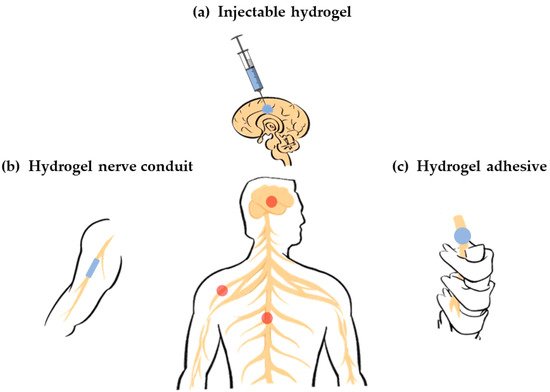
A hydrogel exhibits several advantages in its application as a nerve repair material. First of all, it is an ideal carrier that can load cells, drugs, nutrients, growth factors and other bioactive components, as well as maintain its activity for a long time to promote nerve repair. Second, it has good biodegradability so that it can provide support for the growth of new tissues at the initial stage of repair and provide space for nerve regeneration through gradual degradation. Third, it has a higher moisture content and better biocompatibility, making it a suitable substrate for endogenous or transplanted cell growth. Forth, it has an adjustable mechanical strength, which can flexibly fit the modulus of the damaged nerve tissue. Finally, it has abundant pore space that is conducive to cell growth and material exchange. Therefore, the application of hydrogels in the field of nerve repair has been widely studied.
From different angles, hydrogels have various classification standards. For instance, based on their physical shape, hydrogels can generally be classified as blocks, microspheres, fibrous, and films. Based on their physical structure, hydrogels can be divided into three categories: amorphous, crystalline and semicrystalline phases, which is a mixture of amorphous and crystalline. Based on the presence or absence of electrical charge located on the cross-linked chains, hydrogels can be divided into four categories: nonionic, ionic, amphoteric electrolyte and zwitterionic. Based on the cross-linking mechanism, hydrogels can be divided into two categories. The chemical cross-linked hydrogel network is a permanent connection. The physical cross-linked hydrogel network is a transient connection that arises from molecular/polymer chain entanglement or under the action of weak interactions, such as hydrogen bonding and electrostatic interactions. Based on their polymeric composition, hydrogels can be divided into three categories. Homopolymeric hydrogels are referred to as polymer networks derived from a single species of monomer. Copolymeric hydrogels are referred to as polymer networks that comprise two or more different monomer species with at least one hydrophilic component. Multipolymer interpenetrating polymeric hydrogels are referred to as polymer networks made of two independent cross-linked components where one component is a cross-linked polymer and other component is a non-cross-linked polymer [
11].
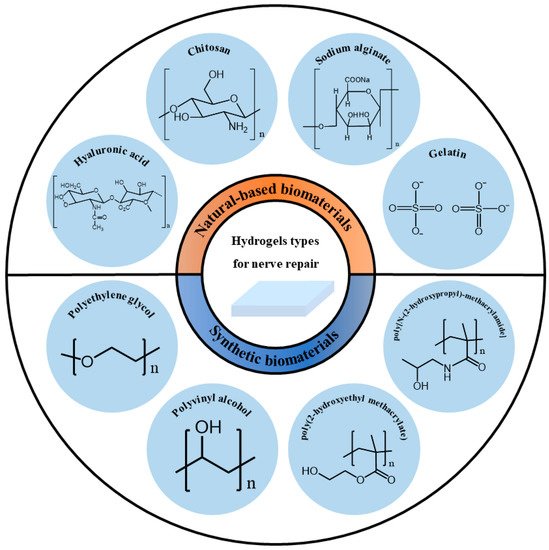
Based on their source, hydrogels can be divided into natural-based biomaterials and synthetic biomaterials. The source of the polymer has a greater impact on the biocompatibility and safety of the hydrogel when considered as a functional material for biological tissues. Therefore, this article introduces the classification of hydrogels based on their sources as shown in Figure 2.
2.1. Natural-Based Biomaterials
Natural-based biomaterials refer to biomaterials generated under natural conditions, usually in the form of naturally occurring macromolecular substances derived from animals, plants or humans. Natural-based biomaterials can be gradually degraded into small molecular substances existing in the body through hydrolysis, enzymatic hydrolysis and other methods under the physiological environment of the body, and then completely absorbed or excreted through metabolism without causing toxic side effects on the body itself. Because of their large availability, large quantities of natural-based biomaterials are accessible at reasonable prices. Thus, they have been widely used as hydrogels for nerve repair. However, their mechanical properties are usually poor, and some of them are insoluble in water or common organic solvents. It is difficult to purify them for large-scale production [
16]. The following will briefly introduce and discuss some natural-based biomaterials used in nerve repair.
Hyaluronic acid (HA) is a macromolecular linear polysaccharide and the main component of an extracellular matrix, especially abundant in the central system. It is involved in the regulation of a variety of cell activities. Its unique molecular structure and physicochemical properties endows it with a variety of important physiological functions in the body [
17]. Thus, it has been widely used for nerve repair. In vitro experiments have shown that HA hydrogel can promote strong neurite outgrowth [
18]. Neurite is an elongated part extending from the cell body of a nerve cell (neuron), and can be divided into dendrites and axons. The dendrite is the entrance that receives information, and the axon is the exit that sends out information. Both are used together for information transfer between cells. HA hydrogels also support the viability of neural precursor cells [
19,
20], neural stem cells [
21] and Schwann cells [
22], maintain their activities, and enable them to proliferate and differentiate. HA hydrogels have a high water-absorbency and enzyme degradability. In vivo, they usually exhibit rapid erosion and degradation behavior. They mainly form a hydration open mesh at the site of injury to inhibit the formation of a glial scar. Glial scarring is a compensatory reaction product after injury. In the early post-injury period, glial scarring can limit the spread of inflammation. However, with the progression of the disease, a glial scar can form a physical and chemical barrier, secrete a variety of axon regeneration inhibitory factors, and hinder the recovery of neural function. By reducing its formation, glial scarring can promote neuroprotection and functional recovery. In addition, HA hydrogels support the cell infiltration of cells into the hydrogel and angiogenesis [
23]. Broguiere cross-linked high-molecular-weight hyaluronic acid and transglutaminase to prepare a new type of hydrogel, which could be covalently bound to the protein in the body when it was injected in vivo, so that the hydrogel could covalently bind to brain or spinal cord defects. Thus, it showed a fast neurite outgrowth, axonal and dendritic speciation, strong synaptic connectivity in 3D networks, and rapidly occurring and long-lasting coordinated electrical activity [
24]. The healing potential of HA hydrogels may be partly attributed to hyaluronic acid promoting interactions between hydrogels and the central nervous system [
25].
Collagen is the most important extracellular water-insoluble fibrin. It is the skeletal component of the extracellular matrix and the most abundant protein in the human body. Collagen hydrogel can be used as the inner filling of nerve conduits to repair broken nerve tissue [
26] or independent tissue engineering material to fill tissue defect [
27]. It is an excellent carrier of neural stem cells, mesenchymal stem cells and other cells. It can be used for cell transplantation to repair brain or spinal cord injury [
28,
29]. It can also be used as a sustained-release drug carrier [
30].
Gelatin is obtained by the thermal denaturation of collagen. Gelatin has a relatively low antigenicity compared with collagen. The body’s immune system response can be avoided or reduced during its use. Animal-derived gelatin has been widely studied in medical applications due to its biocompatibility, plasticity and adhesion. Gelatin hydrogel is beneficial for cell adhesion and activity, and has significant effects on nerve healing and reconstruction [
31]. Experimental results have shown that the nerve conduit containing gelatin hydrogel and a basic fibroblast growth factor promoted axonal regeneration after a 15 mm sciatic nerve injury in rats. The nerve regeneration rate was significantly increased, and a large number of regenerated nerve axons were induced [
32]. However, this effect was not as good as autologous transplantation [
33]. Due to its weak mechanical strength and rapid degradation, most gelatins will be prepared in a combination with other biomaterials to improve the comprehensive properties of hydrogels [
34,
35,
36].
Fibrin is a protein formed during blood coagulation and is also a component of the extracellular matrix. Fibrin has an obvious advantage over other types of protein hydrogels in the repair of the nervous system: when autologous fibrin is used, it has a complete biocompatibility [
37]. Fibrin hydrogel can improve the survival rate of neural stem cells [
38,
39], support the neurogenic differentiation of mesenchymal stem cells and the regeneration of axons [
40]. It also has a satisfactory effect in loading drugs [
41]. In order to overcome the application limitation of fibrin, Robinson crosslinked it with genipin to reduce its degradation rate, so that the hydrogel scaffold could support the neural aggregates derived from neural progenitor cells and induce neurite outgrowth [
42].
Keratin is a kind of fibrin with abundant sources and biological activities [
43]. Keratin hydrogel not only has a good cell adhesion [
44] but can also maintain the high activity of cells [
45,
46]. Keratin hydrogel also has a high nerve induction effect [
47,
48]. In a mouse tibial nerve model, keratin hydrogel significantly improved the electrophysiological recovery in the early stage. At 6 months, the keratin hydrogel group even produced electrical and histological results superior to the empty conduits and sensory nerve autografts [
49]. In 1 cm rat sciatic nerve injury, keratin hydrogel could promote debris clearance in the distal stump by Schwann cell activation [
50].
Chitosan is formed by the deacetylation of chitin in crustacean shells. It is a widely used tissue engineering material with a low production cost. It has the potential to interact with regeneration-related cells and neural microenvironments, so as to improve axon regeneration and reduce the formation of neuroma. Chitosan can effectively maintain the biological activity of loaded cells and promote their proliferation and differentiation [
51], such as promoting the growth of Schwann cells [
52], supporting the differentiation of neural progenitor cells [
53], and inducing mesenchymal stromal cells to differentiate into Schwann-like cells [
54]. It allows a functional and morphological nerve regeneration similar to autologous nerve transplantation [
55]. Jafar prepared chitosan scaffolds containing human endometrial stem cells and platelet plasma. It promoted nerve fiber regeneration and angiogenesis and prevented the formation of scar tissue in a rat spinal cord injury model [
56]. In addition, chitosan oligosaccharide, the degradation product of chitosan, showed a neuroprotective effect in the process of peripheral nerve regeneration. It could promote cell proliferation, prevent cell apoptosis and accelerate peripheral nerve regeneration [
57,
58,
59].
Alginate is a polysaccharide found in brown algae. It has an antioxidant capacity and can protect nerve cells from H
2O
2 damage [
60,
61]. Alginate hydrogel can not only promote the adhesion and proliferation of nerve cells [
62], but also enhance the differentiation of pluripotent cells (mouse embryonic stem cells) into neural lineages [
63]. It can also trigger the regeneration of up and down axon fibers and effectively form synapses, which play a supporting role in axon regeneration [
64]. However, alginate hydrogel still has some limitations. Since mammals do not produce endogenous seaweed enzymes, the alginate hydrogel implanted in vivo may take several months to complete degradation. This poor degradation will reduce the penetration ability of cells [
65]. In addition, pure alginate hydrogel is bioinert and cannot promote the activity of encapsulated cells [
66]. For its applications, it can be improved by compounding with other materials [
67], or adding substances such as nanofibers [
68].
Silk fibroin and sericin are two main proteins in silk. Sericin has neurotrophic and neuroprotective effects, can promote axon extension and branching, and prevent the cell death of primary neurons in a hypoxia environment [
69,
70]. Sericin hydrogel can promote the adhesion and differentiation of neurons and facilitate the repair of nerves [
71]. In addition, the degradation products of sericin protein are rich in glycine and serine, which play an important role as neurotransmitters in neurotransmission [
72]. Silk fibroin has excellent nerve biocompatibility and can support nerve regeneration. It has a high potential in nerve tissue engineering. At present, it is mainly used as a nerve conduit to bridge damaged nerves by electrospinning [
73,
74,
75]. Silk fibroin hydrogel has good biocompatibility with ganglion, and is beneficial for maintaining the activity of Schwann cells [
76], promoting axonal growth and guiding axonal sprouting [
77].
2.2. Synthetic Biomaterials
Polyethylene glycol (PEG) is a stable, nontoxic and biocompatible polymer synthetic material with a wide range of clinical applications. When PEG is used for spinal cord repair, it has many advantages, such as no accumulation in the body, inhibiting the formation of vacuoles and scars, reducing inflammation and so on [
78]. PEG can be used to synthesize hydrogels and modify other hydrogels [
79]. When repairing sciatic nerve transection injury in rats, PEG hydrogels can effectively reduce scar tissue formation [
80], support axon regeneration [
81], and promote limb function by promoting more and more myelin regeneration, enabling a faster recovery [
82]. Myelin refers to the protective shell formed by oligodendrocytes on the outside of nerve fibers. Its main function is to protect nerves and participate in the conduction of nerve impulses. Changing the end groups of PEG and introducing different functional groups, such as toluene sulfonate, amino group, carboxyl group and aldehyde group, can further expand the application field of PEG. By introducing biological ingredients, such as natural biomaterials and nutritional factors, into synthetic hydrogels, PEG can effectively improve the function of nerve cells in inert PEG hydrogels, and promote the survival and growth of nerve cells [
83,
84,
85]. Zhang prepared hydrogels made from graphene oxide and four-arm polyethylene glycol terminated with two acetyl glycerol and loaded with the anti-inflammatory agent, diacerein. The hydrogel was delivered to the spinal cord in rats, causing injury by a minimally invasive injection. Free diacerein can minimize inflammatory responses and prevent the formation of an inhibitory microenvironment. Graphene oxide gives proper conductivity to hydrogels, which promotes the neuronal growth and remyelination of axons [
86].
Methacrylate-based hydrogels are widely used in the field of nerve repair. Hydrogels based on poly(2-hydroxyethyl methacrylate) (PHEMA) and poly[N-(2-hydroxypropyl)-methacrylamide] (PHPMA) belong to a group of synthetic, highly biocompatible polymers [
87]. The high water content of the PHEMA hydrogel and its mechanical compatibility with host tissues allows it to restore the anatomical continuity of damaged nerve structures. Porous PHEMA hydrogels inhibit scarring [
88] and provide scaffolds for the growth of connective tissue elements, blood vessels, neurofilaments, and Schwann cells [
89]. In addition, it can also be used as a platform for continuous drug delivery. The PHEMA also has some disadvantages. First, as a non-degradable polymer, hydrogel calcification and prolonged inflammatory responses might limit long-term axonal regeneration [
90]. Biodegradable hydrogels were prepared by compounding PHEMA with other polymers. Pertici combined PHEMA with PLA to obtain block copolymer. The PLA-b-PHEMA block copolymer can indeed display desired properties, such as biodegradability and an improvement in rat motor function, which were observed after implantation following a thoracic spinal cord hemisection in rats [
91]. In addition, PHEMA itself is not adhesive or attractive to neurons, so it can be modified using substances with biorecognition sites, thereby improving the biocompatibility of the hydrogels. Šárka used laminin-derived peptide sequences to modify PHEMA to promote cell adhesion and neural differentiation [
92]. The modification of PHEMA hydrogels with lysine can prolong the release time of its loading factor, increase the survival rate of neurons, and induce favorable biological reactions [
93].
PHPMA is more biocompatible than PHEMA [
94]. The PHPMA hydrogel implantation into the hemisected T10 rat spinal cord induced locomotor and neurophysiological improvements [
95]. Six months after the PHPMA hydrogel was implanted into the spinal cord of transected adult cats, the interface formed between the hydrogel and the spinal stumps prevented scar formation. The presence of the hydrogel implant led to a considerable reduction in damage to distal caudal portions of the spine and provided an adequate environment for growth of myelinated fibers [
96]. Peptides- and aminosugar-modified PHPMA hydrogels could increase adhesion properties with host neural tissue, promote the migration and reorganization of local wound repair cells, and be filtrated by host nonneuronal cells [
97]. Woerly prepared a PHPMA hydrogel with a bulk-modified saccharidic portion of ganglioside GM3 (The 3′-sialyllactose is a bioactive epitope recognized by many cell surface receptors on cells). The modified hydrogel was well-tolerated by the neural tissue and had the capacity to store endogenous self-migrating stem cells and, for a proportion of them, to support their neural differentiation in a non-neurogenic region [
98]. Similar to PHEMA, PHPMA is also a non-degradable polymer, which can conjugate with degradable polymers to expand its application range [
99].
Polyvinyl alcohol (PVA) is a crystalline polymer prepared from polyvinyl acetate. Although PVA can be hydrolyzed, it cannot be completely degraded, which is the main disadvantage limiting its application in biomedical fields. PVA conduit does not support the adhesion and proliferation of human neuroblastoma cells in vitro [
100]. However, in vivo experiments showed that PVA hydrogel prevented the migration of inflammatory cells after the laminectomy of cats. At the same time, it reduced the formation of scar tissue [
101]. Heang synthesized PVA/HA hydrogels, which can promote the differentiation of human-bone-marrow mesenchymal stem cells into specific cell types by adjusting the hardness of hydrogels [
102].
In short, natural-based biomaterials possess an excellent biocompatibility and some may have a bioactive sequence in the moiety, which can benefit nerve repair to some extent. However, there are slight differences in the performances of natural-based biomaterials between different batches as they are extracted from animals and plants, limiting their further translation into the clinic. Thus, it is important for natural-based biomaterials to pay attention to maintaining performance uniformity.