Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joao Batista Fernandes | -- | 2876 | 2022-04-20 15:29:21 | | | |
| 2 | Amina Yu | -17 word(s) | 2859 | 2022-04-21 05:24:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Fernandes, J.B.; Araújo, S.; Seibert, J.; , .; Alcántara-De La Cruz, R.; Pereira, A.; Forim, M.; Silva, M. Leucoagaricus gongylophorus and Leaf-Cutting Ants. Encyclopedia. Available online: https://encyclopedia.pub/entry/22028 (accessed on 07 February 2026).
Fernandes JB, Araújo S, Seibert J, , Alcántara-De La Cruz R, Pereira A, et al. Leucoagaricus gongylophorus and Leaf-Cutting Ants. Encyclopedia. Available at: https://encyclopedia.pub/entry/22028. Accessed February 07, 2026.
Fernandes, Joao Batista, Sean Araújo, Janaina Seibert, , Ricardo Alcántara-De La Cruz, Alana Pereira, Moacir Forim, Maria Silva. "Leucoagaricus gongylophorus and Leaf-Cutting Ants" Encyclopedia, https://encyclopedia.pub/entry/22028 (accessed February 07, 2026).
Fernandes, J.B., Araújo, S., Seibert, J., , ., Alcántara-De La Cruz, R., Pereira, A., Forim, M., & Silva, M. (2022, April 20). Leucoagaricus gongylophorus and Leaf-Cutting Ants. In Encyclopedia. https://encyclopedia.pub/entry/22028
Fernandes, Joao Batista, et al. "Leucoagaricus gongylophorus and Leaf-Cutting Ants." Encyclopedia. Web. 20 April, 2022.
Copy Citation
Leaf-cutting ants are eusocial insects, as they show a highly developed social structure, manifesting ecological relationships. Their complex structure is characterized by an organized social behavior, the cultivation of a fungus garden and high levels of hygiene, which hinders the management of leaf-cutting ants compared to other insects. Leaf-cutting ants cause damage in agricultural and silviculture areas, mainly in monocultures.
pesticide
antifungal activity
chemical control
biological control
natural products
synthetic compound
1. Introduction
Large-scale insect populations that damage crops are considered pests and include Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae), the maize weevil, which impairs the storage of corn grains (Zea mays, Poales: Poaceae) with a large occurrence in Brazil [1]; Spodoptera frugiperda Smith (Lepidoptera: Noctuidae), the fall armyworm, which causes damage to soybean (Glycine max, Fabales: Fabaceae), sorghum (Sorghum bicolor, Poales: Poaceae), sugar cane (Saccharum officinarum, Poales: Poaceae) and so on. and is native to the tropical regions of North and South America [2][3]; Plutella xylostella L. (Lepidoptera: Plutellidae), the diamondback moth, the main pest in the cultivation of cabbage (Brassica oleracea var. capitata, Brassicales: Brassicaceae) and cauliflower (B. oleracea var. botrytis, Brassicales: Brassicaceae), it has an extensive distribution in tropical and subtropical areas [4][5]; and ants of genera Atta and Acromyrmex (Formicidae: Attini), which attack a great diversity of plants [6].
The fungus Leucoagaricus gongylophorus (Möller) Singer (Agaricales, Agaricaceae) is the symbiotic partner of several species of Attine ants, whose development on leaves cuts inside the nests to form the fungus gardens. The fungus produces enzymes that degrade leaf polysaccharides into nutrients assimilable by ants. Among these nutrients, glucose produced from plant material in the fungus garden appears to be the main source of food for ants along with proteins and amino acids [7]. Leaf-cutting ants from the Myrmicinae family, tribe Attini (Hymenoptera: Formicidae), are native to the Neotropics and belong to two genera: Atta and Acromyrmex [6][8][9].
The use of chemical pesticides for insect pest control, often applied without legal supervision, has led to environmental issues such as the death of beneficial insects, parasitoids, and predators, and pesticide residues in the soil [10]. It was to discover active compounds from natural sources have contributed to the identification of new agrochemical substances [11].
The characterization of plant extracts and natural compounds that have shown direct lethal activity on leaf-cutting ants is well explored, such as the extracts of Asclepias curassavica (Asclepidaceae) [12] and Virola sebifera (Myristicaceae) [13] and isolated compounds such as alkaloids, limonoids [14], sesquiterpenes [15], and flavonoids [16].
Another approach to control leaf-cutting ants would be the application of extracts or compounds acting on the symbiotic fungus L. gongylophorus, an important microorganism in the management and survival of the nest [14][16][17]. Based on a mutualistic relationship with the leaf-cutting ants, the control of this insect can be carried out at the insecticide or fungicide level, either individually or by combining these two strategies to provide an efficient integrated control [18][19]. Baits with insecticides such as chlorpyrifos, deltamethrin, fipronil, and permethrin, but mainly sulfluramid, based on citrus pulp on which the mutualistic fungus feed, have been the main tool for the control of leaf-cutting ants [20][21]. Sulfluramid is one of the most successful active ingredients for the control of leaf-cutting ants because it is chemically and physically stable and has a delayed toxic action [22][23]. In addition, these eusocial insects do not detect it as a hazardous material, that is, sulfluramid is not repellent and is distributed by the ants themselves in a large number of fungus garden compartments inside the nest contaminating as many ants as possible when they feed [24]. However, sulfluramid was listed as a persistent organic pollutant at the Stockholm Convention in 2015 [25][26]. Therefore, it is necessary to find alternatives to sulfluramid for the control of leaf-cutting ants, including methods or active compounds that act on the mutualistic fungus; however, this control approach has been little explored and there are no baits with fungicide that act directly on L. gongylophorus.
Products used in pest control, in addition to being effective, should be safe for the environment and non-target organisms. Thus, the use of alternative methods involving natural substances or based on them (synthetic compounds), as well as the use of other microbes with fungicidal action, is convenient. The reports examples of chemical and biological controls that have shown potential against the symbiotic fungus L. gongylophorus and that could be better explored.
1.1. Damage Caused by Leaf-Cutting Ants
Leaf-cutting ants are eusocial insects, as they show a highly developed social structure, manifesting ecological relationships [27]. Their complex structure is characterized by an organized social behavior, the cultivation of a fungus garden and high levels of hygiene, which hinders the management of leaf-cutting ants compared to other insects [23].
Leaf-cutting ants cause damage in agricultural and silviculture areas, mainly in monocultures. Plants aged 1 to 3 years are the main targets of these ants and are defoliated, causing irreversible damage, since the seedlings are new and fragile [28], causing significant yield losses [29][30]. Defoliation affects the growth of plants because the increase in diameter is dependent on the current level of photosynthesis, which is reduced by the loss of leaves [31]. In addition, a lesser effect is observed on height loss, since growth is related to the plant reserves.
One carried out in Pinus taeda plantations in Argentina concluded that there was a reduction of 17.3% in the growth in the neck diameter of plants aged up to 12 months attacked by leaf-cutting ants belonging to the genus Acromyrmex [32]. In another one carried out in 10-year-old Pinus caribaea trees in Venezuela, attacks by Atta laevigata reduced wood production up to 50% ha−1 [33]. In this same way, a level of economic damage between 13.4 and 39.2 m2 ha−1 was observed in a eucalyptus site in areas of the Atlantic Forest after infestation by leaf-cutting ants (Atta sp.) [34]. Similar values (7.02 to 34.86 m2 ha−1) were also observed for the same cultivation in Cerrado, another Brazilian biome [26].
Leaf-cutting ants (Hymenoptera: Formicidae) of the genera Atta and Acromyrmex are the main pests in Brazil, affecting Pinus and Eucalyptus plantations [35]. These leaf-cutting ants removed 20–30% of the total leaf area of fruit, cocoa, and crops of other plants documented in Trinidad and in Guadalupe (Central America) [36], resulting in an annual loss higher than USD 250,000. Measurements of four Eucalyptus stands showed a total of 2327 nests of leaf-cutting ants with 4742.27 m2 of surface area (loose soil). The number of leaf-cutting ant nests and their area per hectare were 64.52 and 238.90 m2, respectively [37]. Between 1991 and 1996, the average total cost of combatting attacks by leaf-cutting ants in a 6-month-old eucalyptus forest was USD 12.60 per ha−1 in Brazil [38]. In 2011, the updated values for the control of this pest in Brazil were around USD 1.23 per ha−1, considering a market value of eucalyptus wood of USD 18.00 per m−3 [34][37].
Due to the vast regions affected by leaf-cutting ants, avoiding the damage they cause requires high control costs. Economic losses from leaf-cutting ants, either through reduced yields or through expenses for their control, run into the billions of dollars worldwide, reaching more than 30% of the total costs spent on forest management [39]. Fenitrothion, fipronil, or sulfluramid are examples of the most common synthetic active ingredients used to combat leaf-cutting ants, but they are banned in some countries [36]. Although other methods have been evaluated, these chemicals are the only ones to show really satisfactory effects for controlling this pest [9][23][40]. Thus, a better understanding of how the structure of leaf-cutting ant nests works as well as the division of tasks between their mutualistic organisms are key points in the search for new targets to combat this pest.
1.2. Biological Relationship between Leaf-Cutting Ants and Their Mutualistic Fungus
The description of a complex microbial environment of leaf-cutting ants, their obligatory association with the basidiomycete species L. gongylophorus, and the maintenance of the fungus gardens is well reported [41][42][43]. Through the symbiotic relationship between leaf-cutting ants and the fungus L. gongylophorus, the microorganism offers enzymes that break down plant tissues and detoxify some compounds that may have insecticidal characteristics so that the ant has access to plant material that would not be possible without the work of the fungus [44][45].
Glucose produced from plant material in the fungus garden seems to be the main food source for leaf-cutting ants of the genus Atta [46]. L. gongylophorus produces glucose from plant material through, for example, starch hydrolysis by fungal extracellular α-amylase and maltase. This is an ongoing process inside the ants’ nest by which this symbiotic fungus contributes to the ant nutrition with starch [47]. Starch and xylan are consumed by L. gongylophorus faster compared to cellulose. This fungus can efficiently hydrolyze these polysaccharides and assimilate xylose, maltose, and glucose in order to make these nutrients available to the ants [48].
L. gongylophorus produces swollen structures at the tip of the hypha called gongylidia. A grouping of gongylidia forms staphylae, which provide food for the ants and their larvae [49]. The process takes place by the harvesting of a staphylum or bundle of hyphae by the workers and placing it directly in the larval mouthparts, or the workers handle the staphylae or hyphae until they reach the ideal consistency and then deposit them in the larval mouthparts [50]. Staphylae or hyphae are the main sources of nutrients for immature ants. Larvae need a high protein intake for growth, which can only be provided by L. gongylophorus [51]; when they are fed an artificial staphylae-based diet they gain more weight [52]. Larvae of Atta cephalotes obtain 100% of their feed from staphylae, but workers obtain only 4.8% of their respiratory energy needs from this source, while the rest of their needs are presumably supplied by the sap of the plant [52]. In other words, the fungus is not the greatest source of food for workers, since they take up nutrients while harvesting plant material [53].
Workers do not digest many of the fungal enzymes when they consume gongylidia, but they deposit these enzymes in the upper parts of the fungus garden through the fecal fluid of the chewed plant material, thus providing adequate environmental conditions to nourish their fungal partners [54][55]. This allows the cultivation by ants to surpass other microbes present in the garden. For example, ants are generally known to eliminate foreign microbes within their gardens by producing antibiotics in their metapleural and mandibular glands [56][57], such as 3-hydroxydecanoic (myrmicacin) and indoleacetic acid, produced from the genera Atta and Acromyrmex [58]. A schematic summary of the relationship between leaf-cutting ants and their symbiotic fungus L. gongylophorus is shown in Figure 1.
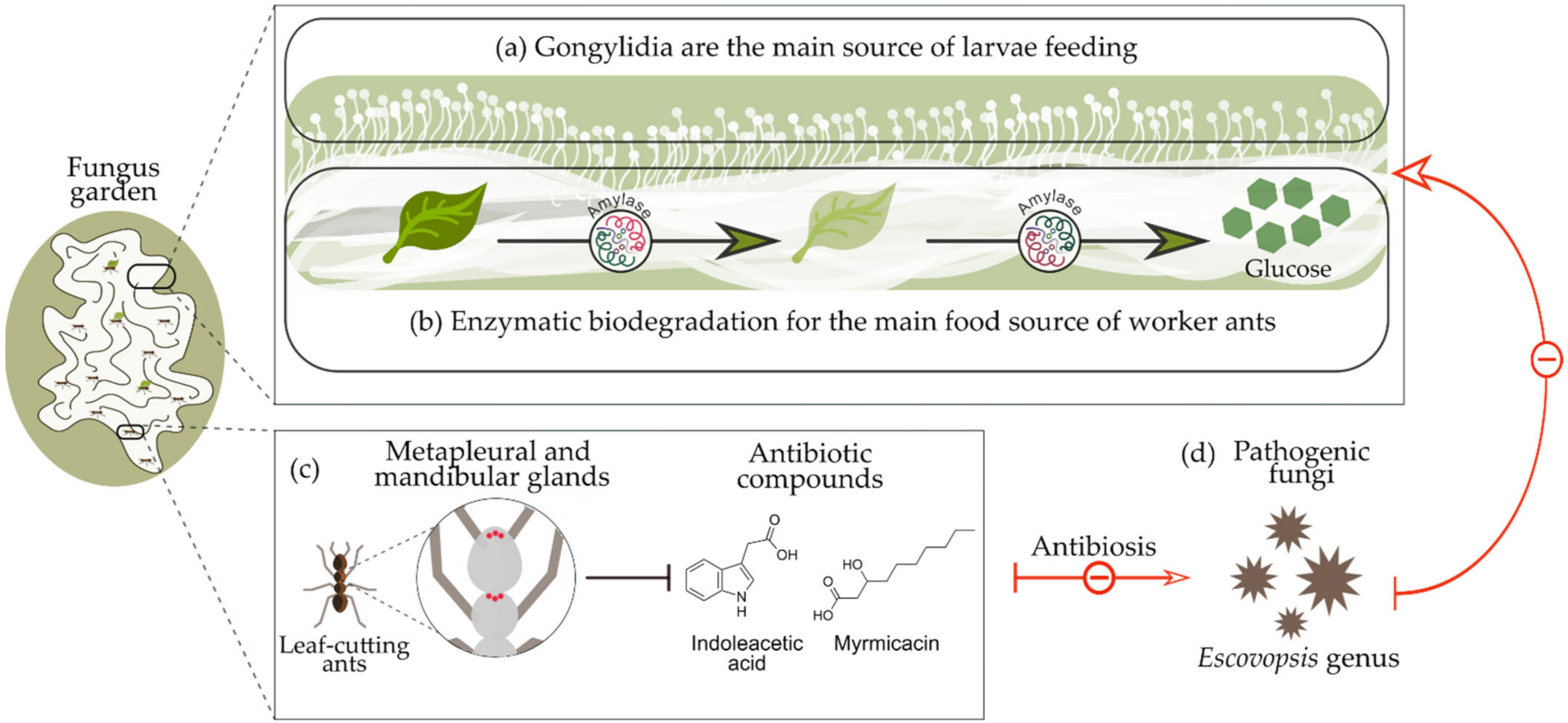
Figure 1. Symbiotic relationship between leaf-cutting ants and Leucoagaricus gongylophorus symbiotic fungus: (a) Gongylidia produced in the fungus garden are the main source of food for the ant larvae; (b) fungus enzymes such as amylase are responsible for the biodegradation of organic material into glucose, the main food source for the worker ants; (c) leaf-cutting ants produce antibiotic compounds that protect the fungus garden against harmful agents; (d) pathogenic microorganisms such Escovopsis fungal genera.
2. Fungal Control
Different compounds have been known as novel alternatives for the control of leaf-cutting ants, since commercially available products, generally synthetics, have disadvantages such as side effects towards non-target organisms and environmental contamination. Searches directed at Brazilian Forest Stewardship Council-certified forestry companies showed that chemical control (82%) is the most important method for pest management, followed by biological control (71.4%), tree resistance (54%), cultural control (37.5%), and mechanical control (34.5%) [59]. Based on this, a comparison between the main chemical and biological controls with effects on the fungus garden was carried out in order to propose new agents to replace the commercially synthetic available products.
“Leaf-cutting ant *”, “fungus growing ant *”, and “leaf cutter ant *” were used as query keywords in the PubMed and Web of Science databases. The use of the symbol “*” in the databases is not to limit the search only to the characters of keywords, but to search for words with similar spellings. Those published through 31 August 2020 and written in the English, Portuguese, and Spanish languages were selected. The total search found 1929 (1924 in English, 1 in Portuguese, and 4 in Spanish); after a complete reading of the abstracts, only 26 were included. Those related to chemical characterization analysis only were excluded. Extracts and isolated and synthetic compounds as well as microorganisms reported in trials against L. gongylophorus were tabulated and classified according to the type of control. The selected materials were considered active according to the methodology applied in each.
2.1. Chemical Control
2.1.1. Natural Compound
The chemical complexity of natural compounds is an important source in the search for new antifungal agents; however, some naturally occurring structures can be difficult to produce synthetically [60], but they can be used as sources of semi-synthetic compounds [61]. In addition, natural compounds can be less dangerous and persistent, which can lessen the risks compared to conventional products used in crop pest control [62]. These qualities, added to the antimicrobial potential presented by essential oils and plant extracts, reinforce the promise of these products as alternatives for agricultural use. In this sense, several works have already demonstrated the potential of natural compounds as fungicidal agents on the symbiotic microorganism of leaf-cutting ants. Although all the studies reported here were based on in vitro methods, the results are promising and the data can be used for future trials in in vivo systems to confirm the effect on leaf-cutting ant control.
2.1.2. Synthetic Compounds
Although the chemical variety of natural products is extensive, synthetic compounds have advantages related to purity as well as the ease of large-scale production [63]. Various synthetic products showed toxic effects against L. gongylophorus, including fatty acids with 6–12 carbons [61]. Although no differences in activity were observed among them, the inhibition of fungal growth can vary according to the number of carbon atoms: the longer the chain, the greater the biological effect. This observation can be proven from the synthesis of piperonyl compounds, since an increase in the length of the side chain from two to eight carbons reduced the concentration effective against the symbiotic fungus almost 10-fold. However, this relationship is not continuous, as a reduction in antifungal activity was observed for side chains with more than 10 carbons [64].
2.2. Biological Control Using Microorganisms
As previously discussed, leaf-cutting ants live in symbiosis with other microorganisms that assist in their feeding and protection. However, drastic changes in the environment in which they live can affect the balance of the nest, favoring the development of other invading organisms [65]. Among them, the species of the genera Escovopsis [65][66][67] and Trichoderma [65][68][69] can be highlighted as the main threats. Escovopsioides nivea is also effective in reducing fungal mycelium development [66], and lower values of inhibition were demonstrated for Acremonium kiliense [65] and Gliocladium sp. [69] species. However, Syncephalastrum sp. showed a harmful effect on sub-colonies regardless of the occurrence of the disturbance to the ants’ nest, suggesting that it may be susceptible to attack by pathogenic microorganisms even under normal conditions (healthy sub-colonies) [70].
When the nest is under attack by invading microorganisms, there is a reduction in biomass in the fungus garden since they consume the nutritional content of L. gongylophorus [65]. Inhibitory effect on the fungus garden shown by A. kiliense is related to this nutritional competition [65]. The degradation of the symbiotic fungus produces substances that can also be used as a food source for the species of Escovopsis and Trichoderma, indicating a necrotrophic relationship between them [65]. A combination of mechanisms of action can also be found for the same microorganism, such as Gliocladium, which both inhibits development and competes for nutrients [69]. Other mechanisms of action, such as the production of substances with antibiotic potential or that degrade the cell wall, may also be involved in the antifungal control [68].
Different microorganism strains, fungi, or bacteria may have different mechanisms on L. gongylophorus since the genetic homogeneity of this mutualistic fungus restricts its capacity for defense against different organisms [67]. This can be observed in the increase in the percentage of mycelium growth inhibition from 43 to 78% after the attack by Escovopsis strains [65][66][67]. Trichoderma species also showed large divergences (1–75%) in inhibition of the growth of L. gongylophorus [65][68][69].
Although L. gongylophorus is susceptible to other microorganisms, their direct application in the field would not be viable since this effect would probably be lost over time. This is because leaf-cutting ants exhibit highly specialized hygiene behavior that allows the creation of barriers to invasion by other microorganisms as well as being able to eliminate them [65][66][67][70]. However, microorganisms have primary and secondary metabolisms similar to those of plants; therefore, they can be considered a rich source of active substances for chemical control. As examples, the lactones antimycins A1–A4 (N5–N8), obtained from Streptomyces sp. [71], and extracts of E. nivea and Escovopsis [66] suppress the growth of L. gongylophorus.
Conventional biological control methods using the direct application of microorganisms in the environment do not seem to be the ideal way to combat this crop pest due to the hygienic behavior of the leaf-cutting ants. Thus, metabolomics research could be an alternative in the search for novel compounds active against L. gongylophorus. On the other hand, pathogenic fungi have already shown insecticidal action better than that of chemical controls, suggesting that the application of a biological control on leaf-cutting ants may be possible and applicable [72]. Ideally, pathogenic fungi would act faster than the ability of leaf-cutting ants to eliminate them, and that would have an action specific to these insects; so, microorganisms that avoid any environmental imbalance would be preferable for biological control. Little is known about how these organisms interact in the fungus garden, and further are needed into the mechanism of the antifungal action.
References
- Zunino, M.P.; Herrera, J.M.; Pizzolitto, R.P.; Rubinstein, H.R.; Zygadlo, J.A.; Dambolena, J.S. Effect of selected volatiles on two stored pests: The fungus Fusarium verticillioides and the maize weevil Sithophilus zeamais. J. Agric. Food Chem. 2015, 63, 7743–7749.
- Ramalho, S.R.; Bezerra, C.S.; Lourenço de Oliveira, D.G.; Souza Lima, L.; Maria Neto, S.; Ramalho de Oliveira, C.F.; Verbisck, N.V.; Rodrigues Macedo, M.L. Novel peptidase kunitz inhibitor from Platypodium elegans seeds is active against Spodoptera frugiperda larvae. J. Agric. Food Chem. 2018, 66, 1349–1358.
- Yang, X.M.; Song, Y.F.; Sun, X.X.; Shen, X.J.; Wu, Q.L.; Zhang, H.W.; Zhang, D.D.; Zhao, S.Y.; Liang, G.M.; Wu, K.M. Population occurrence of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), in the winter season of China. J. Integr. Agric. 2021, 20, 772–782.
- Reddy, G.V.P.; Guerrero, A. Behavioral responses of the diamondback moth, Plutella xylostella, to green leaf volatiles of Brassica oleracea subsp. capitata. J. Agric. Food Chem. 2000, 48, 6025–6029.
- Chapman, J.W.; Reynolds, D.R.; Smith, A.D.; Riley, J.R.; Pedgley, D.E.; Woiwod, I.P. High-altitude migration of the diamondback moth Plutella xylostella to the U.K.: A study using radar, aerial netting, and ground trapping. Ecol. Entomol. 2002, 27, 641–650.
- Ward, P.S.; Brady, S.G.; Fisher, B.L.; Schultz, T.R. The evolution of myrmicine ants: Phylogeny and biogeography of a hyperdiverse ant clade (Hymenoptera: Formicidae). Syst. Entomol. 2014, 40, 61–81.
- Mehdiabadi, N.J.; Schultz, T.R. Natural history and phylogeny of the fungus-farming ants (Hymenoptera: Formicidae: Myrmicinae: Attini). Myrmecol. News 2010, 13, 37–55.
- Pagnocca, F.; Da Silva, O.; Hebling-Beraldo, M.; Bueno, O.; Fernandes, J.B.; Vieira, P.C. Toxicity of sesame extracts to the symbiotic fungus of leaf-cutting ants. Bull. Entomol. Res. 1990, 80, 349–352.
- Zanetti, R.; Zanuncio, J.; Santos, J.; Da Silva, W.; Ribeiro, G.; Lemes, P. An overview of integrated management of leaf-cutting ants (Hymenoptera: Formicidae) in Brazilian forest plantations. Forests 2014, 5, 439–454.
- Forim, M.R.; Costa, E.S.; da Silva, M.F.G.F.; Fernandes, J.B.; Mondego, J.M.; Boiça Junior, A.L. Development of a new method to prepare nano-/microparticles loaded with extracts of Azadirachta indica, their characterization and use in controlling Plutella xylostella. J. Agric. Food Chem. 2013, 61, 9131–9139.
- Song, X.; Zhu, X.; Li, T.; Liang, C.; Zhang, M.; Yu, S.; Tao, J.; Sun, R. Dehydrozingerone inspired discovery of potential broad-spectrum fungicidal agents as ergosterol biosynthesis inhibitors. J. Agric. Food Chem. 2019, 67, 11354–11363.
- Ramos, V.M.; Ferreira Leite, R.G.; Almeida, V.T.; Camargo, R.S.; Souza Cruz, J.V.; Leão, R.M.; Prado, M.V.; Sousa Pereira, M.C. Bioactivity of Asclepias curassavica, equisetum spp. and Rosmarinus officinalis extracts against leaf-cutting ants. Sociobioly 2019, 66, 536–544.
- Bicalho, K.U.; Terezan, A.P.; Martins, D.C.; Freitas, T.G.; Fernandes, J.B.; Silva, M.F.G.F.; Vieira, P.C.; Pagnocca, F.C.; Bueno, O.C. Evaluation of the toxicity of Virola sebifera crude extracts, fractions and isolated compounds on the nest of leaf-cutting ants. Psyche J. Entom. 2011, 2012, 785424.
- Terezan, A.P.; Rossi, R.A.; Almeida, R.N.A.; Freitas, T.G.; Vieira, P.C.; Fernandes, J.B.; Silva, M.F.G.F.; Bueno, O.C.; Pagnocca, F.C.; Pirani, J.R. Activities of extracts and compounds from Spiranthera odoratissima St. Hil. (Rutaceae) in leaf-cutting ants and their symbiotic fungus. J. Braz. Chem. Soc. 2010, 21, 882–886.
- Marsaro, A.L.; Souza, R.C.; Della Lucia, T.M.C.; Vieira, P.C.; Fernandes, J.B.; Silva, M.F.G.F. Behavioral changes in workers of the leaf-cutting ant Atta sexdens rubropilosa induced by chemical components of Eucalyptus maculata leaves. J. Chem. Ecol. 2004, 30, 1771–1780.
- Almeida, R.; Peñaflor, M.; Simote, S.; Bueno, O.; Hebling, M.J.A.; Pagnocca, F.; Fernandes, J.B.; Vieira, P.C.; Silva, M.F.G.F. Toxicity of substances isolated from Helietta puberula RE Fr. (Rutaceae) to the Leaf-cutting Ant Atta sexdens L. (Hymenoptera: Formicidae) and the Symbiotic Fungus Leucoagaricus gongylophorus (Singer) Möller. BioAssay 2009, 2.
- Biavatti, M.W.; Vieira, P.C.; Silva, M.F.G.F.; Fernandes, J.B.; Victor, S.R.; Pagnocca, F.C.; Albuquerque, S.; Caracelli, I.; Zukerman-Schpector, J. Biological activity of quinoline alkaloids from Raulinoa echinata and X-ray structure of flindersiamine. J. Braz. Chem. Soc. 2002, 13, 66–70.
- Boulogne, I.; Petit, P.; Ozier-Lafontaine, H.; Desfontaines, L.; Loranger-Merciris, G. Insecticidal and antifungal chemicals produced by plants: A review. Environ. Chem. Lett. 2012, 10, 325–347.
- Boulogne, I.; Ozier-Lafontaine, H.; Germosén-Robineau, L.; Desfontaines, L.; Loranger-Merciris, G. Acromyrmex octospinosus (Hymenoptera: Formicidae) Management: Effects of TRAMILs fungicidal plant extracts. J. Econ. Entomol. 2012, 105, 1224–1233.
- Vinha, G.L.; Alcántara-de la Cruz, R.; Della Lucia, T.M.C.; Wilcken, C.F.; da Silva, E.D.; Lemes, P.G.; Zanuncio, J.C. Leaf-cutting ants in commercial forest plantations of Brazil: Biological aspects and control methods. South. For. J. For. Sci. 2020, 82, 95–103.
- Cherrett, J.M. Fire Ants and Leaf-Cutting Ants; CRC Press: New York, NY, USA, 2019; pp. 357–368.
- Forti, L.C.; Pretto, D.R.; Nagamoto, N.S.; Padovani, C.R.; Camargo, R.S.; Andrade, A.P.P. Dispersal of the delayed action insecticide sulfluramid in nests of the leaf-cutting ant Atta sexdens rubropilosa (Hymenoptera: Formicidae). Sociobiology 2007, 50, 1149–1163.
- Della Lucia, T.M.C.; Gandra, L.C.; Guedes, R.N.C. Managing leaf-cutting ants: Peculiarities, trends and challenges. Pest Manag. Sci. 2014, 70, 14–23.
- Rust, M.K.; Reierson, D.A.; Klotz, J.H. Delayed toxicity as a critical factor in the efficacy of aqueous baits for controlling Argentine ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2004, 97, 1017–1024.
- Gilljam, J.L.; Leonel, J.; Cousins, I.T.; Benskin, J.P. Additions and correction to is ongoing sulfluramid use in South America a significant soure of perfluorooctanesulfonate (PFOS)? Production Inventories, environmental fate, and local occurance. Environ. Sci. Technol. 2016, 50, 653–659.
- Nascimento, R.A.; Nunoo, D.B.O.; Bizkarguenaga, E.; Schultes, L.; Zabaleta, I.; Benskin, J.P.; Spanó, S.; Leonel, J. Sulfluramid use in Brazilian agriculture: A source of per-and polyfluoroalkyl substances (PFASs) to the environment. Environ. Pollut. 2018, 242, 1436–1443.
- Toledo, M.A.; Ribeiro, P.L.; Carrossoni, P.S.F.; Tomotani, J.V.; Hoffman, A.N.; Klebaner, D.; Watel, H.R.; Iannini, C.A.N.; Helene, A.F. Two castes sizes of leafcutter ants in task partitioning in foraging activity. Cienc. Rural 2016, 46, 1902–1908.
- Vasconcelos, H.L.; Cherrett, J.M. Leaf-cutting ants and early forest regeneration in central Amazonia: Effects of herbivory on tree seedling establishment. J. Trop. Ecol. 1997, 13, 357–370.
- Zanetti, R.; Zanuncio, J.C.; Mayhé-Nunes, A.J.; Medeiros, A.G.B.; Souza-Silva, A. Combate sistemático de formigas-cortadeiras com iscas granuladas, em eucaliptais com cultivo mínimo. Rev. Arvore 2003, 27, 387–392.
- Zanetti, R.; Zanuncio, J.C.; Souza-Silva, A.; Abreu, L.G. Eficiência de isca formicida aplicada sobre o monte de terra solta de ninhos de Atta sexdens rubropilosa (Hymenoptera: Formicidae). Rev. Arvore 2003, 27, 407–410.
- Matrangolo, C.A.R.; Castro, R.V.O.; Della Lucia, T.M.C.; Della Lucia, R.M.; Mendes, A.F.N.; Costa, J.M.F.N.; Leite, H.G. Crescimento de eucalipto sob efeito de desfolhamento artificial. Pesqui. Agropecu. Bras. 2010, 45, 952–957.
- Cantarelli, E.B.; Costa, E.C.; Pezzutti, R.; Oliveira, L.S. Quantificação das perdas no desenvolvimento de Pinus taeda após o ataque de formigas cortadeiras. Ciência Florestal 2008, 18, 39–45.
- Hernández, J.V.; Jaffé, K. Dano econômico causado por populações de formigas Atta laevigata (F. Smith) em plantações de Pinus caribaea Mor. e elementos para o manejo da praga. An. Soc. Entomol. Bras. 1995, 24, 287–298.
- Souza, A.; Zanetti, R.; Calegario, N. Economic damage level for leaf-cutting ants in function of the productivity index of eucapyptus plantations in an Atlantic Forest region. Neotrop. Entomol. 2011, 40, 483–488.
- Zanetti, R.; Zanuncio, J.C.; Vilela, E.F.; Leite, H.G.; Jaffé, K.; Oliveira, A.C. Level of economic damage for leaf-cutting ants (Hymenoptera: Formicidae) in Eucalyptus plantations in Brazil. Sociobioly 2003, 42, 433–444.
- Zanuncio, J.C.; Lemes, P.G.; Antunes, L.R.; Maia, J.L.S.; Mendes, J.E.P.; Tanganelli, K.M.; Salvador, J.F.; Serrão, J.E. The impact of the Forest Stewardship Council (FSC) pesticide policy on the management of leaf-cutting ants and termites in certified forests in Brazil. Ann. For. Sci. 2016, 73, 205–214.
- Boulogne, I.; Ozier-Lafontaine, G.; Loranger-Merciris, G. Leaf cutting ants, biology and control. Sustain. Agric. Rev. 2015, 13, 450–455.
- Dos Santos, A.; Santos, I.C.L.; Da Silva, N.; Zanetti, R.; Oumar, Z.; Guimarães, L.F.R.; De Camargo, M.B.; Zanuncio, J.C. Mapping defoliation by leaf-cutting ants Atta species in Eucalyptus plantations using the Sentinel-2 sensor. Int. J. Remote Sens. 2020, 41, 1542–1554.
- Zanetti, R.; Zanuncio, J.C.; Vilela, E.F.; Leite, H.G.; Della Lucia, T.M.C.; Couto, L. Efeito da espécie de eucalipto e da vegetação nativa circundante sobre o custo de combate a sauveiros em eucaliptais. Rev. Arvore 1999, 23, 321–325.
- Montoya-Lerma, J.; Giraldo-Echeverri, C.; Armbrecht, I.; Farji-Brener, A.; Calle, Z. Leaf-cutting ants revisited: Towards rational management and control. Int. J. Pest Manag. 2012, 58, 225–247.
- Biedermann, P.H.; Rohlfs, M. Evolutionary feedbacks between insect sociality and microbial management. Curr. Opin. Insect Sci. 2017, 22, 92–100.
- De Fine Licht, H.H.; Schiøtt, M.; Rogowska-Wrzesinska, A.; Nygaard, S.; Roepstorff, P.; Boomsma, J.J. Laccase detoxification mediates the nutritional alliance between leaf-cutting ants and fungus-garden symbionts. Proc. Natl. Acad. Sci. USA 2013, 110, 583–587.
- Goes, A.C.; Barcoto, M.O.; Kooij, P.W.; Bueno, O.C.; Rodrigues, A. How Do Leaf-Cutting Ants Recognize Antagonistic Microbes in Their Fungal Crops? Front. Ecol. Evol. 2020, 8, 95.
- Bacci, M., Jr.; Bueno, O.C.; Rodrigues, A.; Pagnocca, F.C.; Somera, A.F.; Silva, A. A metabolic pathway assembled by enzyme selection may support herbivory of leaf-cutter ants on plant starch. J. Insect Physiol. 2013, 59, 525–531.
- Aylward, F.O.; Burnum-Johnson, K.E.; Tringe, S.G.; Teiling, C.; Tremmel, D.M.; Moeller, J.A.; Scott, J.J.; Barry, K.W.; Piehowski, P.D.; Nicora, C.D.; et al. Leucoagaricus gongylophorus produces diverse enzymes for the degradation of recalcitrant plant polymers in leaf-cutter ant fungus gardens. Appl. Environ. Microbiol. 2013, 79, 3770–3778.
- Silva, A.; Bacci, M.; Siqueira, C.G.; Bueno, O.C.; Pagnocca, F.C.; Hebling, M.J.A. Survival of Atta sexdens on different food sources. J. Insect Physiol. 2003, 49, 307–313.
- Silva, A.; Bacci, M.; Bueno, O.C.; Pagnocca, F.C.; Hebling, M.J.A. Starch metabolism in Leucoagaricus gongylophorus, the symbiotic fungus of leaf-cutting ants. Microbiol. Res. 2006, 161, 299–303.
- Khadempour, L.; Burnum-Johnson, K.E.; Baker, E.S.; Nicora, C.D.; Webb-Robertson, B.-J.M.; White, R.A.; Monroe, M.E.; Huang, E.L.; Smith, R.D.; Currie, C.R. The fungal cultivar of leaf-cutter ants produces specific enzymes in response to different plant substrates. Mol. Ecol. 2016, 25, 5795–5805.
- Aylward, F.O.; Burnum, K.E.; Scott, J.J.; Suen, G.; Tringe, S.G.; Adams, S.M.; Barry, K.W.; Nicora, C.D.; Piehowski, P.D.; Purvine, S.O.; et al. Metagenomic and metaproteomic insights into bacterial communities in leaf-cutter ant fungus gardens. ISME J. 2012, 6, 1688–1701.
- Mikheyev, A.S.; Mueller, U.G.; Abbot, P. Comparative Dating of Attine Ant and Lepiotaceous Cultivar Phylogenies Reveals Coevolutionary Synchrony and Discord. Am. Nat. 2010, 175, E126–E133.
- Erthal, M.; Peres Silva, C.; Ian Samuels, R. Digestive enzymes in larvae of the leaf cutting ant, Acromyrmex subterraneus (Hymenoptera: Formicidae: Attini). J. Insect Physiol. 2007, 53, 1101–1111.
- Bass, M.; Cherret, J.M. Fungal hyphae as a source of nutrients for the leaf-cutting ant Atta sexdens. Physiol. Entomol. 1995, 20, 1–6.
- Ford, L.C.; Andrade, A.P.P. Ingestão de líquidos por Atta sexdens (L.) (Hymenoptera, Formicidae) durante a atividade forrageira e na preparação do substrato em condições de laboratório. Naturalia 1999, 24, 61–63.
- De Fine Licht, H.H.; Boomsma, J.; Tunlid, A. Symbiotic adaptations in the fungal cultivar of leaf-cutting ants. Nat. Commun. 2014, 5, 5675.
- Bizarria, R., Jr.; Kooij, P.W.; Rodrigues, A. Climate Change Influences Basidiome Emergence of Leaf-Cutting Ant Cultivars. J. Fungi 2021, 7, 912.
- Rodrigues, A.; Carletti, C.D.; Bueno, O.C.; Pagnocca, F.C. Leaf-cutting ant faecal fluid and mandibular gland secretion: Effects on microfungi spore germination. Braz. J. Microbiol. 2008, 39, 64–67.
- Fernández-Marín, H.; Zimmerman, J.K.; Rehner, S.A.; Wcislo, W.T. Active use of the metapleural glands by ants in controlling fungal infection. Proc. Biol. Sci. 2006, 273, 1689–1695.
- Currie, C.R. A Community of ants, fungi, and bacteria: A multilateral aproach to studying symbiosis. Annu. Rev. Microbiol. 2001, 55, 357–380.
- Lemes, P.G.; Zanuncio, J.C.; Serrão, J.E.; Lawson, S.A. Forest stewardship council (FSC) pesticide policy and integrated pest management in certified tropical plantations. Environ. Sci. Pollut. Res. 2017, 24, 1283–1295.
- Aldholmi, M.; Marchand, P.; Ourliac-Garnier, I.; Le Pape, P.; Ganesan, A. A decade of antifungal leads from natural products: 2010–2019. Pharmaceuticals 2019, 12, 182.
- Katiyar, C.; Gupta, A.; Kanjilal, S.; Katiyar, S. Drug discovery from plant sources: An integrated approach. Ayu 2012, 33, 10–19.
- Peñaflor, M.F.G.V.; Victor, S.R.; Bueno, O.C.; Hebling, M.J.A.; Pagnocca, F.C.; Leita, A.C.; Fernandes, J.B.; Vieira, P.C.; da Silva, M.F.G.F. Toxicity of straight-chain fatty acids to leaf-cutting ants Atta sexdens rubropilosa (Hymenoptera: Formicidae) and the symbiotic fungus Leucoagaricus gongylophorus. Sociobiology 2006, 47, 843–858.
- Fernandes, C.M.; Da Silva, D.; Haranahalli, K.; McCarthy, J.B.; Mallamo, J.; Ojima, I.; Poeta, M. The future of antifungal drug therapy: Novel compounds and targets. Antimicrob. Agents Chemother. 2020, 65, e01719-20.
- Victor, S.R.; Crisóstomo, F.R.; Bueno, F.C.; Pagnocca, F.C.; Fernandes, J.B.; Correa, A.G.; Bueno, O.C.; Hebling, M.J.A.; Bacci, M.; Vieira, P.C.; et al. Toxicity of synthetic piperonyl compounds to leaf-cutting ants and their symbiotic fungus. Pest. Manag. Sci. 2001, 57, 603–608.
- Silva, A.; Rodrigues, A.; Bacci, M.; Pagnocca, F.C.; Bueno, O.C. Susceptibility of the ant-cultivated fungus Leucoagaricus gongylophorus (Agaricales: Basidiomycota) towards microfungi. Mycopathologia 2006, 162, 115–119.
- Varanda-Haifig, S.S.; Albarici, T.R.; Nunes, P.H.; Haifig, I.; Vieira, P.C.; Rodrigues, A. Nature of the interactions between hypocrealean fungi and the mutualistic fungus of leaf-cutter ants. Antonie Van Leeuwenhoek 2017, 110, 593–605.
- Wallace, D.E.E.; Asensio, J.G.V.; Tomás, A.A.P. Correlation between virulence and genetic structure of Escovopsis strains from leaf-cutting ant colonies in Costa Rica. Microbiology 2014, 160, 1727–1736.
- Castrillo, M.L.; Bich, G.A.; Zapata, P.D.; Villalba, L.L. Biocontrol of Leucoagaricus gongylophorus of leaf-cutting ants with the mycoparasitic agent Trichoderma koningiopsis. Mycosphere 2016, 7, 810–819.
- Ortiz, A.; Orduz, S. In vitro evaluation of Trichoderma and Gliocladium antagonism against the symbiotic fungus of the leaf-cutting ant Atta cephalotes. Mycopathologia 2001, 150, 53–60.
- Barcoto, M.O.; Pedrosa, F.; Bueno, O.C.; Rodrigues, A. Pathogenic nature of Syncephalastrum in Atta sexdens rubropilosa fungus gardens. Pest. Manag. Sci. 2017, 73, 999–1009.
- Schoenian, I.; Spiteller, M.; Ghaste, M.; Wirth, R.; Herz, H.; Spiteller, D. Chemical basis of the synergism and antagonism in microbial communities in the nests of leaf-cutting ants. Proc. Natl. Acad. Sci. USA 2011, 108, 1955–1960.
- Lopez, E.; Orduz, S. Metarhizium anisopliae and Trichoderma viride for control of nests of the fungus-growing ant, Atta cephalotes. Biol. Control. 2003, 27, 194–200.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
2 times
(View History)
Update Date:
21 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




