Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sergio Bydlowski | -- | 4341 | 2022-04-20 14:53:12 | | | |
| 2 | Peter Tang | Meta information modification | 4341 | 2022-04-21 03:51:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bydlowski, S.; De Freitas, F.; Levy, D.; Reichert, C.; , . Effects of Oxysterols on Immune Cells. Encyclopedia. Available online: https://encyclopedia.pub/entry/22026 (accessed on 07 February 2026).
Bydlowski S, De Freitas F, Levy D, Reichert C, . Effects of Oxysterols on Immune Cells. Encyclopedia. Available at: https://encyclopedia.pub/entry/22026. Accessed February 07, 2026.
Bydlowski, Sergio, Fabio De Freitas, Debora Levy, Cadiele Reichert, . "Effects of Oxysterols on Immune Cells" Encyclopedia, https://encyclopedia.pub/entry/22026 (accessed February 07, 2026).
Bydlowski, S., De Freitas, F., Levy, D., Reichert, C., & , . (2022, April 20). Effects of Oxysterols on Immune Cells. In Encyclopedia. https://encyclopedia.pub/entry/22026
Bydlowski, Sergio, et al. "Effects of Oxysterols on Immune Cells." Encyclopedia. Web. 20 April, 2022.
Copy Citation
Oxysterols are the products of cholesterol oxidation. They have a wide range of effects on several cells, organs, and systems in the body. Oxysterols also have an influence on the physiology of the immune system, from immune cell maturation and migration to innate and humoral immune responses.
oxysterols
25-hydroxycholesterol
7α,25-dihydroxycholesterol
immune cells
EBI2
LXR
1. Introduction
Cholesterol is a vital component of cellular membranes [1][2] comprising about 20% of lipids present in plasma membrane [3][4]. Consequently, cholesterol plays a key role in maintaining the membrane integrity and fluidity, as well as having an impact on cellular physiology [3][4].
Oxysterols are oxidized derivatives of cholesterol [5][6][7][8], being intermediate compounds in the biosynthesis of bile acids, steroid hormones, and 1,25-dihydroxyvitamin D3 [5][9][10]. Oxysterols can be formed either enzymatically, by the action of some members of the CYP (cytochrome P450) family, or non-enzymatically, by the action of ROS (Figure 1) [3][9][10]. Oxysterols present in the diet may contribute to the total pool of oxysterols in the body [9][10].
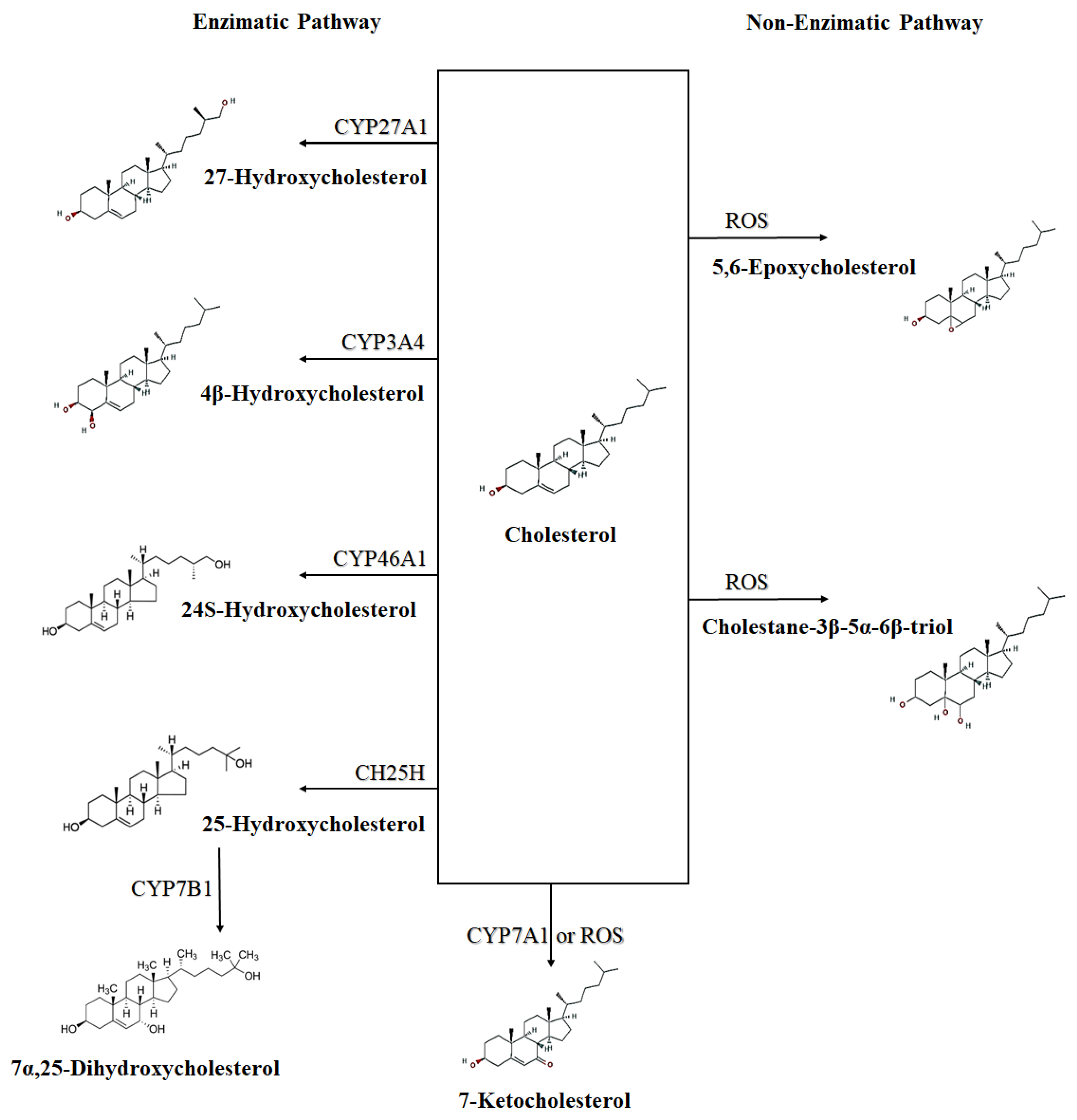
Figure 1. Schematic representation of enzymatic and non-enzymatic synthesis of some oxysterols. ROS—Reactive oxygen species; CYP27A1—Cytochrome P450 Family 27 Subfamily A Member 1 (sterol 27-hydroxylase); CYP3A4—Cytochrome P450 Family 3 Subfamily A Member 4; CYP7A1—Cytochrome P450 Family 7 Subfamily A Member 1 (cholesterol 7-alpha-monooxygenase); CH25H—cholesterol 25-hydroxylase; CYP46A1—Cytochrome P450 Family 46 Subfamily A Member 1 (cholesterol 24-hydroxylase); CYP7B1—Cytochrome P450 Family 7 Subfamily B Member 1 (25-hydroxycholesterol 7-alpha-hydroxylase). CYP7B1 synthetize a secondary oxysterol (7α,25-Dihydroxycholesterol) from a primary oxysterol (25-Hydroxycholesterol).
Oxysterols can also be classified into those synthesized directly from cholesterol, the primary oxysterols, which are formed by side-chain changes (such as 24S-, 25-, (25R)-26 and 27-hydroxycholesterols), and by ring changes (which includes 7α-hydroxycholesterol and 7β-hydroxycholesterol); and those derived from primary oxysterols, the secondary oxysterols, such as 7α,25-dihydroxycholesterol (Figure 1) and 7α,(25R)-26-dihydroxycholesterol, which are generated from 25-hydroxycholesterol and (25R)-26-hydroxycholesterol, respectively [11].
Although oxysterols are metabolic intermediates, several of them are bioactive, and their absence or excess can significantly affect the pathophysiology of some diseases [5]. Indeed, different oxysterols can have different actions, depending on their concentration and the type of cell or tissue, from changes in gene expression and lipid metabolism [12] to promotion of cell death, proliferation or differentiation [13][14][15][16][17][18][19]. In this way, some oxysterols have effects on several cells of the immune system, contributing to the development of several diseases. In the following sections the effects of some oxysterols on immune cells and related diseases will be discussed.
Oxysterols such as 7-ketocholesterol, 7β-hydroxycholesterol, 24-hydroxycholesterol, cholestane-3β,5α, 6β-triol, and mainly 25-hydroxycholesterol, have proinflammatory properties, stimulating THP-1 (human monocyte-derived macrophages) cells, and porcine retinal pigment epithelial cells, to produce IL-8 [20], involving the MEK/ERK1/2 cell signaling pathway [21]. In addition, when U937 (human promonocytic leukemia cells) and THP-1 cells are in the presence of oxysterols, mainly 7β-hydroxycholesterol and 25-hydroxycholesterol, these cells secrete several chemokines involved in the recruitment of immunocompetent cells at the subendothelial level such as MCP-1, MIP-1β, TNF-α, IL-1β and IL-8 [21]. 25-Hydroxycholesterol can potentiate LPS-induced IL-1β secretion in human mononuclear cells, principally under hypoxic conditions [22].
2. Oxysterols and the Immune System
The plasticity of immune cells has many implications in the pathogenesis and resolution of several diseases, such as chronic inflammatory disorders, metabolic disorders, cancers, and autoimmune diseases [23]. It is known that nutritional status and metabolic disorders such as obesity can influence the immune response, as immune cells can interact with different lipids, which can affect the plasticity of cells such as macrophages and T lymphocytes [23]. Pathways that promote lipid synthesis and accumulation tend to drive a proinflammatory phenotype, while pathways that enhance β-oxidation and lipid efflux tend to drive immune cells toward an antiinflammatory phenotype [23]. As examples: (a) in lean adipose tissue, CD4+ T cells and M2 macrophages express antiinflammatory phenotypes; (b) in overnutrition or obesity, there is an elevated level of saturated fatty acids, which drives the regulation of the influx and activation of inflammatory macrophages (M1) and lymphocytes (Th1, CTL and Th17) in adipose tissue; (c) in cardiovascular disease, there is the presence of both M1 and M2 macrophages; macrophages can infiltrate the arteries and engulf oxysterols, giving rise to foam cells; (d) the liver has antiinflammatory immune cells such as M2-associated Kupffer cells and CD4+ T cells (Th2 and Treg); in fatty liver disease, there is an elevate level of lipids, which drives the recruitment of inflammatory monocytes (Ly6Chi) and its differentiation into M1 macrophages [23].
There is a connection between immune system signaling and oxysterols [3]. Oxysterols acts in the regulation of the adaptive immune system [3][24] and have important roles in the innate immune system, such as the direct regulation of the inflammatory programming and participation in the development of immune response [25][26] and signaling [3][24]. Oxysterols and their receptors can regulate and modulate the function and phenotype of immune cell subsets such as macrophage, B and T cells, neutrophils, and dendritic cells [23][27].
In fact, according to some authors, some oxysterols could also be called “immunosterols”, due to their role in the immune system [3].
3. Oxysterol Receptors: LXR and EBI2
Oxysterols are signaling mediators that act in several membrane and nuclear receptors, including estrogen receptor α, and retinoic acid receptor-related orphan receptors. The most important, in terms of the immune system, are the liver X receptors (LXRs) pathway [28] and the G-protein-coupled receptors (GPCRs) EBI2 [27].
3.1. LXR
Oxysterol LXR-dependent activity has a biological influence on immune cells in different pathological contexts, such as infectious diseases, autoimmune diseases, and cancer [29][30]. Oxysterols can bind to LXR receptors from immune cells, modulating their actions [12][27] (Figure 2). Liver X receptors (LXRs) are ligand-activated transcription factors that belong to a superfamily of 48 ligand-dependent transcription factors. There are two LXR isoforms: (1) LXRα—nuclear receptor subfamily 1 group H member 3 (NR1H3); and (2) LXRβ—nuclear receptor subfamily 1 group H member 2 (NR1H2) [29][31][32][33]. Both, LXRα and β, as heterodimers, complex with the retinoid X receptor (RXR), a common partner for several nuclear receptors such as the peroxisome proliferator-activated receptors (PPARs). The LXR/RXR complex links to a LXR response element (LXRE) in the promoter region of target genes, promoting the regulation of gene expression through mechanisms that include direct activation, ligand-independent or ligand-dependent repression, and trans-repression [34].
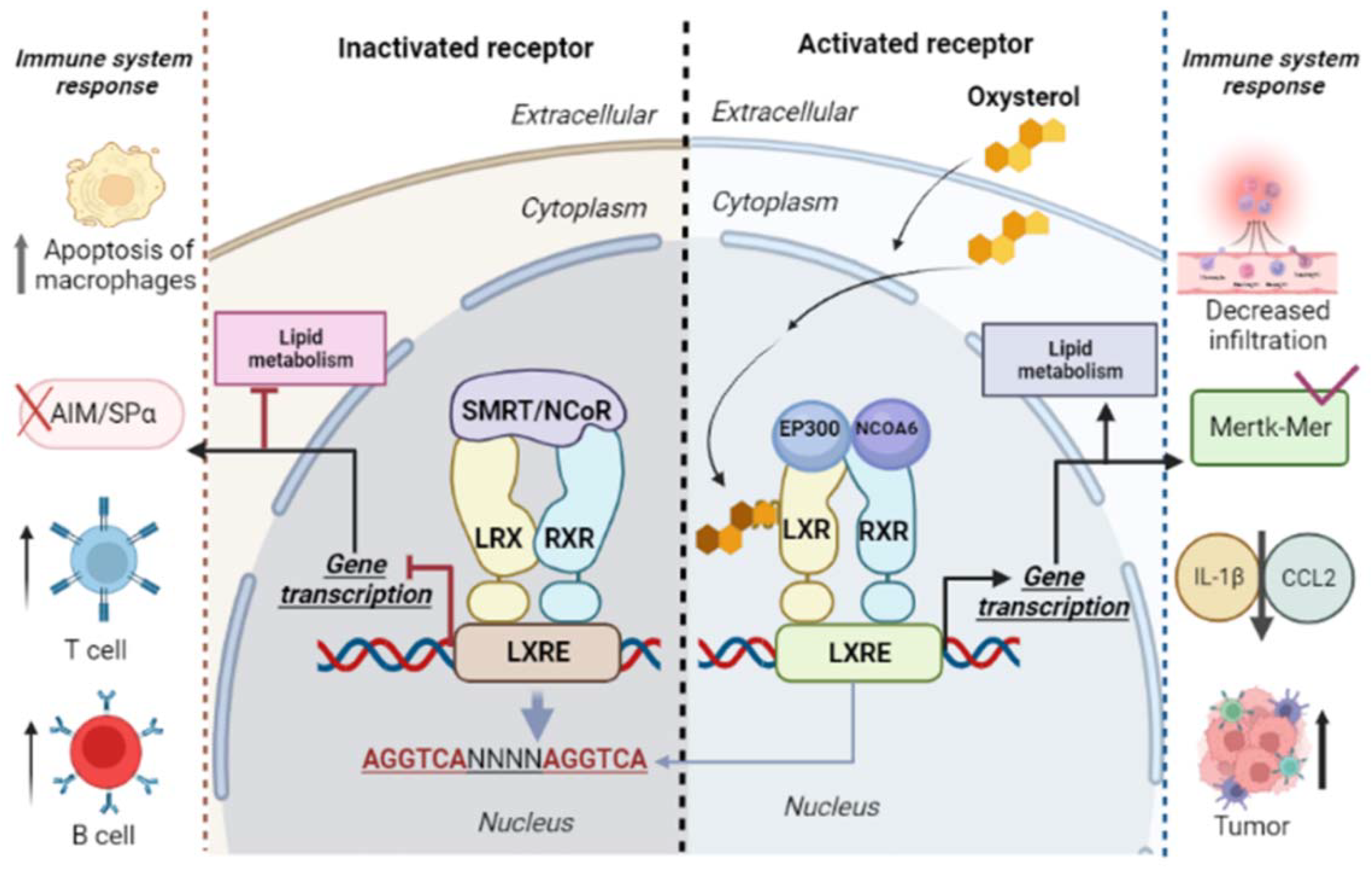
Figure 2. Liver X receptors (LXRs) and retinoid X receptors (RXRs) form heterodimers and bind to LXR response elements (LXREs). The LXR/RXR binding regions are composed of repeated sequences of AGGTCA and four nucleotides (NNNN). This region is responsible for the regulation of activated target genes via LXR/RXR. Inactivation of the LXR/RXR complex occurs by binding with corepressors (nuclear receptor corepressor (NcoR), retinoic acid silencing mediator, and thyroid hormone receptor (SMRT)). In the presence of oxysterols, coactivators (nuclear receptor coactivator 6 (NCOA6) and histone acetyltransferase p300 (EP300)) bind to the LXR/RXR complex, activating the expression of genes involved in lipid metabolism and in innate and cellular immune response. Both the inactivation and the activation of LXR receptors are associated with immune responses.
LXR signaling, mainly LXRβ, inhibits the proliferation of T and B cells. The activation of T and B cells triggers mechanism for cell proliferation: the induction of the sulfotransferase family 2B member enzyme (1SULT2B1), which inactivates oxysterols as LXR ligands by a sulfation process; and the promotion of sterol regulatory element-binding protein (2SREBP-2) pathway for cholesterol synthesis [30].
Oxysterols, in an LXR-independent manner, can recruit protumor immune cells within the tumor microenvironment [27]. However, these chemoattractant tumor-derived oxysterols (e.g., 22R-hydroxycholesterol and 25-hydroxycholesterol) also activate LXRs, inhibiting the CC chemokine receptor-7 (CCR7) expression in maturing dendritic cells, and impairing their migration to draining lymph nodes [35].
In addition, oxysterols such as 24S-hydroxycholesterol can induce apoE-mediated cholesterol efflux in astrocytes via an LXR-controlled pathway, a relevant process in chronic and acute neurological diseases [36]. Interestingly, 24S-hydroxycholesterol is also involved in the suppression of synaptic vesicle exocytosis during 20 Hz activity at the neuromuscular junctions, this action being dependent on both LXR activation and upregulation of NO-signaling [37]. These findings show how LXR/oxysterol studies are important for clarifying the development of some diseases.
In contrast with the traditional knowledge of LXRs’ action on cell nucleus, these receptors can also have non-nuclear functions such as: LXRβ action on endothelial cells caveolae/lipid rafts that entails crosstalk with ERα, which promotes NO production and maintains endothelial monolayer integrity in vivo [38]; LXRβ expression in platelets (anucleate), being responsible for the inhibition of platelet function and thrombosis [39]; 25-hydroxycholesterol oxidant capacity and its regulatory action on synaptic vesicle mobilization via the activation of lipid raft-associated LXRs could trigger signaling via estrogen receptor α-Gi-protein-Gβγ-phospholipase C-Ca2+-protein kinase C pathway [40].
3.2. EBI2 (GPR183)
The orphan seven-transmembrane G-protein-coupled receptor 183 (GPR183), also called Epstein–Barr-virus-induced molecule-2 (EBI2) [41][42][43][44], was identified in 1993 by Birkenbach et al. as one of the principal genes induced by Burkitt’s lymphoma cell line BL41 when these cells are infected by the Epstein–Barr virus (EBV) [42][45]. In 2011, Hannedouche et al., and Liu et al. reported that the oxysterol 7α,25-dihydroxycholesterol is a potent and selective EBI2 agonist and its most likely endogenous ligand [46][47]. EBI2 is coupled with Gαi, and ligand engagement leads to the activation of RHO family GTPases and mitogen-activated protein kinases such as the extracellular signal-regulated kinase (ERK) and P38 to intracellular calcium flux [25].
Although the EBI2 expression was firstly identified in B cells, it is now known that EBI2 is expressed in several cells such as natural killer cells [47][48], B cells [46][47][48][49], T cells [47][48], monocytes/macrophages [47][48][49][50], dendritic cells [46][47], eosinophils [48][51], platelets [52], osteoclasts [50], and neutrophils [47]. In addition, EBI2 expression has also been characterized in astrocytes [53] and in the early developmental stages of immune cells, including hematopoietic stem and progenitor cells and thymocytes [54][55]. Figure 3 shows the EBI2 receptor and its activation pathway.
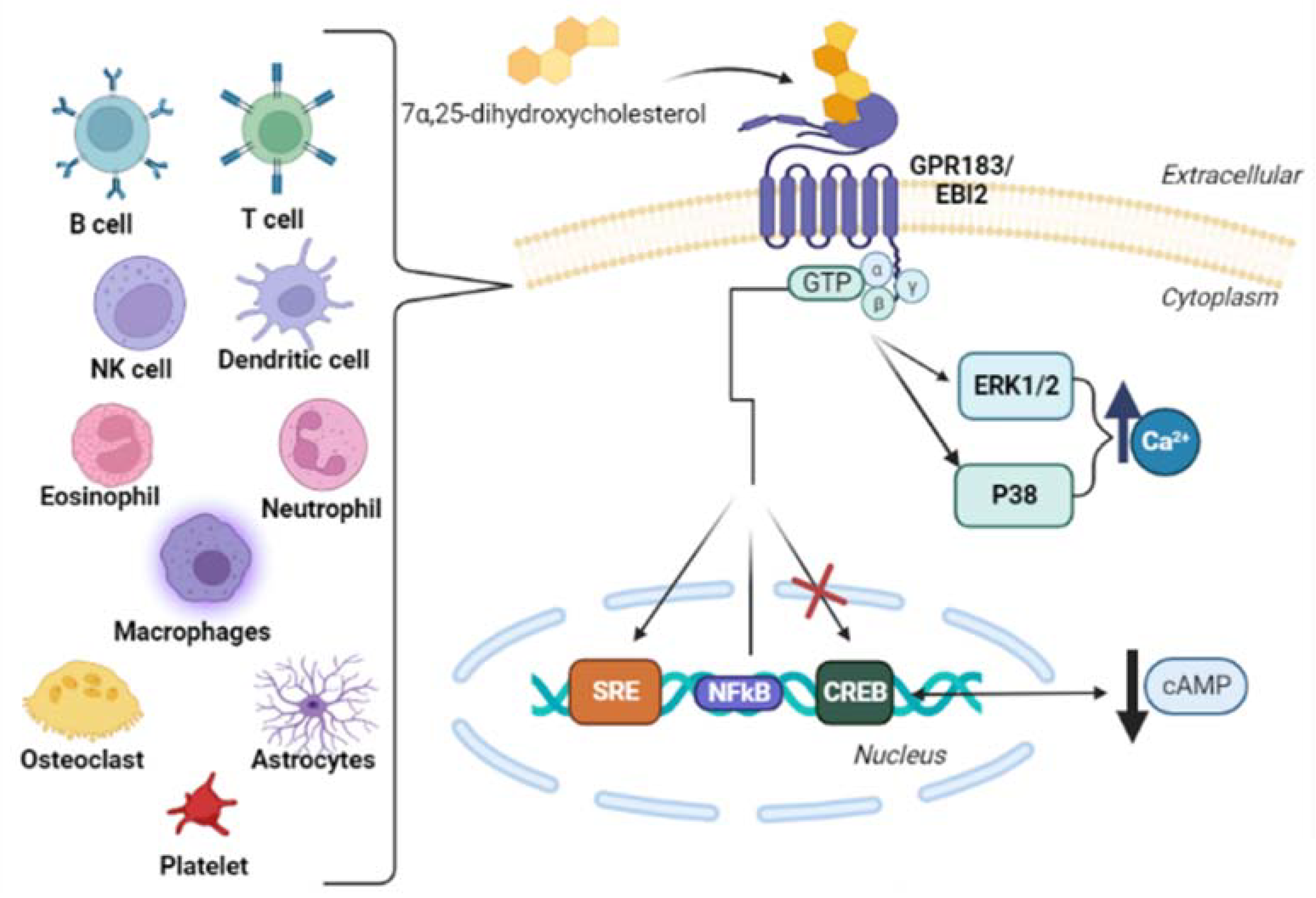
Figure 3. Schematic pathway of EBI2 receptor activation in cells that express this receptor. 7α,25-dihydroxycholesterol, a ligand of EBI2 (Epstein–Barr-virus-induced molecule-2), activates the GTPases family and mitogen-activated protein kinases/extracellular signal-regulated kinase (ERK) and P38 to intracellular calcium flux. In the nucleus, GTPases stimulate the expression of SRE (Serum Response Element) and the expression of NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells) (weak stimulation), and inhibit the expression of CREB (cAMP Response Element-Binding Protein), downregulating the production of cAMP (cyclic Adenosine Monophosphate) [48][56].
EBI2 is responsible for regulating the positioning of immune cells in secondary lymphoid organs. Polymorphisms in this receptor have been associated with some inflammatory autoimmune diseases [46], such as type 1 diabetes [49]. Furthermore, EBI2 participates in: the stimulation of migration of B, T and dendritic cells, monocytes [57], and astrocytes; the increase of B cell proliferation; and in the negative regulation of type I interferon in dendritic cells and monocytes [57][58]. EBI2 deficiency can result in reduced early immunoglobulin-M (IgM) and IgG antibody response to a T cell dependent antigen, in addition to interfering with the B cell migration to the outer follicular niche in the spleen and lymph nodes [41]. In fact, EBI2 also represents another key chemotactic receptor, together with CXCR5, CXCR4, and CCR7, directing B cells migration within secondary lymphoid tissues [57].Chemoattractant receptors of the GPR family have essential roles in coordinating the migration of lymphocytes to produce an efficient response against microorganisms [49], such as in the spleen, where EBI2 plays an important role controlling the immune cell migration during the course of a T-dependent antibody response [42]. However, when these receptors are dysregulated, an initiation or progression of inflammatory and autoimmune disorders can occur [49]. Polymorphisms in the gene encoding EBI2 have been associated with inflammatory processes. In addition, EBI2 was shown to be involved in the regulation of the inflammatory response of macrophages in rats [49].
The dysregulation of EBI2 expression has also been related to human neoplastic diseases, such as acute myeloid leukemia, chronic lymphocytic leukemia, diffuse large B cell lymphoma, and follicular and germinal center B-like diffuse large B cell lymphoma [59].
4. Main Oxysterols That Are Importance to the Immune System
4.1. 25-Hydroxycholesterol
25-Hydroxycholesterol has been described as promoting inflammatory and anti-inflammatory effects [3]. Cholesterol 25-hydroxylase (CH25H) is the enzyme responsible for oxidizing cholesterol to form 25-hydroxycholesterol [60]. CH25H does not belong to the cytochrome P450 (CYP) family; it is a member of a family of enzymes that uses diiron cofactors to catalyze hydroxylation [60].
25-Hydroxycholesterol plays a key role in the regulation of B cells. 25-Hydroxycholesterol enhances the expression of IL-8, IL-6 and macrophage colony-stimulating factor (MCSF) and inhibit the production of IL1-β by inhibiting the Sterol Regulatory Enhancer Binding Protein (SREBP) [61]. The inhibition of SREBP also modulates sterol pathway flux resulting in the formation of STING/cGAMP complex that phosphorylates TBK, which further phosphorylates IFR3 to activate the expression of IL1-β [3]. 25-Hydroxycholesterol also induces the production and release of cytokine C-C Motif Chemokine Ligand 5 (CCL5) [61]. CCL5 is a pro-inflammatory cytokine involved in the recruitment of cells to the site of infection, thus amplifying the immune response and inflammation [3].
The CH25H gene belongs to the family of interferon-stimulating genes (ISGs), which play key roles in inflammation, innate immunity, and subsequent adaptive immune responses through interferon signaling [62]. Inflammatory mediators promote the upregulation of CH25H in dendritic cells and macrophages; this indicates that 25-hydroxycholesterol has a potential function in innate immune regulation [63]. In addition, 25-hydroxycholesterol and 7α,25-dihydroxycholesterol are synthesized and secreted by macrophages [64][65]
An infection promoted by virus or bacteria leads to the production of type I interferon, rapidly inducing CH25H to generate 25-hydroxycholesterol [66]. The increase in 25-hydroxycholesterol production has also been observed in the lung during M. tuberculosis infection [65] and in acute lung injury models [67]. In addition, in its role as an anti-inflammatory molecule, 25-hydroxycholesterol blocks the activation of SREBP, which regulates cholesterol biosynthesis and inflammasome activity, as stated above [66]. Specifically, 25-hydroxycholesterol has been shown to decrease inflammasome activity via NLRP3, and subsequent IL-1β production [66].
4.2. 7α,25-Dihydroxycholesterol
CH25H and CYP7B1 (25-hydroxycholesterol 7-alpha-hydroxylase) are the 2 enzymes involved sequentially in the main route of 7α,25-dihydroxycholesterol synthesis from cholesterol [25][68]: 25-hydroxylation of cholesterol by CH25H, followed by 7α-hydroxylation by CYP7B1 [60][69] (Figure 1). In addition, CYP3A, CYP27A1, and CYP46A1 enzymes also have sterol 25-hydroxylation activity [60]. An alternative synthesis route for 7α,25-dihydroxycholesterol is via 7α-hydroxylation of cholesterol by CYP7A1 followed by 25-hydroxylation of 7α-hydroxycholesterol (7α-HC) [46]. Another enzyme, HSD3B7, is responsible for 7α,25-dihydroxycholesterol metabolization to a 3-oxo derivative [25][68]. CH25H, CYP7B1, and HSD3B7 are also involved in the control of EBI2-ligand concentration in lymphoid tissues and are an extrahepatic pathway that regulates oxysterol production in these tissues [68].
Oxysterols are ligands (orthosteric or allosteric) for G-protein-coupled receptors (GPCRs): EBI2, C-X-C Motif Chemokine Receptor 2 (CXCR2), G-protein-coupled receptor 17 (GPR17), and the Smoothened receptor [70][71][72][73].
EBI2 and key enzymes involved in 7α,25-dihydroxycholesterol synthesis are highly regulated during inflammation and could be involved in autoimmune diseases, cardiovascular diseases, neurodegenerative diseases, some metabolic diseases (dyslipidemia, obesity, and diabetes), in addition to some types of cancer and inflammation [57].
5. Oxysterols and Immune System Cells
Oxysterols can influence several immune system cells, changing their functions, such as immunoglobulin production, formation of neutrophil extracellular traps, and differentiation and migration of lymphocytes [74].
5.1. B Cells
B cells undergo a series of migratory events, which guide them to the appropriate microenvironment for the adequate activation and differentiation [59].
7α,25-dihydroxycholeserol is a EBI2 ligand, a cellular receptor expressed by B cells. The expression of EBI2 is increased when B cells are activated and downregulated in the germinal center, the specialized microstructure formed in secondary lymphoid tissues, responsible for producing long-lived antibody-secreting plasma cells and memory B cells [41]. In addition, EBI2 is responsible for mediating the correct location of B cells in humoral immune responses [43]. The overexpression of EBI2 promotes the localization of B cells in the outer follicle which are highly specialized histological structures [41].
EBI2 plays a key role in the regulation of B cell migration during immune activation and response [41][57][75]. Just a few hours after encountering the antigen, EBI2 is responsible for the migration of B cells to the external follicles of lymphoid tissue in the region where the antigen enters the structure [25]. Th cells send CD40 signals, which sustain EBI2 expression on activated B cells while promoting CCR7 downregulation, which leads to EBI2-dependent B cell migration to the outer follicle, but now with a preference for interfollicular regions [25]. During differentiation into germinal center B cells, downregulation of EBI2 has an important role for precursor cell migration to the follicle center. Downregulation of EBI2 may occur due to B cell lymphoma 6 (BCL6)-mediated repression, and may involve signaling through signal transducer and activator of transcription 6 (STAT6)-dependent cytokine receptors. However, the expression of low levels of EBI2 can be maintained in some germinal center B cells, such that the receptor can potentially influence cells even in this compartment [25].
The HSD3B7 enzyme, produced by stromal cells, are responsible for inactivating 7α,25-dihydroxycholesterol. However, the absence of this enzyme causes an increase in the availability of EBI2-ligand, resulting in the failure of B cell positioning. The same mechanism that controls the EBI2-ligand production by the HSD3B7 enzyme is observed in dendritic cells. Therefore, considering the crucial role that CYP7B1, CH25H and HSD3B7 enzymes have in the maintenance of an adequate EBI2-ligand gradient, decreased production of these enzymes could be associated with impaired humoral immune responses [68].
5.2. T Cells
The T cell-dependent humoral immune response is highly linked to EBI2. Dysregulation of this receptor contributes to B cell diseases such as diffuse large B cell lymphomas and chronic lymphocytic leukemia [56]. In these cases, EBI2 expression is downregulated while it is upregulated in post-transplantation lymphoproliferative disorders [56].
A subset of T CD8+ cells and T CD4+ cells also express EBI2 and are responsive to 7α,25-dihydroxycholesterol, which promotes migratory process of these cells [47]. For example, 7α,25-dihydroxycholesterol promotes the migration of activated CD4+ T cells to tissues with inflammation in experimental autoimmune encephalomyelitis through its interaction with EBI2 [76].
7β,27-dihydroxycholesterol is a selective activator of nuclear receptor RAR-related orphan receptor γ (RORγ). In addition, RORγ is the master nuclear transcription factor needed for Th17 differentiation, which is affected by changes in the enzymes related to 7β,27-dihydroxycholesterol [77].
The nuclear receptor RAR-related orphan receptor γ t (RORγt) is required for the generation of IL-17-producing CD4+ Th17 cells, which are essential for hosting defense, as they are also involved in the development of autoimmune diseases [77]. Soroosh et al., (2014) identified the 7β,26-dihydroxycholesterol as the most potent and selective activator for RORγt. Both 7β,26-dihydroxycholesterol and its isomer 7α,26-dihydroxycholesterol can promote the differentiation of murine and human IL-17 producing Th17 cells in a RORγt-dependent manner [77]. These findings could contribute to a new design for RORγt modulators, and also provide new targets for inhibiting IL-17 production [77]. In addition, RORα (NR1F1), RORγ (NR1F3), NR2F6, and a ligand-regulated PAS superfamily member, Ahr, play important roles during the differentiation of Th17 inflammatory T cells [78].
5.3. Macrophages
Macrophages are functional cells that play central roles in both innate and adaptive immunity and homeostasis [79][80]. There are differences in mature macrophages from each tissue, due to their high sensitivity to microenvironments, therefore differing in their morphology, expression of cell surface receptors, secretome, and functional capabilities [81].
Three important aspects of macrophage biology are regulated by oxysterols: (1) regulation of lipid transport and metabolism; (2) inflammatory responses; (3) cytotoxicity [80]. In addition, LXRs, insulin-induced genes (Insigs), and oxysterol-binding protein (OSBP)/OSBP-related protein (ORP) family members have been identified as key acceptors for these functions of oxysterols [80]. In addition, activated macrophages are classified into: M1 (pro-inflammatory), which are polarized in infections by pathogenic microorganisms, IFN-γ, and/or TLR ligands; and M2 (anti-inflammatory), which are activated by IL-4 or IL-13 [82]. In this way, Krüppel-like factor 4 (KLF4) in the macrophage favors the transition from the pro-inflammatory M1 to anti-inflammatory M2 macrophage phenotype [83]. Interestingly, Lamtor1, v-ATPase and mTORC1 integrate the intracellular amino-acid sufficiency signal and the extrinsic IL-4 signal, leading to the production of 25-hydroxycholesterol and subsequent activation of LXR, ultimately resulting in the polarization of M2 macrophages [82]. In addition, 27-hydroxycholesterol can also stimulate M2 macrophage polarization toward the immunomodulatory functional phenotype [84]
Macrophages can produce and secrete high amounts of 25-hydroxycholesterol in response to the activation of Toll-like receptors [63][85]. However, its expression is notably silent in quiescent immune cells [63].
Interferon (IFN) regulates the gene CH25H, at least in rats. This is responsible for synthesizing 25-hydroxycholesterol in macrophages, after IFN-stimulation or viral infection, in this way acting as a potent paracrine inhibitor of viral infection for different viruses. This response to IFN is via the direct recruitment of signal transducer and activator of transcription 1 (Stat1) to the promoter proximal region of the CH25H gene. This mechanism shows the importance of Ch25h in the innate immune pathway [64]. In addition, the Stat1 binding to the CH25H promoter provides a critical molecular link between innate immune stimulation, infection, and the secretion of 25-HC by macrophages [64]. By using transcriptional regulatory-network analyses, genetic interventions and chromatin immunoprecipitation experiments, Blanc et al., (2013) have shown that Stat1 is strongly linked to CH25H regulation to IFN in macrophages [64].
24,25-Epoxycholesterol and 25-hydroxycholesterol are ligands for the LXR [86]. Activation of LXR by 24,25-epoxycholesterol enhances the expression of genes encoding Abca1 and Abcg1 transporters that mediate cholesterol efflux, and inhibit the expression of inflammatory response genes [87].
Diczfalusy et al., (2009) [61] have shown that CH25H is strongly upregulated by lipopolysaccharide (LPS). The injection of LPS into healthy volunteers increased 25-hydroxycholesterol concentration in their plasma [61]. In addition, Kdo2-lipid A, the active component of an inflammatory LPS, acts as a Toll-like receptor 4 (TLR4) agonist, and promotes a 4-fold increase of CH25H mRNA expression on RAW264.7 cell line (mouse macrophage) [85], while the increase of CH25H mRNA in bone marrow-derived macrophages has been reported to be 15-fold [63].
In addition, Ngo at al., (2022) [65] have described an upregulation of the CH25H enzyme and CYP 7B1 in alveolar and infiltrating macrophages of dysglycemic mice lung infected by M. tuberculosis even with elevated in 25-hydroxycholesterol levels. This finding was linked with the increased EBI2 expression, responsible for the oxysterol-mediated recruiting of immune cells to the lung [65]. Barlett et al., (2020) [58] have shown that EBI2 activation by 7α,25-dihydroxycholesterol reduces both M. tuberculosis and M. bovis growth in primary human monocytes, via the reduction of IFN-β and IL-10 expression and enhanced autophagy [58].
In a lipidomic analysis using mouse macrophage RAW264.7 (RAW) activated by Kdo2-lipid A (KDO), the active component of LPS, a 3-fold increase in intracellular 25-hydroxycholesterol concentration was observed, with a 4-fold increase in CH25H mRNA expression [85][88]. A total of 24 h after stimulation, both cellular cholesterol and 24S,25-epoxycholesterol levels had doubled [85]. The increase in both cellular cholesterol and 24S,25-epoxycholesterol levels involves LPS stimulation of the complex 1 of the mammalian targets of rapamycin (mTORC1), leading to the activation of the SREBP-2 pathway [89][90].
The stimulation of macrophage Toll-like receptor 4 (TLR4) increases CH25H expression and 25-hydroxycholesterol synthesis. This leads to the suppression of interleukin-2 (IL-2), which mediates the stimulation of B cell proliferation, inhibits the activation of cytidine deaminase (AID) expression, leading to a decreased IgA production [63]. The IgA suppression by B cells in response to TLR activation is responsible for a mechanism that negatively regulates the local and systemic adaptive immune response by the innate immune system [63].
In macrophages, the connection between LXR and TLR signaling is TLR3- and TLR4-mediated, and the IRF3-dependent activation of macrophages blocks the induction of LXR target genes and inhibits cholesterol efflux [91]. In contrast, TLR3 and TLR4 agonists induce CH25H and, consequently, oxysterol synthesis. Therefore, it is possible that immune modulation can be accompanied by LXR target gene induction and enhanced cholesterol efflux [12].
LXR activation in macrophages, mediated by oxysterols, promotes macrophage survival and suppresses TLR-mediated innate immune responses, characterized by the reduced production of IL-6, IL-1β, MCP-1, MCP-3, iNOS, COX-2, and MMP-9 [92].
Activated macrophages in mice, in the absence of IFN-stimulation of the CH25H gene, are unable to produce 25-hydroxycholesterol, and overproduce inflammatory interleukin-1 (IL-1)-family cytokines. 25-Hydroxycholesterol is an antagonist of SREBP, reducing Il1β transcription and repressing IL-1–activating inflammasomes. Therefore, 25-hydroxycholesterol is a critical mediator in the negative-feedback pathway of IFN signaling on IL-1-family cytokine production and inflammasome activity [66].
Type 1 IFN restrains IL-1β driven inflammation in macrophages by upregulating CH25H and 25-hydroxycholesterol and repressing the sterol-sensing transcription factor SREBP2-driven cholesterol synthesis. In the absence of CH25H, cholesterol overload triggers mitochondrial DNA release and activation of AIM2 inflammasomes in activated macrophages [93]. Therefore, the anti-inflammatory action of 25-hydroxycholesterol in activated macrophages maintains mitochondrial integrity and prevents the AIM2 inflammasome activation [93].
5.4. Dendritic Cells
Similar to macrophages, CH25H is upregulated in dendritic cells in response to cell surface TLR4 activation by LPS and intracellular TLR3 ligands [12]. In addition, TLR-mediated expression of CH25H is dependent on TIR-domain-containing adapter-inducing interferon-β (TRIF), production of type I interferon (IFN-1), and signaling through the IFN α/β receptor/Janus kinase/Signal transducer and activator of transcription 1 (IFNR/JAK/STAT1) pathway. In addition, CH25H is an IFN-responsive gene in dendritic cells, as in macrophages, during innate immune responses, and the early expression of CH25H implies a role for oxysterols in the regulation of innate immunity [12].
Tumor-derived oxysterols such as 22(R)-hydroxycholesterol and 27-hydroxycholesterol can exert opposite effects on the expression of CCR7 in dendritic cells. The different action of oxysterols on dendritic cells is related to the differentiation stage of these cells (immature versus mature), possibly through the differential activation of LXRα and/or LXRβ isoforms [29]. In addition, the stimulation of LXR in dendritic cells during innate response activation decreases the production of CD86 and IL-12, enhances the secretion of IL-10, and blocks the activation of T cells [94].
5.5. Oligodendrocytes
Oxysterols have been shown to promote adverse effects on oligodendrocyte viability in vitro. The treatment of 158N (oligodendrocyte) cell line with 25-hydroxycholesterol or 22(S)-hydroxycholesterol induce cell death and morphological changes independently of LXR signaling [95]. In addition, the presence of oxysterol biosynthetic enzymes and oxysterols has been shown in oligodendrocytes, indicating that oxysterols may signal in an autocrine/paracrine manner in these cells [95].
5.6. Astrocytes
Oxysterols play important roles in astrocyte biology. For instance, they inhibit astrogliosis and there is a correlation between cholesterol synthesis in the central nervous system and reactive astrocyte proliferation. Human and mouse astrocytes express EBI2 as well as the enzymes necessary for the synthesis and degradation of 7α,25-dihidroxycholesterol, CH25H, CYP7B1 and HSD3B7. The receptor expressed in astrocytes is functional and signals via ERK1/2 phosphorylation and Ca2+ influx. In this way, EBI2/oxysterol signaling is involved in normal myelin development as well as in the release of proinflammatory cytokines and communication with macrophages [96].
References
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. Srebps: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131.
- Griffiths, W.J.; Wang, Y. Sterols, oxysterols and accessible cholesterol: Signalling for homeostasis, in immunity and during development. Front. Physiol. 2021, 12, 723224.
- Bah, S.Y.; Dickinson, P.; Forster, T.; Kampmann, B.; Ghazal, P. Immune oxysterols: Role in mycobacterial infection and inflammation. J. Steroid Biochem. Mol. Biol. 2017, 169, 152–163.
- Shahoei, S.H.; Nelson, E.R. Nuclear receptors, cholesterol homeostasis and the immune system. J. Steroid Biochem. Mol. Biol. 2019, 191, 105364.
- Griffiths, W.J.; Wang, Y. An update on oxysterol biochemistry: New discoveries in lipidomics. Biochem. Biophys. Res. Commun. 2018, 504, 617–622.
- York, A.G.; Bensinger, S.J. Subverting sterols: Rerouting an oxysterol-signaling pathway to promote tumor growth. J. Exp. Med. 2013, 210, 1653–1656.
- Ruiz, J.L.; Fernandes, L.R.; Levy, D.; Bydlowski, S.P. Interrelationship between atp-binding cassette transporters and oxysterols. Biochem. Pharmacol. 2013, 86, 80–88.
- Rosa-Fernandes, L.; Maselli, L.M.F.; Maeda, N.Y.; Palmisano, G.; Bydlowski, S.P. Outside-in, inside-out: Proteomic analysis of endothelial stress mediated by 7-ketocholesterol. Chem. Phys. Lipids 2017, 207, 231–238.
- Griffiths, W.J.; Abdel-Khalik, J.; Hearn, T.; Yutuc, E.; Morgan, A.H.; Wang, Y. Current trends in oxysterol research. Biochem. Soc. Trans. 2016, 44, 652–658.
- Reinmuth, L.; Hsiao, C.C.; Hamann, J.; Rosenkilde, M.; Mackrill, J. Multiple targets for oxysterols in their regulation of the immune system. Cells 2021, 10, 2078.
- Duc, D.; Vigne, S.; Pot, C. Oxysterols in autoimmunity. Int. J. Mol. Sci. 2019, 20, 4522.
- Park, K.; Scott, A.L. Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type i interferons. J. Leukoc. Biol. 2010, 88, 1081–1087.
- Levy, D.; Ruiz, J.L.; Celestino, A.T.; Silva, S.F.; Ferreira, A.K.; Isaac, C.; Bydlowski, S.P. Short-term effects of 7-ketocholesterol on human adipose tissue mesenchymal stem cells in vitro. Biochem. Biophys. Res. Commun. 2014, 446, 720–725.
- Rosa Fernandes, L.; Stern, A.C.; Cavaglieri, R.C.; Nogueira, F.C.; Domont, G.; Palmisano, G.; Bydlowski, S.P. 7-ketocholesterol overcomes drug resistance in chronic myeloid leukemia cell lines beyond mdr1 mechanism. J. Proteom. 2017, 151, 12–23.
- Levy, D.; de Melo, T.C.; Ohira, B.Y.; Fidelis, M.L.; Ruiz, J.L.M.; Rodrigues, A.; Bydlowski, S.P. Oxysterols selectively promote short-term apoptosis in tumor cell lines. Biochem. Biophys. Res. Commun. 2018, 505, 1043–1049.
- Favero, G.M.; Paz, J.L.; Otake, A.H.; Maria, D.A.; Caldini, E.G.; de Medeiros, R.S.S.; Deus, D.F.; Chammas, R.; Maranhão, R.C.; Bydlowski, S.P. Cell internalization of 7-ketocholesterol-containing nanoemulsion through ldl receptor reduces melanoma growth in vitro and in vivo: A preliminary report. Oncotarget 2018, 9, 14160–14174.
- Silva, S.F.; Levy, D.; Ruiz, J.L.M.; de Melo, T.C.; Isaac, C.; Fidelis, M.L.; Rodrigues, A.; Bydlowski, S.P. Oxysterols in adipose tissue-derived mesenchymal stem cell proliferation and death. J. Steroid Biochem. Mol. Biol. 2017, 169, 164–175.
- Levy, D.; de Melo, T.C.; Oliveira, B.A.; Paz, J.L.; de Freitas, F.A.; Reichert, C.O.; Rodrigues, A.; Bydlowski, S.P. 7-ketocholesterol and cholestane-triol increase expression of smo and lxrα signaling pathways in a human breast cancer cell line. Biochem. Biophys. Rep. 2019, 19, 100604.
- De Freitas, F.A.; Levy, D.; Zarrouk, A.; Lizard, G.; Bydlowski, S.P. Impact of oxysterols on cell death, proliferation, and differentiation induction: Current status. Cells 2021, 10, 2301.
- Lemaire-Ewing, S.; Prunet, C.; Montange, T.; Vejux, A.; Berthier, A.; Bessède, G.; Corcos, L.; Gambert, P.; Néel, D.; Lizard, G. Comparison of the cytotoxic, pro-oxidant and pro-inflammatory characteristics of different oxysterols. Cell Biol. Toxicol. 2005, 21, 97–114.
- Prunet, C.; Montange, T.; Véjux, A.; Laubriet, A.; Rohmer, J.F.; Riedinger, J.M.; Athias, A.; Lemaire-Ewing, S.; Néel, D.; Petit, J.M.; et al. Multiplexed flow cytometric analyses of pro- and anti-inflammatory cytokines in the culture media of oxysterol-treated human monocytic cells and in the sera of atherosclerotic patients. Cytometry. Part A J. Int. Soc. Anal. Cytol. 2006, 69, 359–373.
- Rydberg, E.K.; Salomonsson, L.; Hultén, L.M.; Norén, K.; Bondjers, G.; Wiklund, O.; Björnheden, T.; Ohlsson, B.G. Hypoxia increases 25-hydroxycholesterol-induced interleukin-8 protein secretion in human macrophages. Atherosclerosis 2003, 170, 245–252.
- Hubler, M.J.; Kennedy, A.J. Role of lipids in the metabolism and activation of immune cells. J. Nutr. Biochem. 2016, 34, 1–7.
- Lathe, R.; Sapronova, A.; Kotelevtsev, Y. Atherosclerosis and alzheimer—Diseases with a common cause? Inflammation, oxysterols, vasculature. BMC Geriatr. 2014, 14, 36.
- Cyster, J.G.; Dang, E.V.; Reboldi, A.; Yi, T. 25-hydroxycholesterols in innate and adaptive immunity. Nat. Rev. Immunol. 2014, 14, 731–743.
- Varaksa, T.; Bukhdruker, S.; Grabovec, I.; Marin, E.; Kavaleuski, A.; Gusach, A.; Kovalev, K.; Maslov, I.; Luginina, A.; Zabelskii, D.; et al. Metabolic fate of human immunoactive sterols in mycobacterium tuberculosis. J. Mol. Biol. 2021, 433, 166763.
- Traversari, C.; Russo, V. Control of the immune system by oxysterols and cancer development. Curr. Opin. Pharmacol. 2012, 12, 729–735.
- Mutemberezi, V.; Guillemot-Legris, O.; Muccioli, G.G. Oxysterols: From cholesterol metabolites to key mediators. Prog. Lipid Res. 2016, 64, 152–169.
- Traversari, C.; Sozzani, S.; Steffensen, K.R.; Russo, V. Lxr-dependent and -independent effects of oxysterols on immunity and tumor growth. Eur. J. Immunol. 2014, 44, 1896–1903.
- Bensinger, S.J.; Bradley, M.N.; Joseph, S.B.; Zelcer, N.; Janssen, E.M.; Hausner, M.A.; Shih, R.; Parks, J.S.; Edwards, P.A.; Jamieson, B.D.; et al. Lxr signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 2008, 134, 97–111.
- Fang, F.; Li, D.; Zhao, L.; Li, Y.; Zhang, T.; Cui, B. Expression of nr1h3 in endometrial carcinoma and its effect on the proliferation of ishikawa cells in vitro. OncoTargets Ther. 2019, 12, 685–697.
- Yang, X.; Lamia, K.A.; Evans, R.M. Nuclear receptors, metabolism, and the circadian clock. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 387–394.
- Jarvis, S.; Williamson, C.; Bevan, C.L. Liver x receptors and male (in)fertility. Int. J. Mol. Sci. 2019, 20, 5379.
- Glass, C.K.; Rosenfeld, M.G. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000, 14, 121–141.
- Villablanca, E.J.; Raccosta, L.; Zhou, D.; Fontana, R.; Maggioni, D.; Negro, A.; Sanvito, F.; Ponzoni, M.; Valentinis, B.; Bregni, M.; et al. Tumor-mediated liver x receptor-alpha activation inhibits cc chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat. Med. 2010, 16, 98–105.
- Abildayeva, K.; Jansen, P.J.; Hirsch-Reinshagen, V.; Bloks, V.W.; Bakker, A.H.; Ramaekers, F.C.; de Vente, J.; Groen, A.K.; Wellington, C.L.; Kuipers, F.; et al. 24(s)-hydroxycholesterol participates in a liver x receptor-controlled pathway in astrocytes that regulates apolipoprotein e-mediated cholesterol efflux. J. Biol. Chem. 2006, 281, 12799–12808.
- Mukhutdinova, K.A.; Kasimov, M.R.; Zakyrjanova, G.F.; Gumerova, M.R.; Petrov, A.M. Oxysterol modulates neurotransmission via liver-x receptor/no synthase-dependent pathway at the mouse neuromuscular junctions. Neuropharmacology 2019, 150, 70–79.
- Ishikawa, T.; Yuhanna, I.S.; Umetani, J.; Lee, W.R.; Korach, K.S.; Shaul, P.W.; Umetani, M. Lxrβ/estrogen receptor-α signaling in lipid rafts preserves endothelial integrity. J. Clin. Investig. 2013, 123, 3488–3497.
- Unsworth, A.J.; Flora, G.D.; Gibbins, J.M. Non-genomic effects of nuclear receptors: Insights from the anucleate platelet. Cardiovasc. Res. 2018, 114, 645–655.
- Zakyrjanova, G.F.; Tsentsevitsky, A.N.; Kuznetsova, E.A.; Petrov, A.M. Immune-related oxysterol modulates neuromuscular transmission via non-genomic liver x receptor-dependent mechanism. Free Radic. Biol. Med. 2021, 174, 121–134.
- Pereira, J.P.; Kelly, L.M.; Xu, Y.; Cyster, J.G. Ebi2 mediates b cell segregation between the outer and centre follicle. Nature 2009, 460, 1122–1126.
- Barington, L.; Wanke, F.; Niss Arfelt, K.; Holst, P.J.; Kurschus, F.C.; Rosenkilde, M.M. Ebi2 in splenic and local immune responses and in autoimmunity. J. Leukoc. Biol. 2018, 104, 313–322.
- Benned-Jensen, T.; Smethurst, C.; Holst, P.J.; Page, K.R.; Sauls, H.; Sivertsen, B.; Schwartz, T.W.; Blanchard, A.; Jepras, R.; Rosenkilde, M.M. Ligand modulation of the epstein-barr virus-induced seven-transmembrane receptor ebi2: Identification of a potent and efficacious inverse agonist. J. Biol. Chem. 2011, 286, 29292–29302.
- Daugvilaite, V.; Arfelt, K.N.; Benned-Jensen, T.; Sailer, A.W.; Rosenkilde, M.M. Oxysterol-ebi2 signaling in immune regulation and viral infection. Eur. J. Immunol. 2014, 44, 1904–1912.
- Birkenbach, M.; Josefsen, K.; Yalamanchili, R.; Lenoir, G.; Kieff, E. Epstein-barr virus-induced genes: First lymphocyte-specific g protein-coupled peptide receptors. J. Virol. 1993, 67, 2209–2220.
- Hannedouche, S.; Zhang, J.; Yi, T.; Shen, W.; Nguyen, D.; Pereira, J.P.; Guerini, D.; Baumgarten, B.U.; Roggo, S.; Wen, B.; et al. Oxysterols direct immune cell migration via ebi2. Nature 2011, 475, 524–527.
- Liu, C.; Yang, X.V.; Wu, J.; Kuei, C.; Mani, N.S.; Zhang, L.; Yu, J.; Sutton, S.W.; Qin, N.; Banie, H.; et al. Oxysterols direct b-cell migration through ebi2. Nature 2011, 475, 519–523.
- Rosenkilde, M.M.; Benned-Jensen, T.; Andersen, H.; Holst, P.J.; Kledal, T.N.; Lüttichau, H.R.; Larsen, J.K.; Christensen, J.P.; Schwartz, T.W. Molecular pharmacological phenotyping of ebi2. An orphan seven-transmembrane receptor with constitutive activity. J. Biol. Chem. 2006, 281, 13199–13208.
- Heinig, M.; Petretto, E.; Wallace, C.; Bottolo, L.; Rotival, M.; Lu, H.; Li, Y.; Sarwar, R.; Langley, S.R.; Bauerfeind, A.; et al. A trans-acting locus regulates an anti-viral expression network and type 1 diabetes risk. Nature 2010, 467, 460–464.
- Nevius, E.; Pinho, F.; Dhodapkar, M.; Jin, H.; Nadrah, K.; Horowitz, M.C.; Kikuta, J.; Ishii, M.; Pereira, J.P. Oxysterols and ebi2 promote osteoclast precursor migration to bone surfaces and regulate bone mass homeostasis. J. Exp. Med. 2015, 212, 1931–1946.
- Shen, Z.-J.; Hu, J.; Kashi, V.P.; Kelly, E.A.; Denlinger, L.C.; Lutchman, K.; McDonald, J.G.; Jarjour, N.N.; Malter, J.S. Epstein-barr virus-induced gene 2 mediates allergen-induced leukocyte migration into airways. Am. J. Respir. Crit. Care Med. 2017, 195, 1576–1585.
- Amisten, S.; Braun, O.O.; Bengtsson, A.; Erlinge, D. Gene expression profiling for the identification of g-protein coupled receptors in human platelets. Thromb. Res. 2008, 122, 47–57.
- Rutkowska, A.; Preuss, I.; Gessier, F.; Sailer, A.W.; Dev, K.K. Ebi2 regulates intracellular signaling and migration in human astrocyte. Glia 2015, 63, 341–351.
- Zhang, P.; He, Q.; Chen, D.; Liu, W.; Wang, L.; Zhang, C.; Ma, D.; Li, W.; Liu, B.; Liu, F. G protein-coupled receptor 183 facilitates endothelial-to-hematopoietic transition via notch1 inhibition. Cell Res. 2015, 25, 1093–1107.
- Ki, S.; Thyagarajan, H.M.; Hu, Z.; Lancaster, J.N.; Ehrlich, L.I.R. Ebi2 contributes to the induction of thymic central tolerance in mice by promoting rapid motility of medullary thymocytes. Eur. J. Immunol. 2017, 47, 1906–1917.
- Benned-Jensen, T.; Madsen, C.M.; Arfelt, K.N.; Smethurts, C.; Blanchard, A.; Jepras, R.; Rosenkilde, M.M. Small molecule antagonism of oxysterol-induced epstein-barr virus induced gene 2 (ebi2) activation. FEBS Open Bio 2013, 3, 156–160.
- Sun, S.; Liu, C. 7α, 25-dihydroxycholesterol-mediated activation of ebi2 in immune regulation and diseases. Front. Pharmacol. 2015, 6, 60.
- Bartlett, S.; Gemiarto, A.T.; Ngo, M.D.; Sajiir, H.; Hailu, S.; Sinha, R.; Foo, C.X.; Kleynhans, L.; Tshivhula, H.; Webber, T.; et al. Gpr183 regulates interferons, autophagy, and bacterial growth during mycobacterium tuberculosis infection and is associated with tb disease severity. Front. Immunol. 2020, 11, 601534.
- Gatto, D.; Brink, R. B cell localization: Regulation by ebi2 and its oxysterol ligand. Trends Immunol. 2013, 34, 336–341.
- Honda, A.; Miyazaki, T.; Ikegami, T.; Iwamoto, J.; Maeda, T.; Hirayama, T.; Saito, Y.; Teramoto, T.; Matsuzaki, Y. Cholesterol 25-hydroxylation activity of cyp3a. J. Lipid Res. 2011, 52, 1509–1516.
- Diczfalusy, U.; Olofsson, K.E.; Carlsson, A.M.; Gong, M.; Golenbock, D.T.; Rooyackers, O.; Fläring, U.; Björkbacka, H. Marked upregulation of cholesterol 25-hydroxylase expression by lipopolysaccharide. J. Lipid Res. 2009, 50, 2258–2264.
- Wilkins, C.; Gale, M., Jr. Sterol-izing innate immunity. Immunity 2013, 38, 3–5.
- Bauman, D.R.; Bitmansour, A.D.; McDonald, J.G.; Thompson, B.M.; Liang, G.; Russell, D.W. 25-hydroxycholesterol secreted by macrophages in response to toll-like receptor activation suppresses immunoglobulin a production. Proc. Natl. Acad. Sci. USA 2009, 106, 16764–16769.
- Blanc, M.; Hsieh, W.Y.; Robertson, K.A.; Kropp, K.A.; Forster, T.; Shui, G.; Lacaze, P.; Watterson, S.; Griffiths, S.J.; Spann, N.J.; et al. The transcription factor stat-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity 2013, 38, 106–118.
- Ngo, M.D.; Bartlett, S.; Bielefeldt-Ohmann, H.; Foo, C.X.; Sinha, R.; Arachige, B.J.; Reed, S.; Mandrup-Poulsen, T.; Rosenkilde, M.M.; Ronacher, K. A blunted gpr183/oxysterol axis during dysglycemia results in delayed recruitment of macrophages to the lung during m. Tuberculosis infection. J. Infect. Dis. 2022, jiac102.
- Reboldi, A.; Dang, E.V.; McDonald, J.G.; Liang, G.; Russell, D.W.; Cyster, J.G. 25-hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type i interferon. Science 2014, 345, 679–684.
- Bottemanne, P.; Paquot, A.; Ameraoui, H.; Guillemot-Legris, O.; Alhouayek, M.; Muccioli, G.G. 25-hydroxycholesterol metabolism is altered by lung inflammation, and its local administration modulates lung inflammation in mice. FASEB J. 2021, 35, e21514.
- Yi, T.; Wang, X.; Kelly, L.M.; An, J.; Xu, Y.; Sailer, A.W.; Gustafsson, J.A.; Russell, D.W.; Cyster, J.G. Oxysterol gradient generation by lymphoid stromal cells guides activated b cell movement during humoral responses. Immunity 2012, 37, 535–548.
- Wanke, F.; Moos, S.; Croxford, A.L.; Heinen, A.P.; Gräf, S.; Kalt, B.; Tischner, D.; Zhang, J.; Christen, I.; Bruttger, J.; et al. Ebi2 is highly expressed in multiple sclerosis lesions and promotes early cns migration of encephalitogenic cd4t cells. Cell Rep. 2017, 18, 1270–1284.
- Raccosta, L.; Fontana, R.; Maggioni, D.; Lanterna, C.; Villablanca, E.J.; Paniccia, A.; Musumeci, A.; Chiricozzi, E.; Trincavelli, M.L.; Daniele, S.; et al. The oxysterol-cxcr2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J. Exp. Med. 2013, 210, 1711–1728.
- Sensi, C.; Daniele, S.; Parravicini, C.; Zappelli, E.; Russo, V.; Trincavelli, M.L.; Martini, C.; Abbracchio, M.P.; Eberini, I. Oxysterols act as promiscuous ligands of class-a gpcrs: In silico molecular modeling and in vitro validation. Cell. Signal. 2014, 26, 2614–2620.
- Nachtergaele, S.; Mydock, L.K.; Krishnan, K.; Rammohan, J.; Schlesinger, P.H.; Covey, D.F.; Rohatgi, R. Oxysterols are allosteric activators of the oncoprotein smoothened. Nat. Chem. Biol. 2012, 8, 211–220.
- Myers, B.R.; Sever, N.; Chong, Y.C.; Kim, J.; Belani, J.D.; Rychnovsky, S.; Bazan, J.F.; Beachy, P.A. Hedgehog pathway modulation by multiple lipid binding sites on the smoothened effector of signal response. Dev. Cell 2013, 26, 346–357.
- Spann, N.J.; Glass, C.K. Sterols and oxysterols in immune cell function. Nat. Immunol. 2013, 14, 893–900.
- Gatto, D.; Paus, D.; Basten, A.; Mackay, C.R.; Brink, R. Guidance of b cells by the orphan g protein-coupled receptor ebi2 shapes humoral immune responses. Immunity 2009, 31, 259–269.
- Chalmin, F.; Rochemont, V.; Lippens, C.; Clottu, A.; Sailer, A.W.; Merkler, D.; Hugues, S.; Pot, C. Oxysterols regulate encephalitogenic cd4(+) t cell trafficking during central nervous system autoimmunity. J. Autoimmun. 2015, 56, 45–55.
- Soroosh, P.; Wu, J.; Xue, X.; Song, J.; Sutton, S.W.; Sablad, M.; Yu, J.; Nelen, M.I.; Liu, X.; Castro, G.; et al. Oxysterols are agonist ligands of rorγt and drive th17 cell differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 12163–12168.
- Cui, G.; Qin, X.; Wu, L.; Zhang, Y.; Sheng, X.; Yu, Q.; Sheng, H.; Xi, B.; Zhang, J.Z.; Zang, Y.Q. Liver x receptor (lxr) mediates negative regulation of mouse and human th17 differentiation. J. Clin. Investig. 2011, 121, 658–670.
- Olkkonen, V.M. Macrophage oxysterols and their binding proteins: Roles in atherosclerosis. Curr. Opin. Lipidol. 2012, 23, 462–470.
- Shibata, N.; Glass, C.K. Macrophages, oxysterols and atherosclerosis. Circ. J. 2010, 74, 2045–2051.
- Perry, V.H.; Gordon, S. Macrophages and the nervous system. Int. Rev. Cytol. 1991, 125, 203–244.
- Kimura, T.; Nada, S.; Takegahara, N.; Okuno, T.; Nojima, S.; Kang, S.; Ito, D.; Morimoto, K.; Hosokawa, T.; Hayama, Y.; et al. Polarization of m2 macrophages requires lamtor1 that integrates cytokine and amino-acid signals. Nat. Commun. 2016, 7, 13130.
- Li, Z.; Martin, M.; Zhang, J.; Huang, H.Y.; Bai, L.; Zhang, J.; Kang, J.; He, M.; Li, J.; Maurya, M.R.; et al. Krüppel-like factor 4 regulation of cholesterol-25-hydroxylase and liver x receptor mitigates atherosclerosis susceptibility. Circulation 2017, 136, 1315–1330.
- Marengo, B.; Bellora, F.; Ricciarelli, R.; De Ciucis, C.; Furfaro, A.; Leardi, R.; Colla, R.; Pacini, D.; Traverso, N.; Moretta, A.; et al. Oxysterol mixture and, in particular, 27-hydroxycholesterol drive m2 polarization of human macrophages. BioFactors 2016, 42, 80–92.
- Dennis, E.A.; Deems, R.A.; Harkewicz, R.; Quehenberger, O.; Brown, H.A.; Milne, S.B.; Myers, D.S.; Glass, C.K.; Hardiman, G.; Reichart, D.; et al. A mouse macrophage lipidome. J. Biol. Chem. 2010, 285, 39976–39985.
- Janowski, B.A.; Willy, P.J.; Devi, T.R.; Falck, J.R.; Mangelsdorf, D.J. An oxysterol signalling pathway mediated by the nuclear receptor lxr alpha. Nature 1996, 383, 728–731.
- Ghisletti, S.; Huang, W.; Ogawa, S.; Pascual, G.; Lin, M.E.; Willson, T.M.; Rosenfeld, M.G.; Glass, C.K. Parallel sumoylation-dependent pathways mediate gene- and signal-specific transrepression by lxrs and ppargamma. Mol. Cell 2007, 25, 57–70.
- McDonald, J.G.; Thompson, B.M.; McCrum, E.C.; Russell, D.W. Extraction and analysis of sterols in biological matrices by high performance liquid chromatography electrospray ionization mass spectrometry. Methods Enzymol. 2007, 432, 145–170.
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M.; Leevers, S.; Griffiths, J.R.; Chung, Y.L.; Schulze, A. Srebp activity is regulated by mtorc1 and contributes to akt-dependent cell growth. Cell Metab. 2008, 8, 224–236.
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a metabolic gene regulatory network downstream of mtor complex 1. Mol. Cell 2010, 39, 171–183.
- Castrillo, A.; Joseph, S.B.; Vaidya, S.A.; Haberland, M.; Fogelman, A.M.; Cheng, G.; Tontonoz, P. Crosstalk between lxr and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol. Cell 2003, 12, 805–816.
- Joseph, S.B.; Bradley, M.N.; Castrillo, A.; Bruhn, K.W.; Mak, P.A.; Pei, L.; Hogenesch, J.; O’Connell R, M.; Cheng, G.; Saez, E.; et al. Lxr-dependent gene expression is important for macrophage survival and the innate immune response. Cell 2004, 119, 299–309.
- Dang, E.V.; McDonald, J.G.; Russell, D.W.; Cyster, J.G. Oxysterol restraint of cholesterol synthesis prevents aim2 inflammasome activation. Cell 2017, 171, 1057–1071.e1011.
- Geyeregger, R.; Zeyda, M.; Bauer, W.; Kriehuber, E.; Säemann, M.D.; Zlabinger, G.J.; Maurer, D.; Stulnig, T.M. Liver x receptors regulate dendritic cell phenotype and function through blocked induction of the actin-bundling protein fascin. Blood 2007, 109, 4288–4295.
- Trousson, A.; Bernard, S.; Petit, P.X.; Liere, P.; Pianos, A.; El Hadri, K.; Lobaccaro, J.M.; Ghandour, M.S.; Raymondjean, M.; Schumacher, M.; et al. 25-hydroxycholesterol provokes oligodendrocyte cell line apoptosis and stimulates the secreted phospholipase a2 type iia via lxr beta and pxr. J. Neurochem. 2009, 109, 945–958.
- Rutkowska, A.; Dev, K.K.; Sailer, A.W. The role of the oxysterol/ebi2 pathway in the immune and central nervous systems. Curr. Drug Targets 2016, 17, 1851–1860.
More
Information
Subjects:
Immunology; Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
21 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




