| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wan Zaireen Nisa Yahya | + 3198 word(s) | 3198 | 2020-09-23 04:58:08 |
Video Upload Options
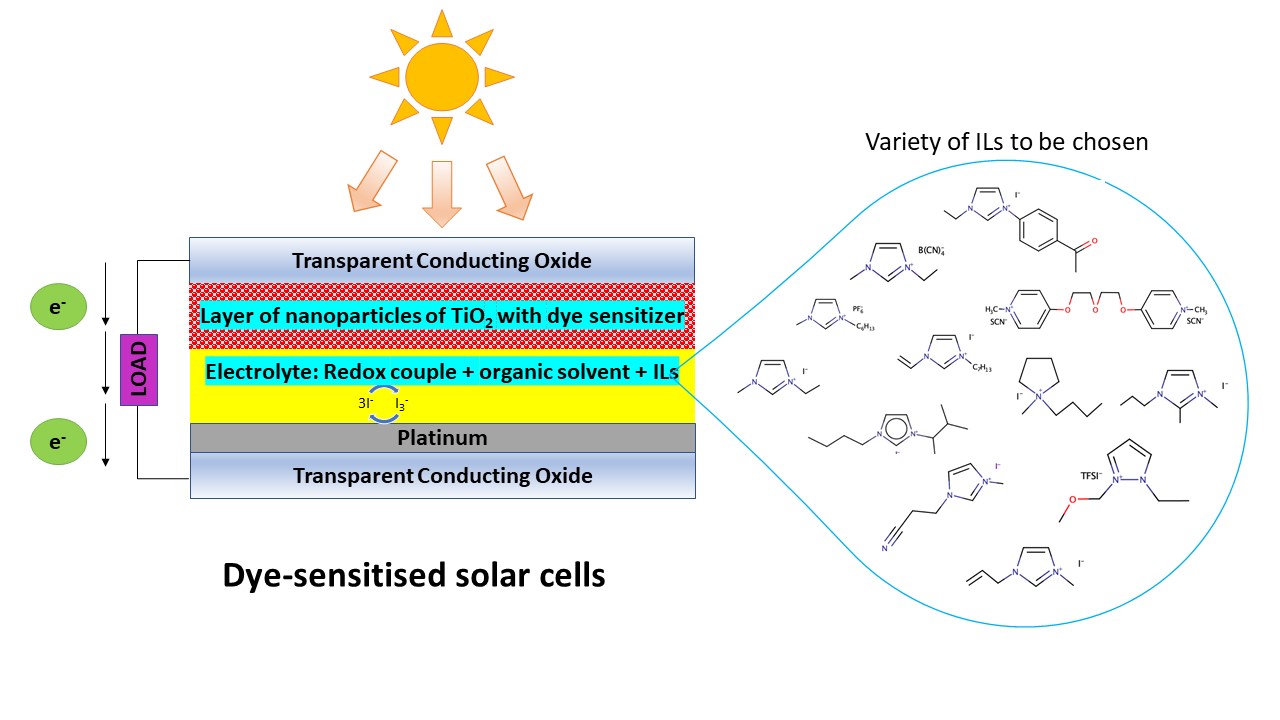
The dye-sensitized solar cells (DSSCs) which are considered as the third-generation solar cells have a huge potential to be commercialized due to their low cost, simplicity in fabrication, and promising photon-to-electrical energy conversion efficiency. Nevertheless, a high cell efficiency can only be achieved when an organic solvent is incorporated into the formulation of the electrolyte, which is prone to evaporation and leakage. As a result, DSSCs become unsuitable for long-run usage due to thermal instability in the electrolyte. The early intention of incorporating ionic liquids (ILs) into the electrolyte was to curb the abovementioned problem and to enable the DSSCs to function as a sustainable energy device. ILs have been incorporated into the electrolyte formulation and the extent of how the ILs can affect the cell efficiency in various electrolyte states is highlighted. This sheds light on the true purpose of introducing ILs into DSSC electrolyte, which is to enhance the ionicity of the electrolyte.
1. Introduction
Solar energy is a clean and the most abundantly available renewable energy. Only 10 min is required for the sun to irradiate the earth’s surface to be equal to annual energy consumption. Photovoltaic technology is a method that converts solar energy to electrical energy [1]. Among the three generations of the photovoltaic cells, the third generation, particularly the dye-sensitized solar cells (DSSCs) have garnered much attention from researchers owing to the costly production and the environmental pollution that had arisen from the commercialized first and second generations of photovoltaic cells [1][2].
The DSSCs have a huge potential to be commercialized due to low cost and simplicity in fabrication. They gained attention when the technology had a breakthrough with its cell efficiency that rose to ~7–8%. This was achieved by introducing nanometer-sized titanium oxide (TiO2) to the working electrode surface in the year 1991 [3]. Before that, the maximum efficiency was stagnant at not more than 1%. Since then, many studies have reported improvement in the DSSC’s main components, such as the dye, the electrolyte, and the electrodes, in the attempt to increase cell efficiency. As a result, the cell efficiency has slowly increased, not drastically but significantly. This reflects the importance of improving every aspect of the DSSCs to gain high cell efficiency.
An organic solvent, namely acetonitrile (ACN), is commonly incorporated to form the liquid electrolyte for DSSCs. It has a low boiling point and easily vaporizes under heat stress. This is a major drawback that makes the DSSCs unsuitable for a long-run usage due to the thermal instability of the electrolyte, thus DSSCs are considered to be unfit as a sustainable energy device. Subsequently, higher boiling point solvents of more than 100 °C have been widely used to replace acetonitrile, namely valeronitrile, 3-methoxypropionitrile (MPN), N-methyl-2-pyrrolidone (NMP), ethylene carbonate, and methylene carbonate, to form a stable electrolyte [4]. Nevertheless, the DSSCs still face the problem of evaporation and leakage through the sealant at elevated temperatures [5]. This indirectly points out the difficulty for DSSCs to be commercialized for outdoor applications.
Also known as designer’s solvents, ILs are usually designed and synthesized for specific purposes. The combinations of various anions with cations, along with a range of pendants or functional groups, enable the synthesis of the desired ILs with specific properties. The cyclic-based ILs, particularly imidazolium-based ILs have become the prevailing choice in studies concerning IL-based electrolytes for the past two decades. Among the members of the heterocyclic family, imidazolium has been commonly used as the cation for ILs due to several properties, such as lower viscosity, higher diffusivity, higher density, and higher thermal stability, when compared to pyridinium, pyrrolidinium, and ammonium [6]. Besides, the electrochemical windows of the ILs were mostly reported at about 4 V, which appeared practical for many electrochemical devices, such as DSSCs [7].
For DSSCs to be commercialized, they should exhibit high efficiency and long-term stability. These two aspects are the technical challenges that demand further investigations. As a multi-component device, each DSSC aspect must be optimized. An electrolyte in DSSCs primarily serves as a medium for a redox reaction to occur. A well-articulated process contributes to cell efficiency [8]. Although the major concerns for DSSCs commercialization are poor long-term stability and low efficiency (due to the direct connection with electrolyte component), surprisingly, only 11% of all studies related to electrolyte had probed into DSSCs until the year 2015 [9]. The search for an ideal solvent for DSSCs electrolytes is crucial as it determines the efficiency of fabricated DSSCs.
2. Ionic Liquids in Liquid, Solid and Quasi-Solid Electrolyte for DSSCs
The three types of electrolytes for DSSCs are liquid, solid, and quasi solid electrolytes. In liquid electrolyte, ACN is the main solvent by far that offers the highest cell efficiency [10]. Being the simplest organic nitrile, ACN (low in viscosity of 0.536 mPa·s at 25 °C) dissolves other electrolyte components including redox couple and additive, to enable the redox reaction to occur. Mass transport is governed by the gradient diffusions of the species present in the electrolyte. A solvent with low viscosity enhances the diffusion coefficient of the redox couple, besides allowing high ionic conductivity, and more importantly, high cell efficiency [6]. The drawback of using an organic solvent as mentioned earlier is that it easily vaporizes despite having a high boiling point, thus unsuitable for long-term usage as the cell is bound to experience solvent evaporation over time. As a result, ILs were introduced to solve the problem posed by organic solvents. Ionic liquids (ILs) have an unprecedented high boiling point with negligible vapor pressure. For each study reviewed here, the function of ILs is translated into two; additives and solvent.
In 2001, Nazeeruddin et al. [11] reported a cell efficiency of 10.4% by introducing a black dye (ruthenium-based) with better light-harvesting properties. In 2005, they [12]reported other ruthenium-based dyes in DSSCs, namely N719/N749 dye, which had resulted in high cell efficiency of ~11–12%. In 2018, a cell efficiency of 14.3% was recorded, which was the highest by far (under standard light intensity) by using cobalt (III/II) tris(1,10-phenanthroline) complex as redox couple in the electrolyte [13] Ionic liquids were also incorporated in the electrolytes but at lower concentrations playing the role of additives. However, the high cell efficiency reported in the aforementioned study was attained through the use of volatile organic solvent as the solvent for the electrolytes. The most obvious solution to this instability issue is the complete elimination of liquid-based electrolyte and the incorporation of solid-state hole transport material (ss HTM) to take up the role of electrolyte and to form solid-state DSSC (ss-DSSC). Recently, an 11%-cell efficiency was achieved by using an inorganic HTM [Cu(4,4′,6,60-tetramethyl-2,2′-bipyridine)2](bis(trifluoromethylsulfonyl)imide)2 with [Cu (4,4′,6,6′- tetramethyl-2,2′-bipyridine)2](bis(trifluoromethylsulfonyl)imide) [14]. However, the noted efficiency was still lower than a liquid-based electrolyte, mainly due to the low conductivity nature of HTM and the inefficient HTM filling onto mesoporous TiO2, thus causing poor contact of the HTM with the mesoporous TiO2 [14]. Besides, the lack of stability data reported on the ss-DSSCs has raised concern on the long-term performance of ss-DSSCs.
Papageorgiou et al. (1996)[15] reported one of the earliest attempts to obtain better photoconversion efficiency by introducing low viscosity ILs mixtures. This was achieved by mixing two or more ILs of different viscosity values. In the study, the viscosity of 1-hexyl-3-methylimidazolium iodide (HMImI) was reduced by adding a low viscosity IL namely 1-ethyl-3-methylimidazolium triflate (EMImTf). The said mixture in the electrolyte of DSSCs resulted in a better photo energy conversion efficiency when compared to HMImI alone. The addition of EMImTf to HMImI had increased the diffusion coefficient of triiodide by 1.6%, in comparison to the use of HMImI alone, wherein the conversion efficiency was ~7%.
Mhd Yusof and Yahya [16]had attempted to overcome the low mass transportation in viscous IL by adding low viscosity 1-butyl-3-methylimidazolium thiocyanate (BMISCN) (56 mPa·s) into IL of higher viscosity, 1-propyl-3-methylimidazolium iodide(PMII) (336 mPa·s). These two ILs were mixed at four different ratios to study their effect on cell efficiency. As a result, the mixture ratio of PMII to BMISCN at 1:0.75 gave the highest power conversion efficiency at 1.89%. Although the mixture ratio of PMII to BMISCN at 1:1 gave the lowest viscosity (68 mPa·s) with the highest triiodide diffusion coefficient, the power conversion efficiency was only 1.52%. This indicated that the viscosity and diffusion coefficient are not the only factors that determine cell efficiency.
In the year 2008, Cao et al. [17] employed the classic choice of redox couple; I3-/I- and explored several known ILs to prepare an efficient electrolyte for DSSCs. They had assessed several ILs of different density, conductivity, and fluidity to combine with I3-/I- redox couple in forming an electrolyte for DSSCs. The ILs employed were 1-hexyl-3-methylimidazolium iodide (HMII), 1-butyl-3-methylimidazolium iodide (BMII), 1-propyl-3-methylimidazolium iodide (PMII), as well as a mixture of 1-ethyl-3-methylimidazolium iodide, 1,3-dimethylimidazolium iodide, and 1-allyl-3-methylimidazolium iodide (EMII/DMII/AMII). The study highlighted the importance of using low viscosity redox couple (triiodide/iodide). As a result, EMII/DMII/AMII-based electrolyte of DSSC displayed better power conversion efficiency when compared to single ILs (HMII, BMII, and PMII), mainly because the mixture had high fluidity and low density.
Kang et al. (2004) [18] reported the usage of pure IL as a solvent of the electrolyte for DSSCs. Novel IL, 1-vinyl-3-heptylimidazolium iodide (VHpII) was used as the solvent for the electrolyte. The thermogravimetric analysis revealed that the VHpII was stable for thermal stress up to 250 °C and non-volatile at 150 °C, thus suggesting the low probability of photovoltaic cell leakage due to long exposure to irradiation, which was suffered by the organic solvent-based DSSCs electrolytes. The conversion efficiency was reported to be 2.63%. Nonetheless, the addition of lithium iodide (LiI) as part of the electrolyte had increased the efficiency to 3.63%. The obvious increase in Jsc value upon the addition of LiI was observed from 6.63 mA×cm−2 (before addition) to 9.61 mA×cm−2 (after addition).
In a countless number of studies, the addition of ILs into organic solvent-based electrolyte was often claimed to solve its instability that could easily vaporize, especially in elevated temperature or long-term condition. Over time, the organic solvent will evaporate and cause deterioration of the solar cells. This has become the main reason for introducing polymer-based electrolyte. The polymer-based electrolytes can be further classified into solid and gel polymer electrolytes (GPEs). These two can be differentiated based on their physical attributes, in which the solid polymer electrolyte has a solid physical characteristic, while GPE is between solid and liquid (gel-like properties). This consistency classically can be achieved by using certain preparation methods, such as heat-induced or UV-cured or simply by adding certain materials, such as plasticizer, into the mixture of polymer host with the electrolyte components[19].
Polymer electrolytes in both solid and gel states have very low ionic conductivity [20]. The presence of salt, such as LiI and KI, together with the polymer host material, is integral to provide the necessary ionicity to the polymer electrolyte. Upon the discovery of IL, it has gained popularity to be incorporated into the polymer electrolyte, to enhance the ionicity and the overall performance of the photovoltaic cell [20]. Due to this, ILs had been substantially mixed into the polymer host to improve the transport properties of these materials towards achieving high cell efficiency. Often, the effect that takes place due to the improvements of the electrolyte properties can be observed on the cell performance parameters, such as Jsc, Voc, and FF.
Polyethylene oxide (PEO) a commonly used polymer in electrolyte studies, which is semi-crystalline, impedes the electron movement and decreases the ionic conductivity. The addition of imidazolium-based IL into GPE was intended to overcome this disadvantage. The presence of ILs in PEO was intended as a plasticizer to decrease the crystallinity and simultaneously increase the probability for the electrolyte to infiltrate into the TiO2 layer. This is beneficial in terms of ionic conductivity. Syairah et al. [21] evaluated the incorporation of imidazolium-based ILs of different alkyl chain lengths into PEO with ethylene carbonate (EC) and propylene carbonate (PC) as solvents. Ionic liquids (ILs), namely 1-methyl-3-propylimidazolium iodide (PMII), 1-butyl-3-methylimidazolium (BMII), and 1-hexyl-3-methylimidazolium iodide (HMII), were used as an additive in the GPE. The study was focused on establishing a connection between the use of imidazolium-based ILs of different alkyl chain lengths and the resulting ionic conductivities of the GPEs and the DSSCs power conversion efficiencies. As a result, the addition of ILs into the GPEs had increased the ionic conductivity, whereby the presence of the lone pair electron in the conjugated system of imidazolium-based IL had facilitated the flow of ions. The highest conductivity was obtained at 9.41 mS·cm−1, which also led to the highest conversion efficiency at 9.35%, in comparison to GPE without IL. From the current versus voltage (I-V) data of the solar cells, it was revealed that the addition of ILs had increased the Voc. Furthermore, the usage of low viscosity ILs had improved the transport properties of the electrolyte.
Li et al. [22] reported the imbibition of ILs into the polymer host poly(acrylic acid/gelatin) [poly(AA/GR)], and poly(acrylic acid/cetyltrimethylammonium ammonium bromide) [poly(AA/CTAB)] matrices to increase ionic conductivity, cell efficiency, and stability. The study compared the efficiency and the stability of the cells that used ILs imbibed polymer-based electrolyte with ACN imbibed polymer-based electrolyte. Their primary concern was on the nature of the organic solvent, ACN, which easily vaporized when incorporated in a GPE. For the preparation of the IL electrolyte, 0.5 M I2 and 0.01 M LiI were added into 40 volume % PMII, 50 volume % [AMIM]BF4, and 10 volume % N-methyl pyrrolidone. The GPEs were prepared by immersing 0.2 g of [poly (AA/GR)] or [poly (AA/CTAB)] into the IL electrolyte formulation. The same procedure was conducted to obtain the ACN-contained iodide imbibed poly (AA/GR) and the ACN-contained iodide imbibed poly (AA/CTAB), except that the ACN was used as a solvent in the electrolyte formulation. The ionic conductivity of ILs imbibed [poly(AA/GR)], ILs imbibed [poly(AA/CTAB)], ACN-contained iodide imbibed poly(AA/GR), and ACN-contained iodide imbibed poly(AA/CTAB) were 15.36, 12.95, 13.84, and 10.73 mS·cm−1, respectively. The cell that had incorporated ILs imbibed [poly (AA/GR)] as electrolyte showed the highest cell efficiency of 7.19%. This value was comparable to the efficiency recorded when the cell employed IL electrolyte alone (without polymer matrix), which was at 7.27%. As for the stability test, the data showed that the DSSCs with ILs imbibed [poly(AA/GR)] and poly(AA/CTAB) retained 97% of their initial efficiencies, which were better than those with ACN-contained iodide imbibed [poly(AA/GR)] and poly(AA/CTAB), which only managed to retain 83% of their initial efficiencies.
The transport mechanism in an electrolyte is principally through conventional or physical diffusion. The viscosity of the electrolyte is a key factor in determining the efficiency of ion transport. This is true for the case of liquid electrolyte, in which the use of ILs with low viscosity is favored as they promote physical diffusion that increases ionic conductivity. Another transport mechanism refers to transfer diffusion or electron exchange[23][24][25], which occurs due to effective collision during physical diffusion. This mechanism is significant mainly in the case of the usage of very high viscosity or solid ILs, wherein physical diffusion is not favorable. In a study by Yamanaka et al. [26], a very ordered structure of highly viscous ILs had positively affected Jsc. It was observed that the use of imidazolium-based ILs with alkyl chain of 12 carbons formed an ordered smectic A phase, which gave better Jsc, when compared to one without smectic A phase (imidazolium-based ILs with alkyl chain of 11 carbons (C11MImI)). In smectic A phase, the ions are well aligned, and this helps to ease the electron exchange to occur. Although the viscosity of C12MImI was high due to the 12-carbon alkyl chain, the nature of C12MImI in ordered smectic A phase eases electron hopping and increases conductivity, when compared to C11MImI with lower viscosity. The addition of gelator to C12MImI with iodine in the electrolyte had increased the Jsc value from 7.0 to 7.7 mA·cm−2. The increment in Jsc is attributed to the organization of the structure.
The study had inspired Pan et al. in 2013 [27] to use ILs with smectic A phase to be incorporated as electrolyte for DSSCs. Thus, ILs with long alkyl chain, 1-dodecyl-3-ethylimidazolium iodide (C12EImI) that physically exists as solid and 1-decyl-3-ethylimidazolium iodide (C10EImI) that exists as a thick liquid, were chosen to be combined with iodine to serve as electrolytes for DSSCs. The cell that employed C12EImI achieved 2.57% power conversion efficiency, which was greater than that of C10EImI. The diffusion coefficient of triiodide in C12EImI was greater than C10EImI due to the presence of the smectic A phase. This phase promotes exchange reaction (Dex), which is beneficial to the transport properties of polyionic liquid that is in solid form. As C10EImI existed physically as a liquid, this study signifies that the physical characteristics of the electrolyte are not the only factor that determines the cell efficiency.
In the works included as part of this review, the incorporation of ILs into the electrolyte formulation had affected, in particular, the transport properties of the electrolyte, such as viscosity, ionic conductivity, and diffusion coefficient [15][16][17][29][30][31]. The combination of a variety of cations with anions, the introduction of certain functional groups into the moiety of cations, or the addition of one or more high fluidity ILs into another IL were intended specifically to improve these properties [15][16][17][19][20][28][29][30][31][32]. The two essential parameters that determine cell efficiency are Jsc and Voc. These parameters were correlated with how well the processes involving the transport of electrons occur, such as the electron injection at the interface of TiO2/electrolyte, the regeneration process of the oxidized dye, and the shielding of the electron recombination [32][33]. The use of an organic solvent, which is less viscous, renders the electron injection at the interface of TiO2/electrolyte and the regeneration of oxidized dye to take place efficiently, thus resulting in high cell efficiency. The use of organic solvent enables to solvate the redox couple, hence causing the diffusion coefficient of triiodide to be high, easing the regeneration of oxidized dye [9] and reducing the possibility of recombination from occurring.
3. Conclusions
A variety of ILs have been used in DSSCs electrolyte. Among them, ILs based on imidazolium cation and iodide anion are preferred. The former is due to the low viscosity of the resulting ILs, whereas the latter serves as one of the redox couples, crucial for the dye regeneration. Other types of cations (e.g., pyridinium, pyrazolium, and pyrrolidinium) and anions (e.g., tetracyanoborate, thiocyanate, bromide, and bis(trifluoromethylsulfonyl)imide) have been reported as well. Most studies that had assessed liquid or polymer type electrolytes emphasized the incorporation of certain functional group(s) to offer desirable properties, such as low viscosity and high ionic conductivity. Improved transport properties are critical, as the ILs are meant to solve the problems that arise due to the usage of organic solvent. Although acetonitrile and other nitrile-based solvents have excellent transport properties, they exhibit poor thermal stability. However, the total elimination of organic solvents from DSSCs electrolyte resulted mostly in lower cell conversion efficiency. Nevertheless, it is to highlight that the utilization of ILs as an excellent ionicity provider is relevant towards providing long term stability and sustainable energy devices.
References
- Parida, B.; Iniyan, S.; Goic, R.; A review of solar photovoltaic technologies. Renew. Sustain. Energy Rev. 2011, 153, 1625–1636, 10.1016/j.rser.2010.11.032.
- Mozaffari, S.; Nateghi, M.R.; Zarandi, M.B; An overview of the Challenges in the commercialization of dye sensitized solar cells. Renew. Sustain. Energy Rev. 2017, 71, 675–686, 10.1016/j.rser.2016.12.096.
- O’Regan, B.; Grätzel, M.; A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737-740, 10.1038/353737a0.
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L.; Luo, G.; Electrolytes in Dye-Sensitized Solar Cells. Chem. Rev. 2015, 115, 2136–2173, 10.1021/cr400675m.
- Harikisun, R.; Desilvestro, H.; Long-term stability of dye solar cells. Sol. Energy 2011, 85, 1179–1188, 10.1016/j.solener.2010.10.016.
- Ahosseini, A.; Scurto, A.M.; Viscosity of Imidazolium-Based Ionic Liquids at Elevated Pressures: Cation and Anion Effects. Int. J. Thermophys. 2008, 29, 1222–1243, 10.1007/s10765-008-0497-7.
- Murugesan, S.; Quintero, O.A.; Chou, B.P.; Xiao, P.; Park, K.; Hall, J.W.; Jones, R.A.; Henkelman, G.; Goodenough, J.B.; Stevenson, K.J.; et al. Wide electrochemical window ionic salt for use in electropositive metal electrodeposition and solid state Li-ion batteries . J. Mater. Chem. A 2014, 2, 2194–2201, 10.1039/c3ta15010k.
- Gorlov, M.; Kloo, L.; Ionic liquid electrolytes for dye-sensitized solar cells. Dalton Trans. 2008, 20, 2655–2666, 10.1039/b716419.
- Iftikhar, H.; Sonai, G.G.; Hashmi, S.G.; Nogueira, A.F.; Lund, P.D.; Progress on Electrolytes Development in Dye-Sensitized Solar Cells . Materials 2019, 12, 1998, 10.3390/ma12121998.
- Nazeeruddin, M.K.; De Angelis, F.; Fantacci, S.; Selloni, A.; Viscardi, G.; Liska, P.; Grätzel, M.; Combined Experimental and DFT-TDDFT Computational Study of Photoelectrochemical Cell Ruthenium Sensitizers. J. Am. Chem. Soc. 2005, 127, 16835–16847, 10.1021/ja052467l.
- Nazeeruddin, M.K.; Péchy, P.; Renouard, T.; Zakeeruddin, S.M.; Humphry-Baker, R.; Comte, P.; Liska, P.; Cevey, L.; Costa, E.; Shklover, V.; et al.et al. Engineering of Efficient Panchromatic Sensitizers for Nanocrystalline TiO2-Based Solar Cells. J. Am. Chem. Soc. 2001, 123, 1613–1624, 10.1021/ja003299u.
- Nazeeruddin, M.K.; De Angelis, F.; Fantacci, S.; Selloni, A.; Viscardi, G.; Liska, P.; Grätzel, M.; Combined Experimental and DFT-TDDFT Computational Study of Photoelectrochemical Cell Ruthenium Sensitizers. J. Am. Chem. Soc. 2005, 127, 16835–16847, 10.1021/ja052467l.
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.I.; Hanaya, M.; Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897, 10.1039/c5cc06759f.
- Cao, Y.; Saygili, Y.; Ummadisingu, A.; Teuscher, J.; Luo, J.; Pellet, N.; Giordano, F.; Zakeeruddin, S.M.; Moser, J.; Freitag, M.; et al.et al. 11% efficiency solid-state dye-sensitized solar cells with copper (II/I) hole transport materials. Nat. Commun. 2017, 8, 15390, 10.1038/ncomms15390.
- Papageorgiou, N.; Athanassov, Y.; Armand, P.; Bonhote, H.; Azam, A.; Grätzel, M.; The Performance and Stability of Ambient Temperature Molten Salts for Solar Cell Applications. J. Electrochem. Soc. 1996, 143, 3099, 10.1149/1.1837171.
- Yusof, S.M.M.; Yahya, W.Z.N.; Binary Ionic Liquid Electrolyte for Dye-Sensitized Solar Cells. Procedia Eng. 2016, 148, 100–105, 10.1016 /j.proeng.2016.06 .453.
- Cao, Y.; Zhang, J.; Bai, Y.; Li, R.; Zakeeruddin, S.M.; Grätzel, M.; Wang, P.; Dye-sensitized solar cells with solvent-free ionic liquid electrolytes . J. Phys. Chem. C 2008, 112, 13775–13781, 10.1021/jp805027v.
- Kang, M.G.; Ryu, K.S.; Chang, S.H.; Park, N.G.; A new ionic liquid for a redox electrolyte of dye-sensitized solar cells. ETRI J. 2004, 26, 647–652, 10.4218/etrij.04.0103.0152.
- Su’ait, M.S.; Rahman, M.Y.A.; Ahmad, A.; Review on Polymer Electrolyte in Dye-Sensitized Solar Cells (DSSCs). Sol. Energy 2015, 115, 452–470, 10.1016/j.solener.2015.02.043.
- Kambe, S.; Nakade, S.; Kitamura, T.; Wada, Y.; Yanagida, S.; Influence of the electrolytes on electron transport in mesoporous TiO2-electrolyte systems. J. Phys. Chem. B 2002, 106, 2967–2972, 10.1021/jp013397h.
- Syairah, A.; Khanmirzaei, M.H.; Saidi, N.M.; Farhana, N.K.; Ramesh, S.; Ramesh, K.; Ramesh, S.; Effect of different imidazolium-based ionic liquids on gel polymer electrolytes for dye-sensitized solar cells. Ionics 2019, 25, 2427–2435, 10.1007/s11581-018-2603-6.
- Li, Q.; Tang, Q.; He, B.; Yang, P.; Full-ionic liquid gel electrolytes: Enhanced photovoltaic performances in dye-sensitized solar cells. Power Sources 2014, 264, 83–91, 10.1016/j.jpowsour.2014.04.095.
- Ruff, I.; Friedrich, V.J.; Transfer Diffusion. I. Theoretical. J. Phys. Chem. 1971, 75, 3297–3302, 10.1021/j100690a016.
- Ruff, I.; Friedrich, V.J.; Demeter, K.; Csillag, K.; Transfer diffusion. II. Kinetics of electron exchange reaction between ferrocene and ferricinium ion in alcohols . J. Phys. Chem. 1971, 75, 3303–3309, 10.1021/j100690a017.
- Ruff, I.; Botár, L.; Effect of exchange reactions on transport processes: A comparison of thermodynamic treatment with random walk on lattice points. J. Chem. Phys. 1985, 83, 1292–1297, 10.1063/1.449445.
- 44. Yamanaka, N.; Kawano, R.; Kubo, W.; Masaki, N.; Kitamura, T.; Wada, Y.; Watanabe, M.; Yanagida, S.; Dye-Sensitized TiO2 Solar Cells Using Imidazolium-Type Ionic Liquid Crystal Systems as Effective Electrolytes. J. Phys. Chem. B 2007, 111, 4763–4769, 10.1021/jp0671446.
- Pan, X.; Wang, M.; Fang, X.; Zhang, C.; Huo, Z.; Dai, S.; Ionic liquid crystal-based electrolyte with enhanced charge transport for dye-sensitized solar cells. Sci. China Chem. 2013, 56, 1463–1469, 10.1007/s11426-013-4904-y.
- Chi, W.S.; Ahn, S.H.; Jeon, H.; Shul, Y.G.; Kim, J.H.; Rubbery copolymer electrolytes containing polymerized ionic liquid for dye-sensitized solar cells. J. Solid State Electrochem. 2012, 16, 3037–3043, 10.1007/s10008-012-1738-z.
- Gao, F.; Wang, Y.; Shi, D.; Zhang, J.; Wang, M.; Jing, X.; Humprey-Baker, R.; Wang, P.; Zakeeruddin, S.M.; Enhance the Optical Absorptivity of Nanocrystalline TiO2 Film with High Molar Extinction Coefficient Ruthenium Sensitizers for High Performance Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2008, 130, 10720–10728, 10.1021/ja801942j.
- Fang, Y.; Ma, P.; Cheng, H.; Tan, G.; Wu, J.; Zheng, J.; Fang, S.; Dai, Y.; Lin, Y.; Synthesis of Low-Viscosity Ionic Liquids for Application in Dye-Sensitized Solar Cells. Chem. Asian J. 2019, 14, 4201–4206, 10.1002/asia.201901130.
- Zheng, Y.; Huang, Q.; Fang, S.; Yang, L.; Gan, Y.; Ether-Functionalized Pyrazolium Ionic Liquids as Electrolytes for Dye Sensitized Solar Cells. Int. J. Electrochem. Sci. 2013, 8, 9558–9567.
- Grätzel, M.; Dye-sensitized solar cells. J. Photochem. Photobiol. C 2003, 4, 145–153, 10.1016/S1389-5567(03)00026-1.
- Carella, A.; Borbone, F.; Centore, R.; Research Progress on Photosensitizers for DSSC. Front. Chem. 2018, 6, 481, 10.3389/fchem.2018.00481.