
Video Upload Options
Nerve regeneration and repair still remain a huge challenge for both central nervous and peripheral nervous system. Although some therapeutic substances, including neuroprotective agents,clinical drugs and stem cells, as well as various growth factors, are found to be effective to promote nerve repair, a carrier system that possesses a sustainable release behavior, in order to ensure high on-site concentration during the whole repair and regeneration process, and high bioavailability is still highly desirable. Hydrogel, as an ideal delivery system, has an excellent loading capacity and sustainable release behavior, as well as tunable physical and chemical properties to adapt to various biomedical scenarios; thus, it is thought to be a suitable carrier system for nerve repair.
1. Introduction
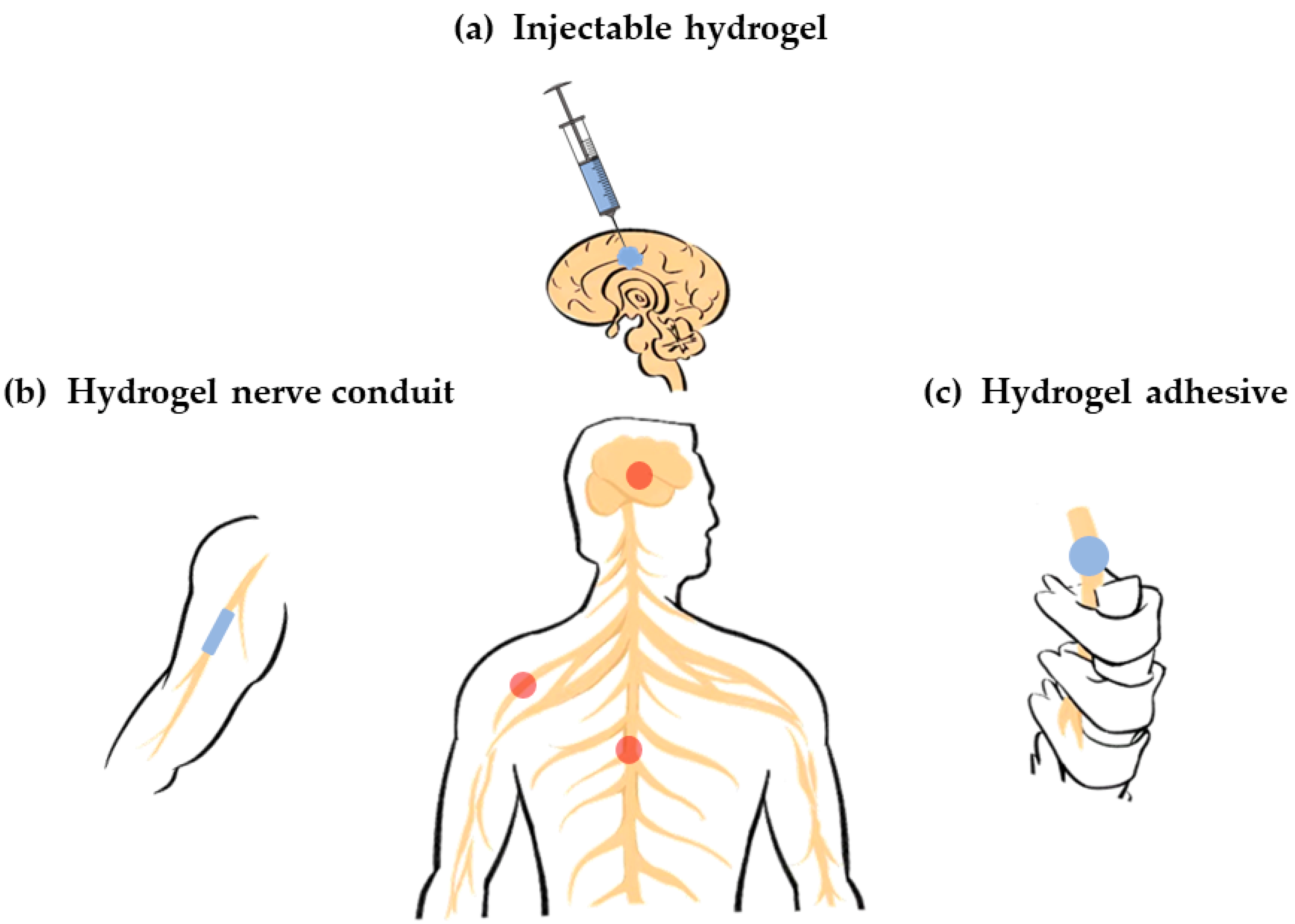 Figure 1. Various hydrogel carriers for nerve repair. (a) Injectable hydrogel carriers for brain nerve repair (central nervous system); (b) Hydrogel carriers as conduits for peripheral nerve repair (peripheral nervous system); (c) Hydrogel carriers as adhesive for spinal cord repair (central nervous system).
Figure 1. Various hydrogel carriers for nerve repair. (a) Injectable hydrogel carriers for brain nerve repair (central nervous system); (b) Hydrogel carriers as conduits for peripheral nerve repair (peripheral nervous system); (c) Hydrogel carriers as adhesive for spinal cord repair (central nervous system).2. Classification of Hydrogel Carriers for Nerve Repair
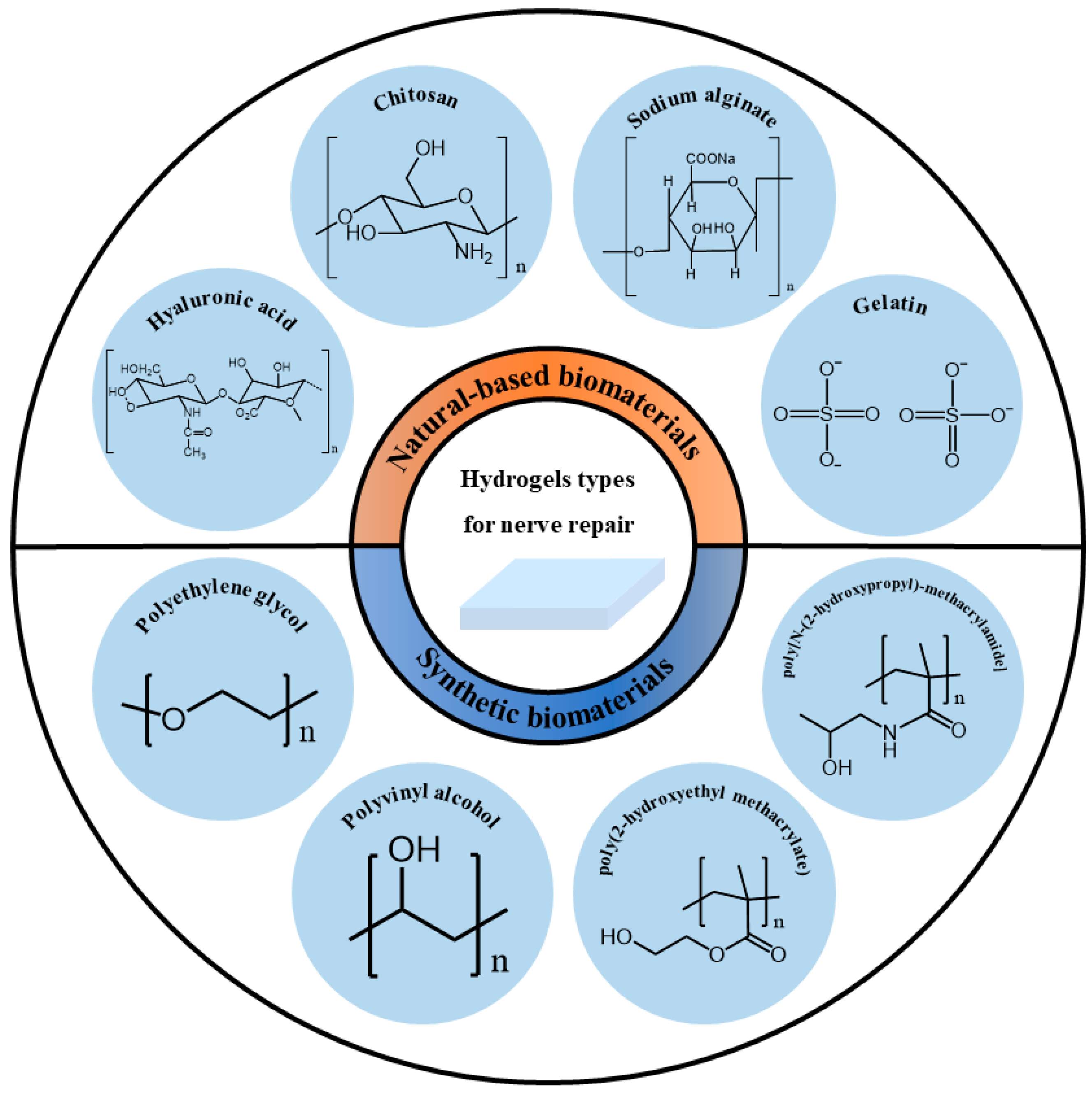 Figure 2. Classification of hydrogel carriers for nerve repair based on their sources.
Figure 2. Classification of hydrogel carriers for nerve repair based on their sources.2.1. Natural-Based Biomaterials
2.2. Synthetic Biomaterials
3. Hydrogels as Carriers for Nerve Repair
3.1. Cell Carrier
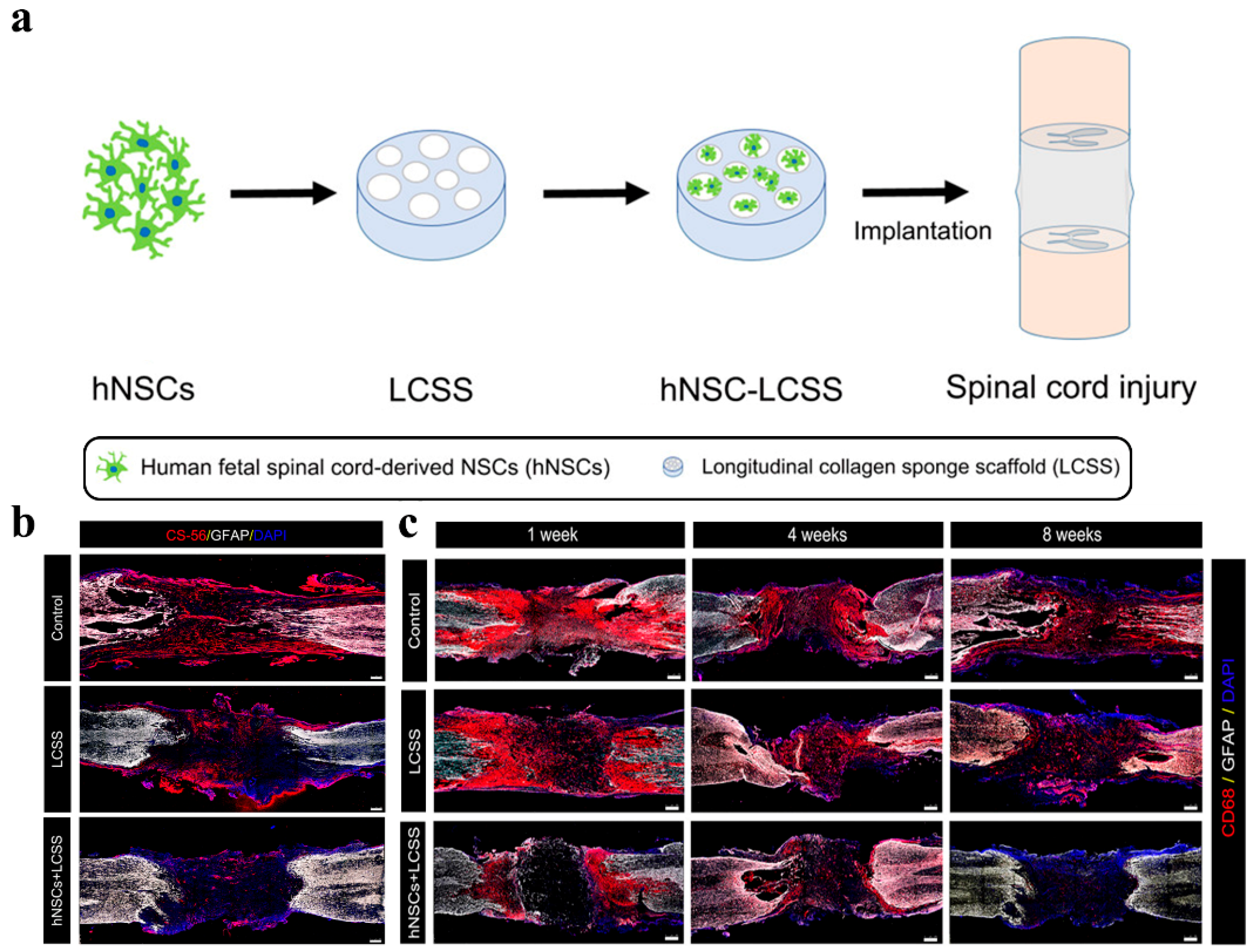 Figure 3. Schematic diagram and repair effect of neural stem cells in spinal cord repair. (a) Scheme of neural stem cells implantation for spinal cord repair; (b) Images of CS-56 immunostaining representing the formation of glial scars in the injury cavity at 8 weeks post-operation. The hNSCs implantation efficiently reduced glial scar forming around the injury cavity; (c) Representative immunofluorescence images of CD68 (activated microglia cell and macrophage marker)-positive signals in damaged sites. Grafted neural stem cells significantly suppress inflammation. (Adapted with permission from Ref. [106]. 2020, American Chemical Society).
Figure 3. Schematic diagram and repair effect of neural stem cells in spinal cord repair. (a) Scheme of neural stem cells implantation for spinal cord repair; (b) Images of CS-56 immunostaining representing the formation of glial scars in the injury cavity at 8 weeks post-operation. The hNSCs implantation efficiently reduced glial scar forming around the injury cavity; (c) Representative immunofluorescence images of CD68 (activated microglia cell and macrophage marker)-positive signals in damaged sites. Grafted neural stem cells significantly suppress inflammation. (Adapted with permission from Ref. [106]. 2020, American Chemical Society).3.2. Bioactive Compounds
References
- Mietto, B.S.; Mostacada, K.; Martinez, A.M.B. Neurotrauma and Inflammation: CNS and PNS Responses. Mediat. Inflamm. 2015, 2015, 251204.
- Echternacht, S.R.; Chacon, M.A.; Leckenby, J.I. Central versus peripheral nervous system regeneration: Is there an exception for cranial nerves? Regen. Med. 2021, 16, 567–579.
- Wang, Q.; Capistrant, B.; Ehntholt, A.; Glymour, M.M. Long-Term Rate of Change in Memory Functioning before and after Stroke Onset. Stroke 2012, 43, 2561–2566.
- Maida, C.D.; Norrito, R.L.; Daidone, M.; Tuttolomondo, A.; Pinto, A. Neuroinflammatory Mechanisms in Ischemic Stroke: Focus on Cardioembolic Stroke, Background, and Therapeutic Approaches. Int. J. Mol. Sci. 2020, 21, 6454.
- Wei, K.; Wang, P.; Miao, C.-Y. A Double-Edged Sword with Therapeutic Potential: An Updated Role of Autophagy in Ischemic Cerebral Injury. CNS Neurosci. Ther. 2012, 18, 879–886.
- Sobesky, J. Akutversorgung des ischämischen Schlaganfalls. Der Internist 2009, 50, 1218–1226.
- Kumar, A.; Aakriti; Gupta, V. A review on animal models of stroke: An update. Brain Res. Bull. 2016, 122, 35–44.
- Liu, C.-L.; Liao, S.-J.; Zeng, J.-S.; Lin, J.-W.; Li, C.-X.; Xie, L.-C.; Shi, X.-G.; Huang, R.-X. dl-3n-butylphthalide prevents stroke via improvement of cerebral microvessels in RHRSP. J. Neurol. Sci. 2007, 260, 106–113.
- Yi, Y.Y.; Shin, H.J.; Choi, S.G.; Kang, J.W.; Song, H.-J.; Kim, S.K.; Kim, D.W. Preventive Effects of Neuroprotective Agents in a Neonatal Rat of Photothrombotic Stroke Model. Int. J. Mol. Sci. 2020, 21, 3703.
- Patel, P.; Yavagal, D.; Khandelwal, P. Hyperacute Management of Ischemic Strokes. J. Am. Coll. Cardiol. 2020, 75, 1844–1856.
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121.
- Carballo-Molina, O.A.; Velasco, I. Hydrogels as scaffolds and delivery systems to enhance axonal regeneration after injuries. Front. Cell. Neurosci. 2015, 9, 13.
- Russo, T.; Tunesi, M.; Giordano, C.; Gloria, A.; Ambrosio, L. Hydrogels for central nervous system therapeutic strategies. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2015, 229, 905–916.
- Gopalakrishnan, A.; Shankarappa, S.A.; Rajanikant, G.K. Hydrogel Scaffolds: Towards Restitution of Ischemic Stroke-Injured Brain. Transl. Stroke Res. 2019, 10, 1–18.
- González-Nieto, D.; Fernández-García, L.; Pérez-Rigueiro, J.; Guinea, G.V.; Panetsos, F. Hydrogels-Assisted Cell Engraftment for Repairing the Stroke-Damaged Brain: Chimera or Reality. Polymers 2018, 10, 184.
- Fornasari, B.E.; Carta, G.; Gambarotta, G.; Raimondo, S. Natural-Based Biomaterials for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2020, 8, 554257.
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743.
- Horn, E.M.; Beaumont, M.; Shu, X.Z.; Harvey, A.; Prestwich, G.D.; Horn, K.M.; Gibson, A.R.; Preul, M.C.; Panitch, A. Influence of cross-linked hyaluronic acid hydrogels on neurite outgrowth and recovery from spinal cord injury. J. Neurosurg. Spine 2007, 6, 133–140.
- Pan, L.; Ren, Y.; Cui, F.; Xu, Q. Viability and differentiation of neural precursors on hyaluronic acid hydrogel scaffold. J. Neurosci. Res. 2009, 87, 3207–3220.
- Seidlits, S.K.; Khaing, Z.; Petersen, R.R.; Nickels, J.; Vanscoy, J.E.; Shear, J.B.; Schmidt, C.E. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 2010, 31, 3930–3940.
- Liang, Y.; Walczak, P.; Bulte, J.W. The survival of engrafted neural stem cells within hyaluronic acid hydrogels. Biomaterials 2013, 34, 5521–5529.
- Wang, M.-D.; Zhai, P.; Schreyer, D.J.; Zheng, R.-S.; Sun, X.-D.; Cui, F.-Z.; Chen, X.-B. Novel crosslinked alginate/hyaluronic acid hydrogels for nerve tissue engineering. Front. Mater. Sci. 2013, 7, 269–284.
- Hou, S.; Xu, Q.; Tian, W.; Cui, F.; Cai, Q.; Ma, J.; Lee, I.-S. The repair of brain lesion by implantation of hyaluronic acid hydrogels modified with laminin. J. Neurosci. Methods 2005, 148, 60–70.
- Broguiere, N.; Isenmann, L.; Zenobi-Wong, M. Novel enzymatically cross-linked hyaluronan hydrogels support the formation of 3D neuronal networks. Biomaterials 2016, 99, 47–55.
- Jensen, G.; Holloway, J.L.; Stabenfeldt, S.E. Hyaluronic Acid Biomaterials for Central Nervous System Regenerative Medicine. Cells 2020, 9, 2113.
- Yoo, J.; Park, J.H.; Kwon, Y.W.; Chung, J.J.; Choi, I.C.; Nam, J.J.; Lee, H.S.; Jeon, E.Y.; Lee, K.; Kim, S.H.; et al. Augmented peripheral nerve regeneration through elastic nerve guidance conduits prepared using a porous PLCL membrane with a 3D printed collagen hydrogel. Biomater. Sci. 2020, 8, 6261–6271.
- Salehi, M.; Naseri-Nosar, M.; Ebrahimibarough, S.; Nourani, M.; Vaez, A.; Farzamfar, S.; Ai, J. Regeneration of sciatic nerve crush injury by a hydroxyapatite nanoparticle-containing collagen type I hydrogel. J. Physiol. Sci. 2018, 68, 579–587.
- Lu, D.; Mahmood, A.; Qu, C.; Hong, X.; Kaplan, D.; Chopp, M. Collagen Scaffolds Populated with Human Marrow Stromal Cells Reduce Lesion Volume and Improve Functional Outcome after Traumatic Brain Injury. Neurosurgery 2007, 61, 596–603.
- Deng, W.-S.; Ma, K.; Liang, B.; Liu, X.-Y.; Xu, H.-Y.; Zhang, J.; Shi, H.-Y.; Sun, H.-T.; Chen, X.-Y.; Zhang, S. Collagen scaffold combined with human umbilical cord-mesenchymal stem cells transplantation for acute complete spinal cord injury. Neural Regen. Res. 2020, 15, 1686–1700.
- Wang, P.; Zhao, H.; Yao, Y.; Lu, C.; Ma, J.; Chen, R.; Pan, J. Repair of facial nerve crush injury in rabbits using collagen plus basic fibroblast growth factor. J. Biomed. Mater. Res. Part A 2020, 108, 1329–1337.
- Hu, Q.; Sun, C.; Zhang, H.; Li, S.; Shen, X.; Liu, S. Design and Fabrication of Tissue-engineering Multiscale and Multichannel Nerve Conduits for Rabbits’ Sciatic Nerve Regeneration. J. Biomater. Tissue Eng. 2019, 9, 10–23.
- Matsumine, H.; Sasaki, R.; Tabata, Y.; Matsui, M.; Yamato, M.; Okano, T.; Sakurai, H. Facial nerve regeneration using basic fibroblast growth factor-impregnated gelatin microspheres in a rat model. J. Tissue Eng. Regen. Med. 2016, 10, E559–E567.
- Takagi, T.; Kimura, Y.; Shibata, S.; Saito, H.; Ishii, K.; Okano, H.J.; Toyama, Y.; Okano, H.; Tabata, Y.; Nakamura, M. Sustained bFGF-Release Tubes for Peripheral Nerve Regeneration. Plast. Reconstr. Surg. 2012, 130, 866–876.
- Tonda-Turo, C.; Gnavi, S.; Ruini, F.; Gambarotta, G.; Gioffredi, E.; Chiono, V.; Perroteau, I.; Ciardelli, G. Development and characterization of novel agar and gelatin injectable hydrogel as filler for peripheral nerve guidance channels. J. Tissue Eng. Regen. Med. 2017, 11, 197–208.
- Lin, C.-C.; Chang, J.-J.; Yung, M.-C.; Huang, W.-C.; Chen, S.-Y. Spontaneously Micropatterned Silk/Gelatin Scaffolds with Topographical, Biological, and Electrical Stimuli for Neuronal Regulation. ACS Biomater. Sci. Eng. 2020, 6, 1144–1153.
- Ye, W.; Li, H.; Yu, K.; Xie, C.; Wang, P.; Zheng, Y.; Zhang, P.; Xiu, J.; Yang, Y.; He, Y.; et al. 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Mater. Des. 2020, 192, 108757.
- Yu, Z.; Li, H.; Xia, P.; Kong, W.; Chang, Y.; Fu, C.; Wang, K.; Yang, X.; Qi, Z. Application of fibrin-based hydrogels for nerve protection and regeneration after spinal cord injury. J. Biol. Eng. 2020, 14, 22.
- Johnson, P.; Tatara, A.; McCreedy, D.A.; Shiu, A.; Sakiyama-Elbert, S.E. Tissue-engineered fibrin scaffolds containing neural progenitors enhance functional recovery in a subacute model of SCI. Soft Matter 2010, 6, 5127–5137.
- Mooney, R.; Tawil, B.; Mahoney, M. Specific Fibrinogen and Thrombin Concentrations Promote Neuronal Rather Than Glial Growth When Primary Neural Cells Are Seeded within Plasma-Derived Fibrin Gels. Tissue Eng. Part A 2010, 16, 1607.
- Yao, S.; Liu, X.; Yu, S.; Wang, X.; Zhang, S.; Wu, Q.; Sun, X.; Mao, H. Co-effects of matrix low elasticity and aligned topography on stem cell neurogenic differentiation and rapid neurite outgrowth. Nanoscale 2016, 8, 10252–10265.
- Yang, W.; Wang, Z.; Zhang, J.; Yang, K.; Lu, C.; Cui, X.; Lu, H.; Cao, S.; Chen, Q.; Lu, X.; et al. Fibrin scaffolds embedded with sonic hedgehog/chitosan microspheres for recovery of spinal cord injury in rats. Mater. Express 2020, 10, 437–445.
- Robinson, M.; Douglas, S.; Willerth, S.M. Mechanically stable fibrin scaffolds promote viability and induce neurite outgrowth in neural aggregates derived from human induced pluripotent stem cells. Sci. Rep. 2017, 7, 6250.
- Rajabi, M.; Ali, A.; McConnell, M.; Cabral, J. Keratinous materials: Structures and functions in biomedical applications. Mater. Sci. Eng. C 2020, 110, 110612.
- Ozaki, Y.; Takagi, Y.; Mori, H.; Hara, M. Porous hydrogel of wool keratin prepared by a novel method: An extraction with guani-dine/2-mercaptoethanol solution followed by a dialysis. Mater. Sci. Eng. C 2014, 42, 146–154.
- Wang, S.; Taraballi, F.; Tan, L.P.; Ng, K.W. Human keratin hydrogels support fibroblast attachment and proliferation in vitro. Cell Tissue Res. 2012, 347, 795–802.
- Wang, S.; Wang, Z.; Foo, S.E.M.; Tan, N.S.; Yuan, Y.; Lin, W.; Zhang, Z.; Ng, K.W. Culturing Fibroblasts in 3D Human Hair Keratin Hydrogels. ACS Appl. Mater. Interfaces 2015, 7, 5187–5198.
- Sierpinski, P.; Garrett, J.; Ma, J.; Apel, P.; Klorig, D.; Smith, T.; Koman, L.A.; Atala, A.; Van Dyke, M. The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials 2008, 29, 118–128.
- Pace, L.A.; Plate, J.F.; Mannava, S.; Barnwell, J.C.; Koman, L.A.; Li, Z.; Smith, T.L.; Van Dyke, M. A Human Hair Keratin Hydrogel Scaffold Enhances Median Nerve Regeneration in Nonhuman Primates: An Electrophysiological and Histological Study. Tissue Eng. Part A 2014, 20, 507–517.
- Apel, P.J.; Garrett, J.P.; Sierpinski, P.; Ma, J.; Atala, A.; Smith, T.L.; Koman, L.A.; van Dyke, M.E. Peripheral Nerve Regeneration Using a Keratin-Based Scaffold: Long-Term Functional and Histological Outcomes in a Mouse Model. J. Hand Surg. 2008, 33, 1541–1547.
- Pace, L.A.; Plate, J.F.; Smith, T.L.; Van Dyke, M.E. The effect of human hair keratin hydrogel on early cellular response to sciatic nerve injury in a rat model. Biomaterials 2013, 34, 5907–5914.
- Zheng, K.; Feng, G.; Zhang, J.; Xing, J.; Huang, D.; Lian, M.; Zhang, W.; Wu, W.; Hu, Y.; Lu, X.; et al. Basic fibroblast growth factor promotes human dental pulp stem cells cultured in 3D porous chitosan scaffolds to neural differentiation. Int. J. Neurosci. 2021, 131, 625–633.
- Yuan, Y.; Zhang, P.; Yang, Y.; Wang, X.; Gu, X. The interaction of Schwann cells with chitosan membranes and fibers in vitro. Biomaterials 2004, 25, 4273–4278.
- Scanga, V.I.; Goraltchouk, A.; Nussaiba, N.; Shoichet, M.S.; Morshead, C.M. Biomaterials for neural-tissue engineering—Chitosan supports the survival, migration, and differentiation of adult-derived neural stem and progenitor cells. Rev. Can. Chim. 2010, 88, 277–287.
- Boecker, A.; Daeschler, S.; Kneser, U.; Harhaus, L. Relevance and Recent Developments of Chitosan in Peripheral Nerve Surgery. Front. Cell. Neurosci. 2019, 13, 104.
- Haastert-Talini, K.; Geuna, S.; Dahlin, L.B.; Meyer, C.; Stenberg, L.; Freier, T.; Heimann, C.; Barwig, C.; Pinto, L.F.; Raimondo, S.; et al. Chitosan tubes of varying degrees of acetylation for bridging peripheral nerve defects. Biomaterials 2013, 34, 9886–9904.
- Ai, J.; Farzin, A.; Zamiri, S.; Hadjighassem, M.; Ebrahimi-Barough, S.; Ai, A.; Mohandesnezhad, S.; Karampour, A.; Farahani, M.S.; Goodarzi, A. Repair of injured spinal cord using platelet-rich plasma- and endometrial stem cells-loaded chitosan scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 1002–1011.
- Zhou, S.; Yang, Y.; Gu, X.; Ding, F. Chitooligosaccharides protect cultured hippocampal neurons against glutamate-induced neurotoxicity. Neurosci. Lett. 2008, 444, 270–274.
- Huang, H.-C.; Hong, L.; Chang, P.; Zhang, J.; Lu, S.-Y.; Zheng, B.-W.; Jiang, Z.-F. Chitooligosaccharides Attenuate Cu2+-Induced Cellular Oxidative Damage and Cell Apoptosis Involving Nrf2 Activation. Neurotox. Res. 2015, 27, 411–420.
- Wang, Y.; Zhao, Y.; Sun, C.; Hu, W.; Zhao, J.; Li, G.; Zhang, L.; Liu, M.; Liu, Y.; Ding, F.; et al. Chitosan Degradation Products Promote Nerve Regeneration by Stimulating Schwann Cell Proliferation via miR-27a/FOXO1 Axis. Mol. Neurobiol. 2016, 53, 28–39.
- Eftekharzadeh, B.; Khodagholi, F.; Abdi, A.; Maghsoudi, N. Alginate protects NT2 neurons against H2O2-induced neurotoxicity. Carbohydr. Polym. 2010, 79, 1063–1072.
- Matyash, M.; Despang, F.; Mandal, R.; Fiore, D.; Gelinsky, M.; Ikonomidou, C. Novel Soft Alginate Hydrogel Strongly Supports Neurite Growth and Protects Neurons Against Oxidative Stress. Tissue Eng. Part A 2012, 18, 55–66.
- Askarzadeh, N.; Nazarpak, M.H.; Mansoori, K.; Farokhi, M.; Gholami, M.; Mohammadi, J.; Mottaghitalab, F. Bilayer Cylindrical Conduit Consisting of Electrospun Polycaprolactone Nanofibers and DSC Cross-Linked Sodium Alginate Hydrogel to Bridge Peripheral Nerve Gaps. Macromol. Biosci. 2020, 20, e2000149.
- Bozza, A.; Coates, E.E.; Incitti, T.; Ferlin, K.M.; Messina, A.; Menna, E.; Bozzi, Y.; Fisher, J.P.; Casarosa, S. Neural differentiation of pluripotent cells in 3D alginate-based cultures. Biomaterials 2014, 35, 4636–4645.
- Suzuki, Y.; Kitaura, M.; Wu, S.; Kataoka, K.; Suzuki, K.; Endo, K.; Nishimura, Y.; Ide, C. Electrophysiological and horseradish peroxidase-tracing studies of nerve regeneration through alginate-filled gap in adult rat spinal cord. Neurosci. Lett. 2002, 318, 121–124.
- Ashton, R.S.; Banerjee, A.; Punyani, S.; Schaffer, D.V.; Kane, R.S. Scaffolds based on degradable alginate hydrogels and poly(lactide-co-glycolide) microspheres for stem cell culture. Biomaterials 2007, 28, 5518–5525.
- Novikova, L.N.; Mosahebi, A.; Wiberg, M.; Terenghi, G.; Kellerth, J.-O.; Novikov, L.N. Alginate hydrogel and matrigel as potential cell carriers for neurotransplantation. J. Biomed. Mater. Res. Part A 2006, 77, 242–252.
- Wang, G.; Wang, X.; Huang, L. Feasibility of chitosan-alginate (Chi-Alg) hydrogel used as scaffold for neural tissue engineering: A pilot studyin vitro. Biotechnol. Biotechnol. Equip. 2017, 31, 766–773.
- Karimi, S.; Bagher, Z.; Najmoddin, N.; Simorgh, S.; Pezeshki-Modaress, M. Alginate-magnetic short nanofibers 3D composite hydrogel enhances the encapsulated human olfactory mucosa stem cells bioactivity for potential nerve regeneration application. Int. J. Biol. Macromol. 2021, 167, 796–806.
- Wang, Z.; Wang, J.; Jin, Y.; Luo, Z.; Yang, W.; Xie, H.; Huang, K.; Wang, L. A Neuroprotective Sericin Hydrogel as an Effective Neuronal Cell Carrier for the Repair of Ischemic Stroke. ACS Appl. Mater. Interfaces 2015, 7, 24629–24640.
- Zhang, L.; Yang, W.; Tao, K.; Song, Y.; Xie, H.; Wang, J.; Li, X.; Shuai, X.; Gao, J.; Chang, P.; et al. Sustained Local Release of NGF from a Chitosan–Sericin Composite Scaffold for Treating Chronic Nerve Compression. ACS Appl. Mater. Interfaces 2017, 9, 3432–3444.
- Cheong, G.M.; Lim, K.; Jakubowicz, A.; Martens, P.; Poole-Warren, L.; Green, R.A. Conductive hydrogels with tailored bioactivity for implantable electrode coatings. Acta Biomater. 2014, 10, 1216–1226.
- Hernandes, M.S.; Troncone, L.R.P. Glycine as a neurotransmitter in the forebrain: A short review. J. Neural Transm. 2009, 116, 1551–1560.
- Zhao, Y.; Liang, Y.; Ding, S.; Zhang, K.; Mao, H.-Q.; Yang, Y. Application of conductive PPy/SF composite scaffold and electrical stimulation for neural tissue engineering. Biomaterials 2020, 255, 120164.
- You, R.; Zhang, Q.; Li, X.; Yan, S.; Luo, Z.; Qu, J.; Li, M. Multichannel Bioactive Silk Nanofiber Conduits Direct and Enhance Axonal Regeneration after Spinal Cord Injury. ACS Biomater. Sci. Eng. 2020, 6, 4677–4686.
- Carvalho, C.R.; Chang, W.; Silva-Correia, J.; Reis, R.L.; Oliveira, J.M.; Kohn, J. Engineering Silk Fibroin-Based Nerve Conduit with Neurotrophic Factors for Proximal Protection after Peripheral Nerve Injury. Adv. Health Mater. 2021, 10, e2000753.
- Yang, Y.; Chen, X.; Ding, F.; Zhang, P.; Liu, J.; Gu, X. Biocompatibility evaluation of silk fibroin with peripheral nerve tissues and cells in vitro. Biomaterials 2007, 28, 1643–1652.
- Gu, X.; Chen, X.; Tang, X.; Zhou, Z.; Huang, T.; Yang, Y.; Ling, J. Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration. Nanotechnol. Rev. 2021, 10, 10–19.
- Chen, X.-Y.; Tu, Y.; Sun, S.-Z.; Kong, X.-B.; Tang, Q.-Y.; Sun, Z. Polyethylene glycol as a promising synthetic material for repair of spinal cord injury. Neural Regen. Res. 2017, 12, 1003–1008.
- Lin, C.-C.; Anseth, K.S. PEG Hydrogels for the Controlled Release of Biomolecules in Regenerative Medicine. Pharm. Res. 2009, 26, 631–643.
- Isaacs, J.; Klumb, I.; McDaniel, C. Preliminary investigation of a polyethylene glycol hydrogel “nerve glue”. J. Brachial Plex. Peripher. Nerve Inj. 2009, 4, e98–e102.
- Estradaa, V.; Brazdaa, N.; Schmitza, C.; Hellera, S.; Blazycab, H.; Martinib, R.; Müller, H.W. Long-lasting significant functional improvement in chronic severe spinal cord injury following scar resection and polyethylene glycol implantation. Neurobiol. Dis. 2014, 67, 165–179.
- Goncharuk-Oleksii, O.; Savosko-Serhii, I.; Petriv-Taras, I.; Medvediev-Volodymyr, V.; Tsymbaliuk-Vitaly, I. Morphometric Study of Rat Sciatic Nerve Recovery after Three Nerve Repair Techniques: Epineural Suture, Polyethylene Glycol Hydrogel and Fibrin Sealant. Int. J. Morphol. 2021, 39, 677–682.
- Mahoney, M.J.; Anseth, K.S. Contrasting effects of collagen and bFGF-2 on neural cell function in degradable synthetic PEG hydrogels. J. Biomed. Mater. Res. Part A 2007, 81A, 269–278.
- Hynes, S.R.; McGregor, L.M.; Rauch, M.F.; Lavik, E.B. Photopolymerized poly(ethylene glycol)/poly(L-lysine) hydrogels for the delivery of neural progenitor cells. J. Biomater. Sci. Polym. Ed. 2007, 18, 1017–1030.
- Burdick, J.A.; Ward, M.; Liang, E.; Young, M.J.; Langer, R. Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials 2006, 27, 452–459.
- Zhang, K.; Li, J.; Jin, J.; Dong, J.; Li, L.; Xue, B.; Wang, W.; Jiang, Q.; Cao, Y. Injectable, anti-inflammatory and conductive hydrogels based on graphene oxide and diacerein-terminated four-armed polyethylene glycol for spinal cord injury repair. Mater. Des. 2020, 196, 109092.
- Hejčl, A.; Lesný, P.; Přádný, M.; Michálek, J.; Jendelová, P.; Štulík, J.; Syková, E. Biocompatible hydrogels in spinal cord injury repair. Physiol. Res. 2008, 57 (Suppl. S3), S121–S132.
- Bakshi, A.; Fisher, O.; Dagci, T.; Himes, B.T.; Fischer, I.; Lowman, A. Mechanically engineered hydrogel scaffolds for axonal growth and angiogenesis after transplantation in spinal cord injury. J. Neurosurg. Spine 2004, 1, 322–329.
- Hejčl, A.; Urdzikova, L.M.; Sedy, J.; Lesny, P.; Pradny, M.; Michálek, J.; Burian, M.; Hajek, M.; Zamecnik, J.; Jendelova, P.; et al. Acute and delayed implantation of positively charged 2-hydroxyethyl methacrylate scaffolds in spinal cord injury in the rat. J. Neurosurg. Spine 2008, 8, 67–73.
- Li, H.Y.; Führmann, T.; Zhou, Y.; Dalton, P.D. Host reaction to poly(2-hydroxyethyl methacrylate) scaffolds in a small spinal cord injury model. J. Mater. Sci. Mater. Electron. 2013, 24, 2001–2011.
- Pertici, V.; Trimaille, T.; Laurin, J.; Felix, M.-S.; Marqueste, T.; Pettmann, B.; Chauvin, J.-P.; Gigmes, D.; Decherchi, P. Repair of the injured spinal cord by implantation of a synthetic degradable block copolymer in rat. Biomaterials 2014, 35, 6248–6258.
- Kubinová, Š.; Horák, D.; Kozubenko, N.; Vaněček, V.; Proks, V.; Price, J.; Cocks, G.; Syková, E. The use of superporous Ac-CGGASIKVAVS-OH-modified PHEMA scaffolds to promote cell adhesion and the differentiation of human fetal neural precursors. Biomaterials 2010, 31, 5966–5975.
- Jhaveri, S.J.; Hynd, M.R.; Dowell-Mesfin, N.; Turner, J.N.; Shain, W.; Ober, C.K. Release of Nerve Growth Factor from HEMA Hydrogel-Coated Substrates and Its Effect on the Differentiation of Neural Cells. Biomacromolecules 2009, 10, 174–183.
- Hejčl, A.; Růžička, J.; Kekulová, K.; Svobodová, B.; Proks, V.; Macková, H.; Jiránková, K.; Kárová, K.; Urdziková, L.M.; Kubinová, Š.; et al. Modified Methacrylate Hydrogels Improve Tissue Repair after Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 2481.
- Pertici, V.; Amendola, J.; Laurin, J.; Gigmes, D.; Madaschi, L.; Carelli, S.; Marqueste, T.; Gorio, A.; Decherchi, P. The Use of Poly(N--Methacrylamide) Hydrogel to Repair a T10 Spinal Cord Hemisection in Rat: A Behavioural, Electrophysiological and Anatomical Examination. ASN Neuro 2013, 5, AN20120082.
- Woerly, S.; Doan, V.D.; Sosa, N.; de Vellis, J.; Espinosa-Jeffrey, A. Prevention of gliotic scar formation by NeuroGel™ allows partial endogenous repair of transected cat spinal cord. J. Neurosci. Res. 2004, 75, 262–272.
- Plant, G.W.; Woerly, S.; Harvey, A.R. Hydrogels Containing Peptide or Aminosugar Sequences Implanted into the Rat Brain: Influence on Cellular Migration and Axonal Growth. Exp. Neurol. 1997, 143, 287–299.
- Woerly, S.; Fort, S.; Pignot-Paintrand, I.; Cottet, C.; Carcenac, C.; Savasta, M. Development of a Sialic Acid-Containing Hydrogel of Poly: Characterization and Implantation Study. Biomacromolecules 2008, 9, 2329–2337.
- Syková, E.; Jendelova, P.; Urdzikova, L.M.; Lesný, P.; Hejčl, A. Bone Marrow Stem Cells and Polymer Hydrogels—Two Strategies for Spinal Cord Injury Repair. Cell. Mol. Neurobiol. 2006, 26, 1113–1129.
- Stocco, E.; Barbon, S.; Lora, L.; Grandi, F.; Sartore, L.; Tiengo, C.; Petrelli, L.; Dalzoppo, D.; Parnigotto, P.P.; Macchi, V.; et al. Partially oxidized polyvinyl alcohol conduitfor peripheral nerve regeneration. Sci. Rep. 2018, 8, 604.
- Matsuura, Y.; Hiraizumi, Y.; Fujimaki, E. Application of a Polyvinyl Alcohol Hydrogel (Pvah) Mem-Brane as an Antiadhesive Interposition after Spinal Surgery. Showa Igakkai Zasshi 1996, 56, 355–362.
- Oh, S.H.; An, D.B.; Kim, T.H.; Lee, J.H. Wide-range stiffness gradient PVA/HA hydrogel to investigate stem cell differentiation behavior. Acta Biomater. 2016, 35, 23–31.
- Assinck, P.; Duncan, G.J.; Hilton, B.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647.
- Ottoboni, L.; de Feo, D.; Merlini, A.; Martino, G. Commonalities in immune modulation between mesenchymal stem cells (MSCs) and neural stem/precursor cells (NPCs). Immunol. Lett. 2015, 168, 228–239.
- Tseng, T.-C.; Tao, L.; Hsieh, F.-Y.; Wei, Y.; Chiu, I.-M.; Hsu, S.-H. An Injectable, Self-Healing Hydrogel to Repair the Central Nervous System. Adv. Mater. 2015, 27, 3518–3524.
- Zou, Y.; Zhao, Y.; Xiao, Z.; Chen, B.; Ma, D.; Shen, H.; Gu, R.; Dai, J. Comparison of Regenerative Effects of Transplanting Three-Dimensional Longitudinal Scaffold Loaded-Human Mesenchymal Stem Cells and Human Neural Stem Cells on Spinal Cord Completely Transected Rats. ACS Biomater. Sci. Eng. 2020, 6, 1671–1680.
- Zhang, S.; Burda, J.E.; Anderson, M.A.; Zhao, Z.; Ao, Y.; Cheng, Y.; Sun, Y.; Deming, T.J.; Sofroniew, M.V. Thermoresponsive Copolypeptide Hydrogel Vehicles for Central Nervous System Cell Delivery. ACS Biomater. Sci. Eng. 2015, 1, 705–717.
- Prang, P.; Mueller, R.; Eljaouhari, A.; Heckmann, K.; Kunz, W.; Weber, T.; Faber, C.; Vroemen, M.; Bogdahn, U.; Weidner, N. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials 2006, 27, 3560–3569.
- Dasari, V.R.; Veeravalli, K.K.; Dinh, D.H. Mesenchymal stem cells in the treatment of spinal cord injuries: A review. World J. Stem Cells 2014, 6, 120.
- Zhang, J.; Cheng, T.; Chen, Y.; Gao, F.; Guan, F.; Yao, M.-H. A chitosan-based thermosensitive scaffold loaded with bone marrow-derived mesenchymal stem cells promotes motor function recovery in spinal cord injured mice. Biomed. Mater. 2020, 15, 35020.
- Rhode, S.C.; Beier, J.P.; Ruhl, T. Adipose tissue stem cells in peripheral nerve regeneration—In vitro and in vivo. J. Neurosci. Res. 2021, 99, 545–560.
- Lin, Y.-J.; Lee, Y.-W.; Chang, C.-W.; Huang, C.-C. 3D Spheroids of Umbilical Cord Blood MSC-Derived Schwann Cells Promote Peripheral Nerve Regeneration. Front. Cell Dev. Biol. 2020, 8, 1632.
- Volarevic, V.; Gazdic, M.; Markovic, B.S.; Jovicic, N.; Djonov, V.; Arsenijevic, N. Mesenchymal stem cell-derived factors: Immuno-modulatory effects and therapeutic potential. BioFactors 2017, 43, 633–644.
- He, J.; Zhang, N.; Zhu, Y.; Jin, R.; Wu, F. MSC spheroids-loaded collagen hydrogels simultaneously promote neuronal differentiation and suppress inflammatory reaction through PI3K-Akt signaling pathway. Biomaterials 2021, 265, 120448.
- Luo, L.; He, Y.; Jin, L.; Zhang, Y.; Guastaldi, F.P.; Albashari, A.A.; Hu, F.; Wang, X.; Wang, L.; Xiao, J.; et al. Application of bioactive hydrogels combined with dental pulp stem cells for the repair of large gap peripheral nerve injuries. Bioact. Mater. 2021, 6, 638–654.
- Kolar, M.K.; Itte, V.N.; Kingham, P.J.; Novikov, L.; Wiberg, M.; Kelk, P. The neurotrophic effects of different human dental mesenchymal stem cells. Sci. Rep. 2017, 7, 12605.
- Pisciotta, A.; Bertoni, L.; Vallarola, A.; Bertani, G.; Mecugni, D.; Carnevale, G. Neural crest derived stem cells from dental pulp and tooth-associated stem cells for peripheral nerve regeneration. Neural Regen. Res. 2020, 15, 373–381.
- Terenghi, G.; Wiberg, M.; Kingham, P.J. Chapter 21 Use of Stem Cells for Improving Nerve Regeneration. Int. Rev. Neurobiol. 2009, 87, 393–403.
- Mosahebi, A.; Simon, M.; Wiberg, M.; Terenghi, G. A Novel Use of Alginate Hydrogel as Schwann Cell Matrix. Tissue Eng. 2001, 7, 525–534.
- Suri, S.; Schmidt, C.E. Cell-Laden Hydrogel Constructs of Hyaluronic Acid, Collagen, and Laminin for Neural Tissue Engineering. Tissue Eng. Part A 2010, 16, 1703–1716.
- Wang, H.; Liu, C.; Ma, X. Alginic acid sodium hydrogel co-transplantation with Schwann cells for rat spinal cord repair. Arch. Med. Sci. 2012, 8, 563–568.
- Guenard, V.; Kleitman, N.; Morrissey, T.; Bunge, R.; Aebischer, P. Syngeneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve regeneration. J. Neurosci. 1992, 12, 3310–3320.
- Tsai, E.; Dalton, P.D.; Shoichet, M.S.; Tator, C.H. Matrix inclusion within synthetic hydrogel guidance channels improves specific supraspinal and local axonal regeneration after complete spinal cord transection. Biomaterials 2006, 27, 519–533.
- Huang, J.; Xiong, J.; Yang, L.; Zhang, J.; Sun, S.; Liang, Y. Cell-free exosome-laden scaffolds for tissue repair. Nanoscale 2021, 13, 874–875.
- Li, L.; Zhang, Y.; Mu, J.; Chen, J.; Zhang, C.; Cao, H.; Gao, J. Transplantation of Human Mesenchymal Stem-Cell-Derived Exosomes Immobilized in an Adhesive Hydrogel for Effective Treatment of Spinal Cord Injury. Nano Lett. 2020, 20, 4298–4305.
- Yang, Z.; Yang, Y.; Xu, Y.; Jiang, W.; Shao, Y.; Xing, J.; Chen, Y.; Han, Y. Biomimetic nerve guidance conduit containing engineered exosomes of adipose-derived stem cells promotes peripheral nerve regeneration. Stem Cell Res. Ther. 2021, 12, 442.
- Sims, S.-K.; Wilken-Resman, B.; Smith, C.J.; Mitchell, A.; McGonegal, L.; Sims-Robinson, C. Brain-Derived Neurotrophic Factor and Nerve Growth Factor Therapeutics for Brain Injury: The Current Translational Challenges in Preclinical and Clinical Research. Neural Plast. 2022, 2022, 3889300.
- Cook, D.J.; Nguyen, C.; Chun, H.N.; Llorente, I.L.; Chiu, A.S.; Machnicki, M.; I Zarembinski, T.; Carmichael, S.T. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J. Cereb. Blood Flow Metab. 2017, 37, 1030–1045.
- Yu, X.; Dillon, G.P.; Bellamkonda, R.V. A Laminin and Nerve Growth Factor-Laden Three-Dimensional Scaffold for Enhanced Neurite Extension. Tissue Eng. 1999, 5, 291–304.
- Zhao, Y.-Z.; Jiang, X.; Xiao, J.; Lin, Q.; Yu, W.-Z.; Tian, F.-R.; Mao, K.-L.; Yang, W.; Wong, H.L.; Lu, C.-T. Using NGF heparin-poloxamer thermosensitive hydrogels to enhance the nerve regeneration for spinal cord injury. Acta Biomater. 2016, 29, 71–80.
- Tong, Y.; Guo, H.; Zhou, H.; Lu, J.; Qu, Y.; Yu, D. Vascular endothelial growth factor: An attractive target in the treatment of hypoxic/ischemic brain injury. Neural Regen. Res. 2016, 11, 174–179.
- Gnavi, S.; di Blasio, L.; Tonda-Turo, C.; Mancardi, A.; Primo, L.; Ciardelli, G.; Gambarotta, G.; Geuna, S.; Perroteau, I. Gelatin-based hydrogel for vascular endothelial growth factor release in peripheral nerve tissue engineering. J. Tissue Eng. Regen. Med. 2014, 11, 459–470.
- Rao, F.; Wang, Y.; Zhang, D.; Lu, C.; Cao, Z.; Sui, J.; Wu, M.; Zhang, Y.; Pi, W.; Wang, B.; et al. Aligned chitosan nanofiber hydrogel grafted with peptides mimicking bioactive brain-derived neurotrophic factor and vascular endothelial growth factor repair long-distance sciatic nerve defects in rats. Theranostics 2020, 10, 1590–1603.
- Fujimaki, H.; Uchida, K.; Inoue, G.; Miyagi, M.; Nemoto, N.; Saku, T.; Isobe, Y.; Inage, K.; Matsushita, O.; Yagishita, S.; et al. Oriented collagen tubes combined with basic fibroblast growth factor promote peripheral nerve regeneration in a 15mm sciatic nerve defect rat model. J. Biomed. Mater. Res. Part A 2016, 105, 8.
- Zhang, J.J.; Zhu, J.J.; Hu, Y.B.; Xiang, G.H.; Xiao, J. Transplantation of bFGF-expressing neural stem cells promotes cell migration and functional recovery in rat brain after transient ischemic stroke. Oncotarget 2017, 8, 102067.
- Midha, R.; Munro, C.A.; Dalton, P.D.; Tator, C.H.; Shoichet, M.S. Growth factor enhancement of peripheral nerve regeneration through a novel synthetic hydrogel tube. J. Neurosurg. 2003, 99, 555.
- Ye, J.; Jin, S.; Cai, W.; Chen, X.; Zheng, H.; Zhang, T.; Lu, W.; Li, X.; Liang, C.; Chen, Q.; et al. Rationally Designed, Self-Assembling, Multifunctional Hydrogel Depot Repairs Severe Spinal Cord Injury. Adv. Health Mater. 2021, 10, 2100242.
- He, Z.; Zang, H.; Zhu, L.; Huang, K.; Yi, T.; Zhang, S.; Cheng, S. An anti-inflammatory peptide and brain-derived neurotrophic factor-modified hyaluronan-methylcellulose hydrogel promotes nerve regeneration in rats with spinal cord injury. Int. J. Nanomed. 2019, 14, 721–732.
- Mahya, S.; Ai, J.; Shojae, S.; Khonakdar, H.A.; Darbemamieh, G.; Shirian, S. Berberine loaded chitosan nanoparticles encapsulated in polysaccharide-based hydrogel for the repair of spinal cord. Int. J. Biol. Macromol. 2021, 182, 82–90.
- Qian, F.; Han, Y.; Hana, Z.; Zhanga, D.; Zhanga, L.; Zhaoa, G.; Lic, S.; Jind, G.; Yuabe, R.; Liuabe, H. In Situ implantable, post-trauma microenvironment-responsive, ROS Depletion Hydrogels for the treatment of Traumatic brain injury. Biomaterials 2021, 270, 120675.
- Luo, J.; Shi, X.; Li, L.; Tan, Z.; Feng, F.; Li, J.; Pang, M.; Wang, X.; He, L. An injectable and self-healing hydrogel with controlled release of curcumin to repair spinal cord injury. Bioact. Mater. 2021, 6, 4816–4829.




