Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Federica Sodano | -- | 2685 | 2022-04-20 08:57:05 | | | |
| 2 | Rita Xu | Meta information modification | 2685 | 2022-04-20 09:45:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sodano, F.; Gazzano, E.; Fruttero, R.; Lazzarato, L. NO in Viral Infections. Encyclopedia. Available online: https://encyclopedia.pub/entry/21978 (accessed on 07 February 2026).
Sodano F, Gazzano E, Fruttero R, Lazzarato L. NO in Viral Infections. Encyclopedia. Available at: https://encyclopedia.pub/entry/21978. Accessed February 07, 2026.
Sodano, Federica, Elena Gazzano, Roberta Fruttero, Loretta Lazzarato. "NO in Viral Infections" Encyclopedia, https://encyclopedia.pub/entry/21978 (accessed February 07, 2026).
Sodano, F., Gazzano, E., Fruttero, R., & Lazzarato, L. (2022, April 20). NO in Viral Infections. In Encyclopedia. https://encyclopedia.pub/entry/21978
Sodano, Federica, et al. "NO in Viral Infections." Encyclopedia. Web. 20 April, 2022.
Copy Citation
Nitric oxide is a ubiquitous signaling radical that influences critical body functions. Its importance in the cardiovascular system and the innate immune response to bacterial and viral infections has been extensively investigated. The overproduction of NO is an early component of viral infections, including those affecting the respiratory tract. The production of high levels of NO is due to the overexpression of NO biosynthesis by inducible NO synthase (iNOS), which is involved in viral clearance. The development of NO-based antiviral therapies, particularly gaseous NO inhalation and NO-donors, has proven to be an excellent antiviral therapeutic strategy.
nitric oxide
viral infections
NO-donors
inhalation therapy
COVID-19
1. Introduction
The enormous cost of viral infections has been made abundantly evident over the last two years, in which researchers have observed the worldwide spread of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which affects every aspect of human life and all economic sectors [1]. After the outbreak of SARS-CoV-1, in 2002, and of Middle East respiratory syndrome coronavirus (MERS-CoV), in 2012, SARS-CoV-2 has infected over 326 million people since 2019, causing the death of nearly 6 million (17 February 2022, source Johns Hopkins Center for Systems Science and Engineering, https://coronavirus.jhu.edu/map.html). Unfortunately, the availability of effective antiviral drugs is limited. Although a great deal of effort has been devoted to the development of new therapeutic approaches since the first approval of an antiviral drug in 1963, only 90 drugs were formally approved up to 2016 [2]. Among the difficulties in antiviral drug research, researchers find the great variety of viral species and their fast mutation rate, which leads to the development of resistance, greatly reducing the efficacy of existing therapies [3]. Resistance to antiviral drugs is a major public health issue and has led to increased mortality, poor quality of life, and enormous social and economic costs [4]. Furthermore, antiviral drugs often also affect host biological processes, further limiting their clinical efficacy. For all of these reasons, the development of new strategies to tackle viral infections is an urgent need and nitric oxide (NO) is an effective approach to this end. NO plays an important role in both cardiovascular and immune systems [5][6], and its antibacterial activity has been well described [7]. Its use against viruses has more recently been proposed and NO-based therapeutic strategies have been investigated [8].
2. Biology of NO
NO is a radical species that is involved in a variety of physiological and pathological processes. It is ubiquitous in mammalian tissues, where it is produced from the conversion of L-arginine into L-citrulline in a reaction catalyzed by three isoforms of the enzyme NO-synthase (NOS). The three isoforms differ in terms of the regulation, amplitude and duration of NO production, and their tissue localization [6]. Neuronal (nNOS) and endothelial (eNOS) NOS are constitutive enzymes found in neuronal and skeletal muscle tissues and vascular endothelial cells, respectively; their activation is calcium-dependent and produces NO on a timescale from seconds to minutes, giving rise to low NO concentrations (pM-nM) [9][10]. The main physiological roles of NO within the vasculature are the inhibition of platelet activation and therefore the prevention of thrombosis and vasodilation [8]. In the nervous system, NO exerts its role by acting as a neurotransmitter and via its vasodilatory properties [11]. Inducible NOS (iNOS) can work in a calcium-independent manner and its activity is associated with immune functions. It is found in macrophages, monocytes and muscle cells, where it produces NO on a timescale from hours to days, leading to high concentrations of the molecule (μM), making NO an important weapon in the immune response against bacteria, viruses and cancer cells [9]. In the immune system, NO is not only produced by activated macrophages but also by dendritic cells, natural killer cells, mast cells and other immune-system cells [6]. The constitutive expression of iNOS has recently been described as occurring in lung epithelial cells, where the enzyme seems to be the principal source of exhaled NO, with activation being calcium-dependent in this case [12]. An alternative synthetic pathway for NO formation, independently of classical NOS enzymes, has been described as passing through the reduction of nitrites thanks to the nitrate reductase activity of xanthine oxidase. This route seems to be particularly important when endothelial functions are reduced due to aging or cardiovascular diseases [13].
NO has direct effects in that it can block the functions of target molecules by reacting with the metal centers (e.g., iron-sulfur centers of proteins and enzymes). It also has indirect effects that are mediated by its reaction with oxygen and the superoxide anion to give reactive nitrogen species (RNS), which then oxidize, nitrate and nitrosate target molecules [14].
The balance between the physiological and pathological effects of NO depends on its concentration, meaning that any proposal to use NO for therapeutic purposes should take its concentration and localization into careful consideration.
3. Role of NO in Immune Defense against Viruses
While endogenous NO is one of the major mediators in the body’s defense against microorganisms in the airway, its antiviral role has been less extensively studied. The non-specific action that NO exerts against viruses was first described in the early 1990s, and, since these pioneering studies, further evidence has demonstrated that NO exerts an action against all types of viruses, including the coronavirus, with broad activity [14][15]. Research in the coronavirus field intensified after the emergence of a new human pulmonary disease in 2002, severe acute respiratory syndrome (SARS), which was caused by a novel coronavirus, SARS-CoV-1. In 2004, the NO donor S-nitroso-N-acetylpenicillamine (SNAP) was demonstrated to have in vitro activity against SARS-CoV-1 [16]. Subsequently, Akerstrom et al. demonstrated the mechanisms by which NO can inhibit the replication cycle of SARS-CoV-1 [17][18]. Studies into NO activity against coronavirus have received even further impetus since the recent outbreak of SARS-CoV-2.
Many studies have shown that a number of diseases caused by viral infection are characterized by an increase in NO levels. Indeed, following infection, the large amounts of NO that are necessary to fight viruses are produced thanks to the upregulation of iNOS via the activation of several pathways that are mediated by: (i) toll-like receptors (TLRs); (ii) signal transducer and activator of transcription 1 (STAT-1); and, (iii) protein kinase-R (PKR) [8], as summarized in Figure 1. TLRs are expressed in immune (macrophages, lymphocytes) and non-immune (epithelial) cells where they detect pathogen-associated molecular patterns (PAMPs), which, in the case of viruses, can be the viral nucleic acid or components of viral envelopes. The recognition of PAMPs by TLRs results in the activation of signaling pathways that converge in the activation of transcription factors, nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) and activator protein 1 (AP-1), which enters the nucleus and upregulate iNOS transcription [19]. A second pathway is mediated by interferon-gamma (IFN-γ), which is produced by immune cells around 24–26 h after infection. IFN-γ induces the phosphorylation and activation of Janus kinase 1 (Jak1), which, in turn, phosphorylates and thus activates STAT-1, leading again to the upregulation of iNOS [20]. Finally, double-stranded RNA-dependent protein kinase (PKR), activated by viral infections, plays a major role in the activation of iNOS through the NF-κB pathway [21].
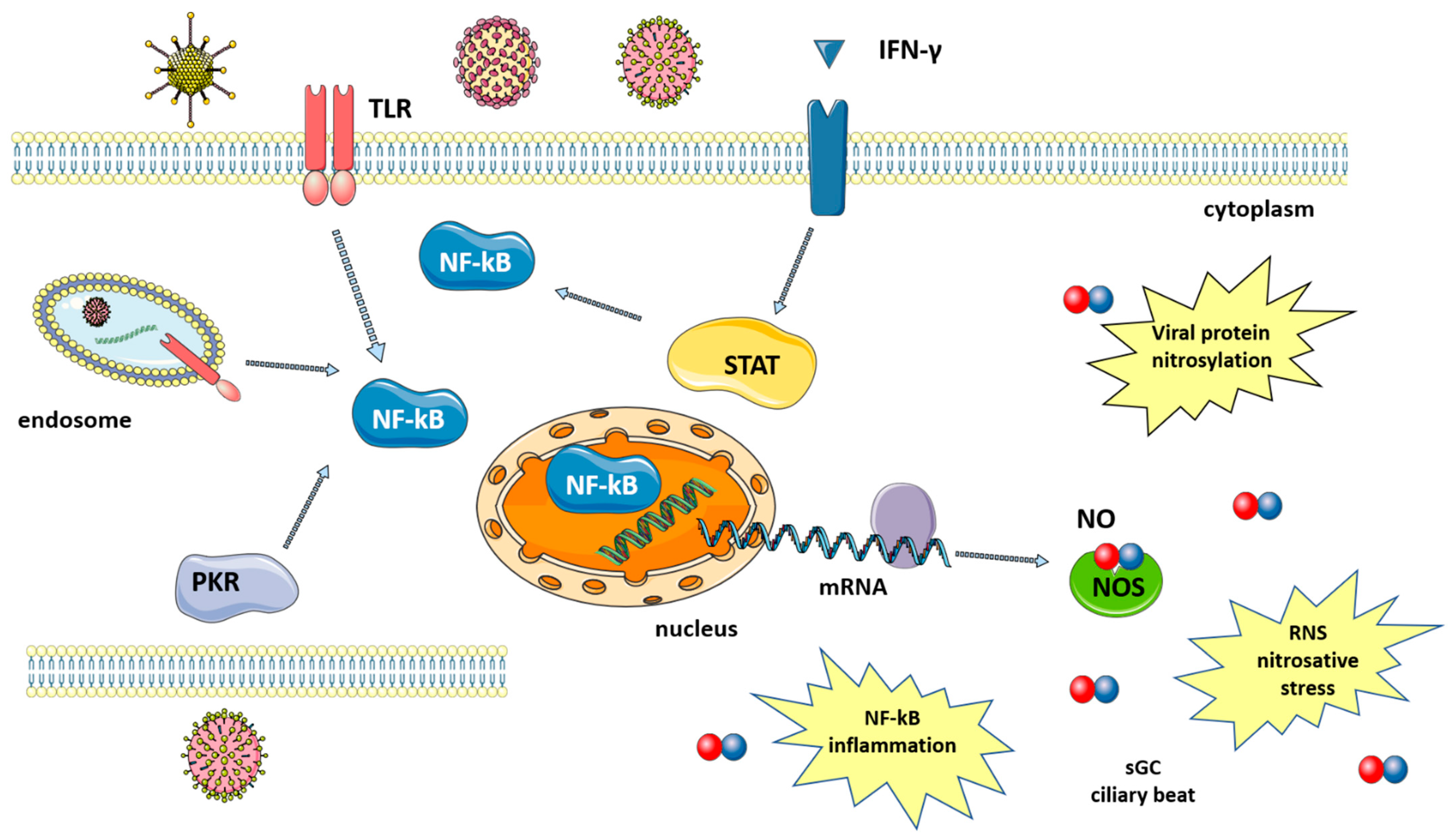
Figure 1. Synthesis of nitric oxide after viral infection. Viruses induce signaling pathways that converge in the activation of NF-kB and iNOS expression. Levels of NO increase, leading to viral protein nitrosylation, nitrosative stress, induction of an inflammatory response, increased ciliary beat, etc. Signaling pathways are described in the text. The figure was created by modifying images obtained from Smart Servier Medical Art (17 February 2022, smart.servier.com) licensed under a Creative Commons Attribution 3.0 Unported License.
iNOS activation results in the increased production of NO, which is effective against both DNA and RNA viruses, including SARS-CoV-2 [22]. NO action against viruses proceeds via several different mechanisms, both direct and indirect, which will be briefly discussed below (Figure 1).
(1) The inactivation of viral proteins via nitrosylation reduces or inhibits enzyme activity. Target proteins include proteases, reverse transcriptases, ribonucleotide reductases, transcription factors and tyrosine-containing enzymes [19]. The mechanism is the S-nitrosylation of the cysteine residues that are directly involved in catalysis or present near the active site [15]. For SARS-CoV in particular, this virus encodes two cysteine proteases that are known to be particularly susceptible to nitrosylation [18]. In fact, a decrease in SARS-CoV-2 protease activity has been reported after treatment with a NO-donor and was explained as acting via the S-nitrosylation of the cysteine in the enzyme active site [23]. Host proteins can also be nitrosylated, and the reaction between NO and either cysteine or thiols is not always detrimental, with it being able to help innate immune response in some cases [24]. Another mechanism for NO-mediated SARS-CoV protein inactivation occurs via the reduced palmitoylation of the spike (S) protein, which affects the interaction between the S protein and the ACE2 receptor [18].
(2) Deamination of viral DNA following nitrosative stress. NO induces DNA damage both to viral and host genetic material, although host DNA can be repaired by the DNA repair machinery [8].
(3) NO increases the clearance of pathogens, including DNA and RNA viruses, by enhancing ciliary beat frequency [8][25]. NO activates soluble guanylyl cyclase (sGC) in airway epithelial cells to produce cyclic guanosine monophosphate (cGMP), which, in turn, activates protein kinase G (PKG). PKG phosphorylates effector proteins leading to an increase in ciliary beat frequency [26].
The high levels of NO produced following iNOS induction also play a role in the inflammatory response. Acute inflammation is a defense mechanism against pathogens and has the aim of destroying or inactivating the invading microorganisms. If microbes are killed and cellular debris removed, inflammation is resolved and the normal tissue is restored. However, when excessive and non-regulated inflammation persists, it leads to tissue damage. During sustained inflammation, NO is produced in high quantities and regulates the activity of a variety of inflammatory mediators, including NF-kB [22]. Other effects of NO include increased blood flow and vascular permeability, further contributing to inflammation [27]. In some viral pathologies, excessive levels of NO are associated with more severe disease course, as has been described in influenza [28], and HIV [29].
4. Role of NO in Vascular Endothelium
The endothelial cells that line vessels control several vascular functions, including blood flow, blood fluidity and vascular permeability. For an in-depth description, see the review by Pober and Sessa [27].
The vascular endothelium acts in response to changes in the microenvironment (i.e., oxygen concentration) and induces the release of specific mediators that act in the control of the tone of smooth muscle cells [27]. These molecules are either vasodilators (such as NO and prostacyclin) or vasoconstrictors (such as endothelin), which exert their action on vascular smooth muscle cells. NO, which promotes vasodilation, is one of the most important of these mediators in the cardiovascular system. The NO produced in low levels by endothelial cells is fundamental for normal vascular tone. The mechanism at the base of the vasodilatory effect is the activation of sGC and the production of cGMP. The reduction of NO, which leads to impaired sGC–cGMP signaling, is associated with vascular dysfunctions, including hypertension and other cardiovascular diseases [30].
Under physiological conditions, the maintenance of blood fluidity, via the inhibition of coagulation is another role of the vascular endothelium [27]. NO also plays a crucial role here by acting as an anticoagulant with a mechanism that again involves the activation of the NO–sGC–cGMP signaling pathway [14]. The synthesis of NO is activated by many stimuli, including shear stress, variations in Ca2+ concentration and mediators such as acetylcholine and bradykinin [31]. NO works in synergy with prostacyclin and inhibits platelet adhesion and activation [27].
Impaired endothelial function, with reduced NO availability, is found in diseases such as diabetes mellitus, obesity and hypertension [32]. The reduction in NO production is probably involved in the pathogenesis of thrombosis [33].
Vascular Endothelium Dysfunctions Associated with Viral Infections
The maintenance of endothelium functionality is essential as it allows for controlled exchanges between blood and the surrounding tissue and corrects vascular tone, while also avoiding the formation of a pro-thrombotic environment. Alterations in the vascular endothelium are therefore associated with pathological conditions, such as those related to viral infections. HIV infection is associated with oxidative stress, which reduces NO availability, leading to endothelium dysfunctions [34]. Similarly, emerging viruses that pose a relevant threat to human and animal health, including SARS-CoV-2, may also affect endothelial cell functions, as recently reviewed in [35]. Coronavirus-like SARS-CoV-1, MERS-CoV and SARS-CoV-2, which have been shown to infect endothelial cells, lead to a decrease in endothelial NO [14]. Indeed, SARS-CoV-2 infection seems to disrupt the signaling for endothelial NO production [36] and significantly decreased endothelial NO levels were measured in patients with COVID-19 [37]. Angiotensin-converting enzyme 2 (ACE2), which is the virus gateway into cells, converts angiotensin II to angiotensin 1-7, activating a pathway that increases NO production, among other effects. Virus infection inactivates this route, and, as a consequence, lowers the availability of NO [36], which has been hypothesized as one of the reasons for COVID-19 deaths [38]. During pathological processes that involve endothelial cell dysfunctions, NO levels decrease, leading to vasoconstriction, impaired blood flow and hypercoagulation. Abnormal coagulation has been reported and associated with poor prognosis in patients infected with SARS-CoV-1 and MERS-CoV [14]. Infection from SARS-CoV-2 is also associated with altered coagulation, and a higher risk for thromboembolism has been observed [39]. This aspect can be exploited in therapy with NO, thanks to its effects on vascular endothelium.
5. NO and the Respiratory System
NO is produced in the human airway by the three isoforms of NOS [20]. A reduction in lung NO levels is found in diseases such as cystic fibrosis and acute respiratory distress syndrome [20]. In the lungs, NO that is produced by eNOS in endothelial cells improves oxygenation; it acts on smooth muscle cells, inducing vessel dilation and, therefore, an increase in local blood flow [40]. The main mechanism of vasodilation is the same as was described in the previous paragraph, with the involvement of sGC and cGMP. A reduced sGC expression, which is related to decreased vasodilation in response to NO, is often found in adult lungs with respect to children [40]. In addition, NO acts also through cGMP-independent mechanisms: it has been reported that direct activation of Ca2+-dependent K+ channels [41] and the involvement in the formation of nitrosothiols, which exhibit bronchodilator activity [42].
Another factor in the respiratory system is the lung surfactant, a complex of proteins and phospholipids with the function of reducing surface tension at the air/liquid interface: evidence suggests a role for NO in surfactant secretion and the preservation of its function [43]. At low doses, NO can scavenge radicals, inhibiting lipid peroxidation and protecting lung surfactant structure [43]. At higher concentrations, NO-driven S-nitrosylation of surfactant proteins can induce a conformational change, leading to inflammatory response [24].
iNOS, which is constitutively expressed in the airway epithelium, also contributes to the production of NO, which, among other functions, helps the regulation of ciliary beat, a defense mechanism of airway epithelium [12]. The effects of NO on ciliary motility have been demonstrated with the use of NOS inhibitors [43] and by the fact that NOS isoforms are expressed in the ciliary epithelium [44].
The use of NO in the treatment of pulmonary diseases is derived from this evidence [45].
6. NO-Based Antiviral Strategies
Although the role of NO in inhibiting viral replication in host defense has been demonstrated, NO-based antiviral strategies have not yet been extensively investigated. To date, no systematic approach has been adopted for the study of NO-based antiviral therapeutics, apart from those active in the cardiovascular system [46][47]. Despite inconsistencies, many researchers have attempted, in reviews, to comprehensively and systematically describe the therapeutic applications of NO following viral infections. Reiss et al. reported the in vitro and in vivo studies that were carried out up to 1998 on NO following viral infection [48], whereas Garren et al. provided a very useful review on the most recent advances in NO-based antiviral therapies [8].
NO-based antiviral strategies can be mainly classified into two groups: gaseous NO (gNO) inhalation-based therapies and NO-donors. As well as chronic inflammatory diseases, viral infections of the upper respiratory tract are related to the presence of higher levels of NO in the air exhaled by human subjects. This is due to an increase in NO production as part of the host response to infection [49], with the increase in the endogenous synthesis of NO being caused by iNOS overexpression that should be involved in viral clearance. Most studies have demonstrated the efficacy of gNO therapies as well as NO-donors in inhibiting RNA replication in viral strains.
References
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193.
- De Clercq, E.; Li, G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016, 29, 695–747.
- Mazur-Marzec, H.; Cegłowska, M.; Konkel, R.; Pyrć, K. Antiviral Cyanometabolites—A Review. Biomolecules 2021, 11, 474.
- Boroumand, H.; Badie, F.; Mazaheri, S.; Seyedi, Z.S.; Nahand, J.S.; Nejati, M.; Baghi, H.B.; Abbasi-Kolli, M.; Badehnoosh, B.; Ghandali, M.; et al. Chitosan-Based Nanoparticles Against Viral Infections. Front. Cell. Infect. Microbiol. 2021, 11, 643953.
- Lei, J.; Vodovotz, Y.; Tzeng, E.; Billiar, T.R. Nitric oxide, a protective molecule in the cardiovascular system. Nitric Oxide 2013, 35, 175–185.
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916.
- Jones, M.L.; Ganopolsky, J.G.; Labbé, A.; Wahl, C.; Prakash, S. Antimicrobial properties of nitric oxide and its application in antimicrobial formulations and medical devices. Appl. Microbiol. Biotechnol. 2010, 88, 401–407.
- Garren, M.R.; Ashcraft, M.; Qian, Y.; Douglass, M.; Brisbois, E.J.; Handa, H. Nitric oxide and viral infection: Recent developments in antiviral therapies and platforms. Appl. Mater Today. 2021, 22, 100887.
- Wink, D.A.; Hines, H.B.; Cheng, R.Y.S.; Switzer, C.H.; Flores-Santana, W.; Vitek, M.P.; Ridnour, L.A.; Colton, C.A. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 2011, 89, 873–891.
- Dallavalle, S.; Dobričić, V.; Lazzarato, L.; Gazzano, E.; Machuqueiro, M.; Pajeva, I.; Tsakovska, I.; Zidar, N.; Fruttero, R. Im-provement of conventional anti-cancer drugs as new tools against multidrug resistant tumors. Drug Resist. Updat. 2020, 50, 100682.
- Džoljić, E.; Grbatinić, I.; Kostić, V. Why is nitric oxide important for our brain? Funct. Neurol. 2015, 30, 159–163.
- Mattila, J.T.; Thomas, A. Nitric Oxide Synthase: Non-Canonical Expression Patterns. Front. Immunol. 2014, 5, 478.
- Peleli, M.; Zollbrecht, C.; Montenegro, M.F.; Hezel, M.; Zhong, J.; Persson, E.G.; Holmdahl, R.; Weitzberg, E.; Lundberg, J.O.; Carlström, M. Enhanced XOR activity in eNOS-deficient mice: Effects on the nitrate-nitrite-NO pathway and ROS homeostasis. Free Radic Biol. Med. 2016, 99, 472–484.
- Fang, W.; Jiang, J.; Su, L.; Shu, T.; Liu, H.; Lai, S.; Ghiladi, R.A.; Wang, J. The role of NO in COVID-19 and potential therapeutic strategies. Free Radic. Biol. Med. 2020, 163, 153–162.
- Lisi, F.; Zelikin, A.N.; Chandrawati, R. Nitric Oxide to Fight Viral Infections. Adv. Sci. 2021, 8, 2003895.
- Keyaerts, E.; Vijgen, L.; Chen, L.; Maes, P.; Hedenstierna, G.; Van Ranst, M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int. J. Infect. Dis. 2004, 8, 223–226.
- Åkerström, S.; Mousavi-Jazi, M.; Klingström, J.; Leijon, M.; Lundkvist, A.; Mirazimi, A. Nitric Oxide Inhibits the Replication Cycle of Severe Acute Respiratory Syndrome Coronavirus. J. Virol. 2005, 79, 1966–1969.
- Akerström, S.; Gunalan, V.; Keng, C.T.; Tan, Y.J.; Mirazimi, A. Dual effect of nitric oxide on SARS-CoV replication: Viral RNA production and palmitoylation of the S protein are affected. Virology 2009, 395, 1–9.
- Abdul-Cader, M.S.; Amarasinghe, A.; Abdul-Careem, M.F. Activation of toll-like receptor signaling pathways leading to nitric oxide-mediated antiviral responses. Arch. Virol. 2016, 161, 2075–2086.
- Xu, W.; Zheng, S.; Dweik, R.A.; Erzurum, S.C. Role of epithelial nitric oxide in airway viral infection. Free Radic. Biol. Med. 2006, 41, 19–28.
- Auch, C.J.; Saha, R.; Sheikh, F.G.; Liu, X.; Jacobs, B.L.; Pahan, K. Role of protein kinase R in double-stranded RNA-induced expression of nitric oxide synthase in human astroglia. FEBS Lett. 2004, 563, 223–228.
- Bayarri, M.A.; Milara, J.; Estornut, C.; Cortijo, J. Nitric Oxide System and Bronchial Epithelium: More Than a Barrier. Front. Physiol. 2021, 12.
- Akaberi, D.; Krambrich, J.; Ling, J.; Luni, C.; Hedenstierna, G.; Järhult, J.D.; Lennerstrand, J.; Lundkvist, Å. Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro. Redox. Biol. 2020, 37, 101734.
- Uehara, E.U.; Shida Bde, S.; De Brito, C.A. Role of nitric oxide in immune responses against viruses: Beyond microbicidal ac-tivity. Inflamm Res. 2015, 64, 845–852.
- Jean, D.; Maitre, B.; Tankovic, J.; Meignan, M.; Adnot, S.; Brun-Buisson, C.; Harf, A.; Delclaux, C. Beneficial effects of nitric oxide inhalation on pulmonary bacterial clearance. Crit. Care Med. 2002, 30, 442–447.
- Wyatt, T.A.; Spurzem, J.R.; May, K.; Sisson, J.H. Regulation of ciliary beat frequency by both PKA and PKG in bovine airway epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 1998, 275, L827–L835.
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007, 7, 803–815.
- Perrone, L.A.; Belser, J.A.; Wadford, D.A.; Katz, J.M.; Tumpey, T.M. Inducible Nitric Oxide Contributes to Viral Pathogenesis Following Highly Pathogenic Influenza Virus Infection in Mice. J. Infect. Dis. 2013, 207, 1576–1584.
- Torre, D.; Ferrario, G. Immunological aspects of nitric oxide in HIV-1 infection. Med Hypotheses 1996, 47, 405–407.
- Klinger, J.R.; Kadowitz, P.J. The Nitric Oxide Pathway in Pulmonary Vascular Disease. Am. J. Cardiol. 2017, 120, S71–S79.
- Chen, J.Y.; Ye, Z.X.; Wang, X.F.; Chang, J.; Yang, M.W.; Zhong, H.H.; Hong, F.F.; Yang, S.L. Nitric oxide bioavailability dys-function involves in atherosclerosis. Biomed. Pharmacother. 2018, 97, 423–428.
- Park, K.-H.; Park, W.J. Endothelial Dysfunction: Clinical Implications in Cardiovascular Disease and Therapeutic Approaches. J. Korean Med. Sci. 2015, 30, 1213–1225.
- Loscalzo, J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ. Res. 2001, 88, 756–762.
- Marincowitz, C.; Genis, A.; Goswami, N.; De Boever, P.; Nawrot, T.S.; Strijdom, H. Vascular endothelial dysfunction in the wake of HIV and ART. FEBS J. 2019, 286, 1256–1270.
- Fosse, J.H.; Haraldsen, G.; Falk, K.; Edelmann, R. Endothelial Cells in Emerging Viral Infections. Front. Cardiovasc. Med. 2021, 8, 619690.
- Ritz, T.; Salsman, M.L.; Young, D.A.; Lippert, A.R.; Khan, D.A.; Ginty, A.T. Boosting nitric oxide in stress and respiratory in-fection: Potential relevance for asthma and COVID-19. Brain Behav. Immun. Health. 2021, 14, 100255.
- Dominic, P.; Ahmad, J.; Bhandari, R.; Pardue, S.; Solorzano, J.; Jaisingh, K.; Watts, M.; Bailey, S.R.; Orr, A.W.; Kevil, C.G.; et al. Decreased availability of nitric oxide and hydrogen sulfide is a hallmark of COVID-19. Redox Biol. 2021, 43, 101982.
- Ozdemir, B.; Yazici, A. Could the decrease in the endothelial nitric oxide (NO) production and NO bioavailability be the crucial cause of COVID-19 related deaths? Med. Hypotheses. 2020, 144, 109970.
- Berkman, S.A.; Tapson, V.F. COVID-19 and Its Implications for Thrombosis and Anticoagulation. Semin. Respir. Crit. Care Med. 2021, 42, 316–326.
- Friebe, A.; Englert, N. NO—Sensitive guanylyl cyclase in the lung. J. Cereb. Blood Flow Metab. 2020.
- Bolotina, V.M.; Najibi, S.; Palacino, J.J.; Pagano, P.J.; Cohen, R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature 1994, 368, 850–853.
- Ricciardolo, F.L.M. Multiple roles of nitric oxide in the airways. Thorax 2003, 58, 175–182.
- Antosova, M.; Mokra, D.; Pepucha, L.; Plevkova, J.; Buday, T.; Sterusky, M.; Bencova, A. Physiology of Nitric Oxide in the Respiratory System. Physiol. Res. 2017, 66, S159–S172.
- Price, M.E.; Sisson, J.H. Redox regulation of motile cilia in airway disease. Redox Biol. 2019, 27, 101146.
- Mehta, P.P.; Dhapte-Pawar, V.S. Novel and Evolving Therapies for COVID-19 Related Pulmonary Complications. Am. J. Med. Sci. 2021, 361, 557–566.
- Llorens, S.; Nava, E. Cardiovascular diseases and the nitric oxide pathway. Curr. Vasc. Pharmacol. 2003, 1, 335–346.
- Ignarro, L.J.; Napoli, C.; Loscalzo, J. Nitric Oxide Donors and Cardiovascular Agents Modulating the Bioactivity of Nitric Oxide. Circ. Res. 2002, 90, 21–28.
- Reiss, C.S.; Komatsu, T. Does nitric oxide play a critical role in viral infections? J. Virol. 1998, 72, 4547.
- McMullin, B.B.; Chittock, D.R.; Roscoe, D.L.; Garcha, H.; Wang, L.; Miller, C.C. The antimicrobial effect of nitric oxide on the bacteria that cause nosocomial pneumonia in mechanically ventilated patients in the intensive care unit. Respir. Care 2005, 50, 1451–1456.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Nitric Oxide: Physiology, Pharmacology, and Therapeutic Applications
Revisions:
2 times
(View History)
Update Date:
20 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




