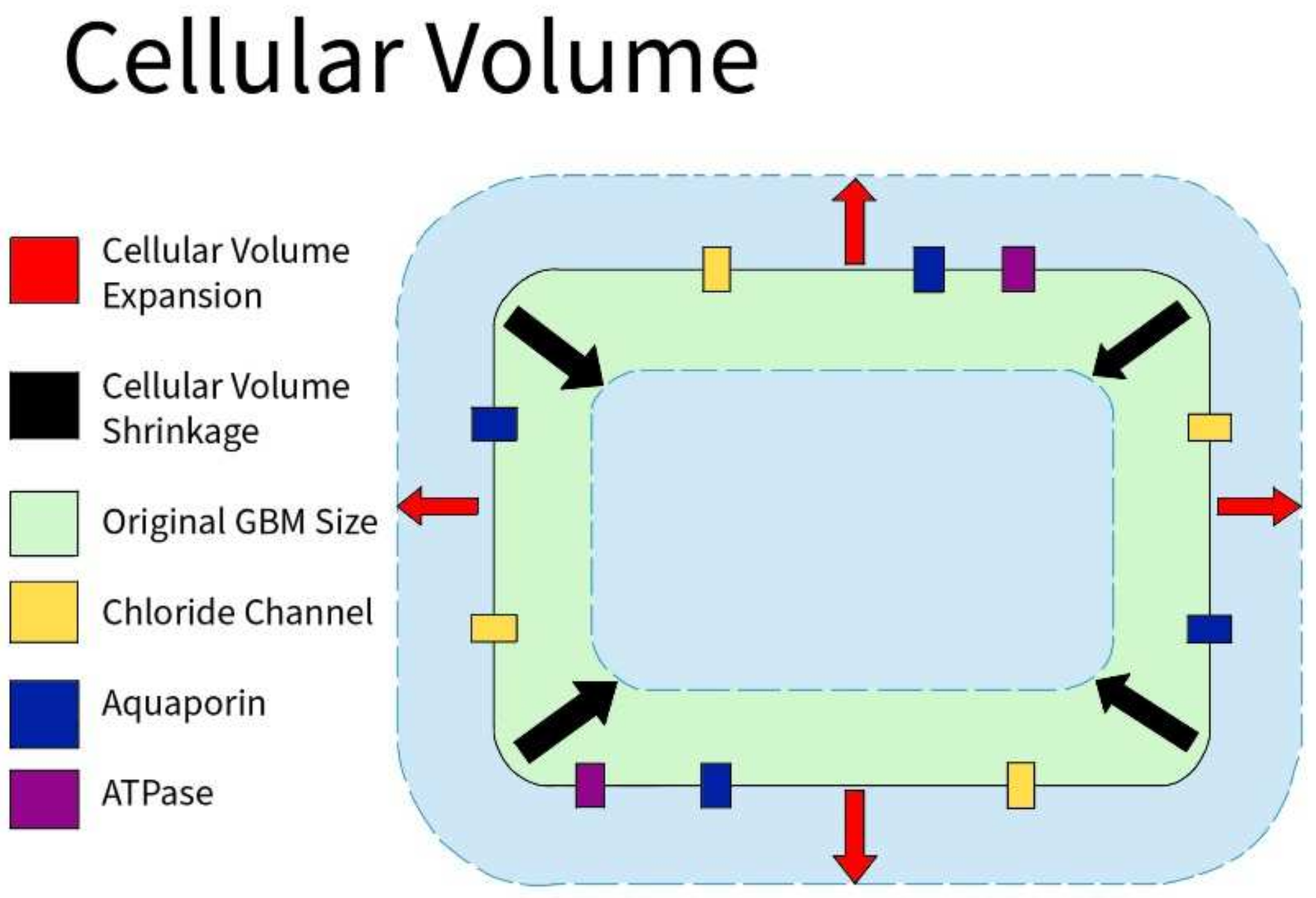
Figure 2. Cellular Volume in GBM. GBM cells express an abundance of chloride ion channels. Along with aquaporin channels and various ATPases, those channels allow the cells to shrink or swell depending on the environment to aid in the survival of GBM cells.
3.3. Hydraulic Conductivity
Hydraulic conductivity refers to the ability to conduct water, or fluids in general. In tumors, blood perfusion is lower than in normal tissue due to the compression of the tumor mass, or due to a higher permeability of vessels [11][12]. Thus, tumors are considered to have a lower hydraulic conductivity than regular tissue. Depending on the cause of the low hydraulic conductivity, vascular normalization occurs because of a decrease in vascular permeability, or vascular decompression to alleviate forces in the tumor [11][12].
3.4. Adhesion Protein Expression
Adhesion proteins are cell membrane proteins that participate in interactions between cells (Figure 3). PIVL, a serine proteinase inhibitor, presents as a monomeric polypeptide chain cross-linked by three disulfide linkages. PIVL has shown the ability to inhibit the adhesion, migration, motility, and invasion of GBM U87 cells. The anti-cancer effect of PIVL is attributed to its (41)RGN(43) motif [13]. Another protein, P311, has also been proven to play a key role in GBM invasion. In human epidermal cells, P311 significantly accelerated cell migration in vitro and enhanced Rho GTPases activity when highly expressed. A RhoA-specific inhibitor and Rac1 inhibitor could both be used to significantly suppress P311-induced human epidermal cells [14].
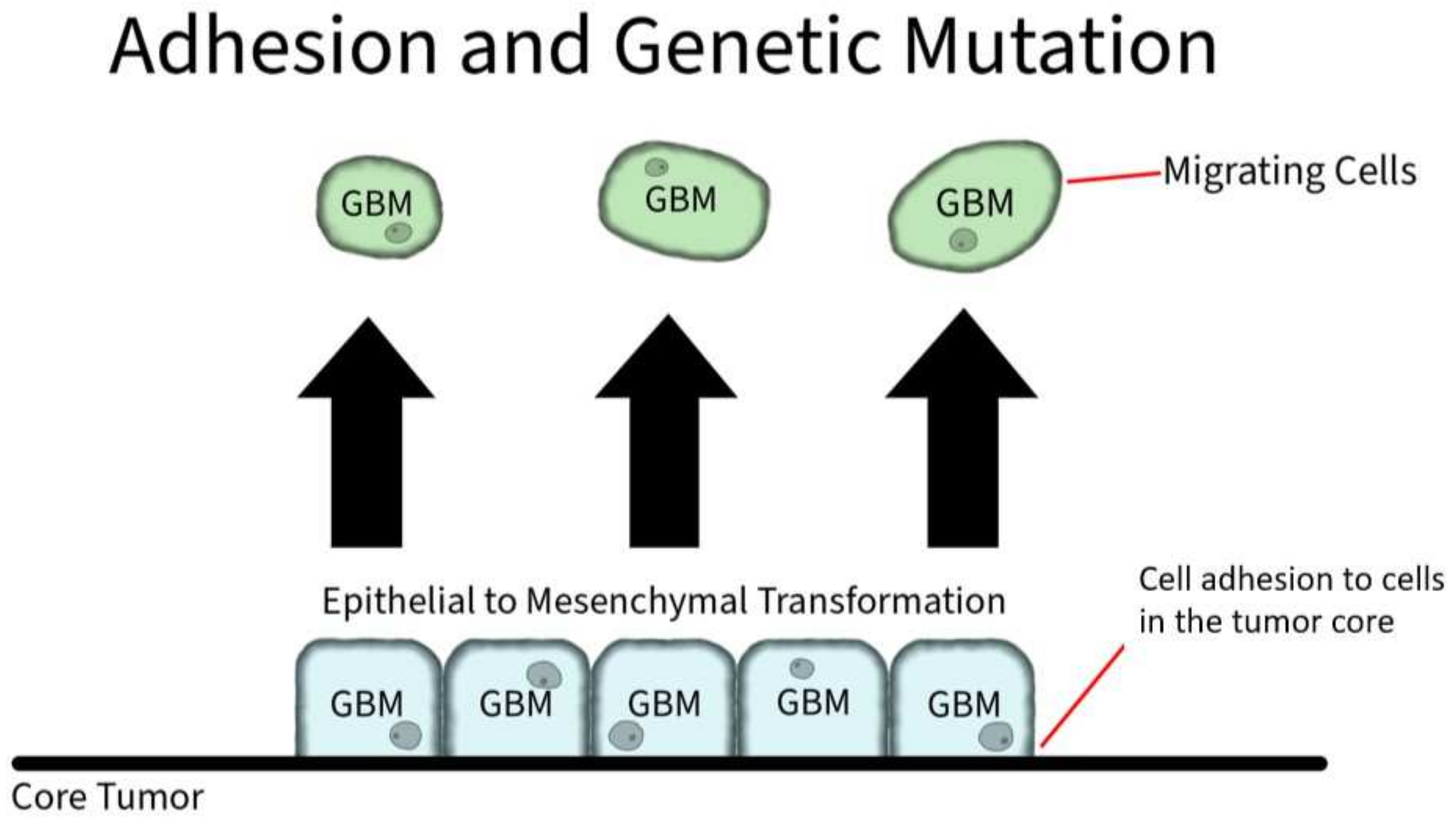
Figure 3. Adhesion and Genetic Mutation in GBM. Cellular membrane proteins play a role in individual GBM cell adhesion to the core tumor. However, through genetic mutation, GBM cells can induce an overexpression of hyaluronic acid, which serves as a ligand for CD-44 receptors. The CD-44 receptors activate SRC complexes that induces a shift to mesenchymal shift in GBM.
4. Major Molecular Mechanisms Associated with Physical Forces in GBM
The researchers have reviewed 11 physical mechanisms involved in GBM aggression, recurrence, migration, and invasion and, in summary, the researchers identified 34 influential molecules and pathways. These molecular influencers were elucidated primarily via the analysis of human cell lines, and included G508, U373 MG, CD133+ GBM cells, U251, U87MG, U87, LN229, HGL21, U343, GL15, U118, and CC2565. The U87 cell lines were used most frequently in the reviewed studies. Three molecular pathways (Piezo/PIEZO1 [5], tenascin C [15], and Talin-1 [16]) were found to be involved in altering GBM stiffness, one (tenascin [11]) was involved in tensile forces, one (PHIP [17]) was involved in traction, four (miR548 [18], caveolin-1, integrin-β1, and Rac1 [19]) were involved in compression, two (PIVL [20] and P311/PTZ17 [13]) was involved in adhesion, ten (swelling-activated chloride current [21], caveolin-1/CAVIN1, UPA, MMPS, AQP1 [22], Snail-1, Snail-2, N-cadherin, Twist, and vimentin [23]) were involved in changes to cellular osmotic pressure, seven (Nestin, vimentin, actin filaments, vinculin, paxillin, and FAK [24]) contributed to shear stress, two (collagen and hyaluronan [25]) contributed to solid stress, and two (HAMLET [12] and swelling-activated chloride currents [26]) were involved in cellular volume changes. Most of the contributing molecular changes were not directly overlapping, though several contributed to more than one physical force: tenascin contributed to both stiffness and tensile forces; swelling-activated chloride channels impacted both changes to cellular osmotic pressure and cellular volume changes; vimentin influenced osmotic pressure and shear stress; hyaluronan impacted solids. The role of multiple molecular pathways influencing single cellular functions highlights GBM redundancy and suggests that the physical forces associated with more molecular pathways are critical to GBM survival. Furthermore, the diversity and variability of molecular changes to GBMs are telling of GBM’s robustness and adaptability.
References
- Spill, F.; Reynolds, D.S.; Kamm, R.D.; Zaman, M.H. Impact of the physical microenvironment on tumor progression and metastasis. Curr. Opin. Biotechnol. 2016, 40, 41–48.
- Shen, Q.; Hill, T.; Cai, X.; Bui, L.; Barakat, R.; Hills, E.; Almugaiteeb, T.; Babu, A.; Mckernan, P.H.; Zalles, M.; et al. Physical confinement during cancer cell migration triggers therapeutic resistance and cancer stem cell-like behavior. Cancer Lett. 2021, 506, 142–151.
- Mierke, C.T. The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells. Rep. Prog. Phys. 2019, 82, 064602.
- Muldoon, L.L.; Alvarez, J.I.; Begley, D.J.; Boado, R.J.; Del Zoppo, G.J.; Doolittle, N.D.; Engelhardt, B.; Hallenbeck, J.M.; Lonser, R.R.; Ohlfest, J.R.; et al. Immunologic privilege in the central nervous system and the blood-brain barrier. J. Cereb. Blood Flow Metab. 2013, 33, 13–21.
- Van Tellingen, O.; Yetkin-Arik, B.; De Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; De Vries, H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updates 2015, 19, 1–12.
- Beckmann, R.; Houben, A.; Tohidnezhad, M.; Kweider, N.; Fragoulis, A.; Wruck, C.J.; Brandenburg, L.O.; Hermanns-Sachweh, B.; Goldring, M.B.; Pufe, T.; et al. Mechanical forces induce changes in VEGF and VEGFR-1/sFlt-1 expression in human chondrocytes. Int. J. Mol. Sci. 2014, 15, 15456–15474.
- Chida, K.; Konno, H.; Sahara, M.; Takase, S. Meningeal seeding of spinal cord glioblastoma multiforme without any signs of myelopathy. Rinsho Shinkeigaku Clin. Neurol. 1995, 35, 1235–1240.
- Knepper, P.A.; McLone, D.G. Glycosaminoglycans and outflow pathways of the eye and brain. Pediatr. Neurosurg. 1985, 12, 240–251.
- Yoo, K.C.; Suh, Y.; An, Y.; Lee, H.; Jeong, Y.J.; Uddin, N.; Cui, Y.; Roh, T.; Shim, J.; Chang, J.H.; et al. Proinvasive extracellular matrix remodeling in tumor microenvironment in response to radiation. Oncogene 2018, 37, 3317–3328.
- Sforna, L.; Cenciarini, M.; Belia, S.; Michelucci, A.; Pessia, M.; Franciolini, F.; Catacuzzeno, L. Hypoxia modulates the swelling-activated cl current in human glioblastoma cells: Role in volume regulation and cell survival. J. Cell Physiol. 2017, 232, 91–100.
- Khan, I.; Bui, L.; Bachoo, R.; Kim, Y.T.; Chuong, C.J. Differences in creep response of GBM cells migrating in confinement. Int. Biomech. 2020, 7, 44–57.
- Stylianopoulos, T.; Jain, R.K. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc. Natl. Acad. Sci. USA 2013, 110, 18632–18637.
- Morjen, M.; Kallech-Ziri, O.; Bazaa, A.; Othman, H.; Mabrouk, K.; Zouari-Kessentini, R.; Sanz, L.; Calvete, J.J.; Srairi-Abid, N.; El Ayeb, M.; et al. PIVL, a new serine protease inhibitor from Macrovipera lebetina transmediterranea venom, impairs motility of human glioblastoma cells. Matrix Biol J. Int. Soc. Matrix Biol. 2013, 32, 52–62.
- Yao, Z.; Li, H.; He, W.; Yang, S.; Zhang, X.; Zhan, R.; Xu, R.; Tan, J.; Zhou, J.; Wu, J.; et al. P311 accelerates skin wound reepithelialization by promoting epidermal stem cell migration through RhoA and Rac1 activation. Stem. Cells Dev. 2017, 26, 451–460.
- Chen, X.; Wanggou, S.; Bodalia, A.; Zhu, M.; Dong, W.; Fan, J.J.; Yin, W.C.; Min, H.; Hu, M.; Draghici, D.; et al. A feedforward mechanism mediated by mechanosensitive ion channel PIEZO1 and tissue mechanics promotes glioma aggression. Neuron 2018, 100, 799–815.
- Miroshnikova, Y.A.; Mouw, J.K.; Barnes, J.M.; Pickup, M.W.; Lakins, J.N.; Kim, Y.; Lobo, K.; Persson, A.I.; Reis, G.F.; McKnight, T.R.; et al. Tissue mechanics promote IDH1-dependent HIF1α-tenascin C feedback to regulate glioblastoma aggression. Nat. Cell Biol. 2016, 18, 1336–1345.
- Barnes, J.M.; Kaushik, S.; Bainer, R.O.; Sa, J.K.; Woods, E.C.; Kai, F.; Przybyla, L.; Lee, M.; Lee, H.W.; Tung, J.C.; et al. A tension-mediated glycocalyx-integrin feedback loop promotes mesenchymal-like glioblastoma. Nat. Cell Biol. 2018, 20, 1203–1214.
- Voutouri, C.; Kirkpatrick, N.D.; Chung, E.; Mpekris, F.; Baish, J.W.; Munn, L.L.; Fukumura, D.; Stylianopoulos, T.; Jain, R.K. Experimental and computational analyses reveal dynamics of tumor vessel cooption and optimal treatment strategies. Proc. Natl. Acad. Sci. USA 2019, 116, 2662–2671.
- Calhoun, M.A.; Cui, Y.; Elliott, E.E.; Mo, X.; Otero, J.J.; Winter, J.O. MicroRNA-mRNA interactions at low levels of compressive solid stress implicate mir-548 in increased glioblastoma cell motility. Sci. Rep. 2020, 10, 311.
- Demou, Z.N. Gene expression profiles in 3D tumor analogs indicate compressive strain differentially enhances metastatic potential. Ann. Biomed. Eng. 2010, 38, 3509–3520.
- Chen, Y.; Hu, Z.; Zhao, D.; Zhou, K.; Huang, Z.; Zhao, W.; Yang, X.; Gao, C.; Cao, Y.; Hsu, Y.; et al. Self-assembled hexagonal superparamagnetic cone structures for fabrication of cell cluster arrays. ACS Appl. Mater. Interfaces 2021, 13, 10667–10673.
- Catacuzzeno, L.; Michelucci, A.; Sforna, L.; Aiello, F.; Sciaccaluga, M.; Fioretti, B.; Castigli, E.; Franciolini, F. Identification of key signaling molecules involved in the activation of the swelling-activated chloride current in human glioblastoma cells. J. Membr. Biol. 2014, 247, 45–55.
- Pu, W.; Qiu, J.; Nassar, Z.D.; Shaw, P.N.; McMahon, K.; Ferguson, C.; Parton, R.G.; Riggins, G.J.; Harris, J.M.; Parat, M. A role for caveola-forming proteins caveolin-1 and CAVIN1 in the pro-invasive response of glioblastoma to osmotic and hydrostatic pressure. J. Cell Mol. Med. 2020, 24, 3724–3738.
- Pu, W.; Qiu, J.; Riggins, G.J.; Parat, M.O. Matrix protease production, epithelial-to-mesenchymal transition marker expression and invasion of glioblastoma cells in response to osmotic or hydrostatic pressure. Sci. Rep. 2020, 10, 2634.
- Ciarletta, P. Buckling instability in growing tumor spheroids. Phys. Rev. Lett. 2013, 110, 158102.
- Fischer, W.; Gustafsson, L.; Mossberg, A.K.; Gronli, J.; Mork, S.; Bjerkvig, R.; Svanborg, C. Human alpha-lactalbumin made lethal to tumor cells (HAMLET) kills human glioblastoma cells in brain xenografts by an apoptosis-like mechanism and prolongs survival. Cancer Res. 2004, 64, 2105–2112.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
534
Revisions:
2 times
(View History)
Update Date:
21 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




