The fungal-like organisms of the Hyphochytriomycota, Oomycota and Labyrinthulomycota all have marine representatives, especially in mangrove habitats (
www.marinefungi.org, accessed on 15 December 2021). Marine representatives of the orders Peronosporales, Pythiales and Saprolegniales (Oomycota) have been widely studied for their growth at various salinities and sodium chloride concentrations (
Table 2 and
Table 3). They are well adapted to the fluctuating salinities found in mangroves with the production of both asexual and sexual stages
[43][44][45].
Few members of the Saprolegniaceae have been reported from marine habitats
[49], and Harrison and Jones
[50] questioned if this was due to their inability to tolerate high salinity levels. Using zoospore suspensions of 17 saprolegniaceous species, they investigated their ability to reproduce asexually at salinities of 0–40% seawater. Normal sporangial development occurred in freshwater, while in 10% seawater only nine produced normal zoospores: e.g.,
Achlya bisexualis, Protoachyla paradoxa, Thraustyotheca clavata, Saprolegnia parasiticaand
a Saprolgenia sp. In three Isoachlya spp. and
Achyla raceomsa, some cytoplasmic cleavage occurred. In 20% seawater only small sporangial primordia were formed, with only S. parasitica and T. clavata producing zoospores. Formation of sexual reproduction was also investigated for the same taxa with most species forming oospheres, but in I. toruloides there was no cleavage of the oogonial cytoplasm. At 20% seawater only
P. paradoxa produced mature oospheres, while at salinities above 20%, sexual reproduction was suppressed. Similar results were reported by Höhnk
[51][52] for a
Saprolegnia sp. when 7.09% salinity inhibited asexual reproduction, although excellent vegetative growth occurred. Clearly members of the Saprolegniales are not well adapted for survival at the higher salinities found in the ocean.
Species of the oomycetous genus
Halophytophthora have wide salinity growth ranges. Isolates of
H. avicenniae and
H. batemanensis were able to grow at 4‰, 8‰, 16‰ and 32‰, although pH and incubation temperature had a combinatorial effect on growth
[45]. The wide salinity growth ranges of Halophytophthora isolates suggest that they are well adapted to the salinity variation daily (high/low tide) and seasonally (summer/winter, rainy/dry seasons) in mangrove habitats.
4. Physiological Response to Salinity
This is a topic that has been widely researched by David Jennings and his students
[53][54][55][56][57][58][59][60][61] who have carried out extensive studies on marine fungi and their ability to tolerate saline conditions in the marine environment. Do marine fungi require sodium chloride for growth and what are the mechanisms that control osmotic pressure within their mycelium? In an effort to understand these phenomena, a wide range of techniques have been applied to elucidate the mechanisms involved. Jennings
[53] highlighted three physiological issues facing a fungus in the marine environment: seawater (1) has a relatively low water potential, (2) contains a relatively high concentration of ions, and (3) has an alkaline pH.
Studies with
Paradendryphiella salina showed that sodium stimulates its growth at low salinities but is toxic at high concentrations
[36]. This inhibition could be overcome by the addition of magnesium, calcium, strontium and barium (in order of effectiveness), and this was also demonstrated for other marine fungi
[36]. The study also demonstrated that the key issue for the growth of the fungus was the permeability of the mycelium to potassium in a high salt medium. This study led to the exploration of a number of factors pertinent to understanding the mechanisms of salt tolerance in marine and other fungi
[53]. These included plasma membrane permeability, accumulation of ions in the vacuoles, and the role of polyols in maintaining turgor in the mycelium.
In seawater, how does
Paradendryphiella salina control the movement of nutrients and ions into the mycelium? It has already been shown that calcium and other bivalent cations play a role in the movement of potassium and exclusion of sodium from the mycelium. Jones and Jennings
[36] proposed that there was a sodium pump for its exclusion from the mycelium. Galpin and Jennings
[62] reported the involvement of ATPase in maintaining the K
+/Na
+ ratio within the mycelium of P. salina, and ATPase is required for the active transport of cations. When
P. salina was grown at high salinity and pH, there was an increased activity of membrane-bound ATPase and this aids in good potassium/sodium balance in the cytoplasm
[53]. Thus, ATPase is required for active transport of cations and fulfilled by glycolysis.
These studies continued by exploring the accumulation of ions in fungal vacuoles
[56][60][63], plasma-membrane permeability
[58][64] and the role of polyols in maintaining turgor in the marine fungal mycelium
[61][63]. Ions contributed some 60% of the solute potential in the 48-h old mycelium of
P. salina grown in the presence of high concentrations of sodium chloride, while polyols contributed 30%
[63]. Jennings and Austin
[60] showed the importance of mannitol and arabitol in maintaining the total in vivo carbohydrate content of mycelium of P. salina which is required for making optimum turgor for growth. Mannitol and arabitol synthesis within the hypha increased with increasing salinity because these sugar alcohols play the main role in maintaining osmotic pressure as well as correct differential water potential in the mycelium
[54]. This was confirmed by Wethered et al.
[63], who provided evidence that the polyol content of the mycelium increased with salinity. Holligan and Jennings
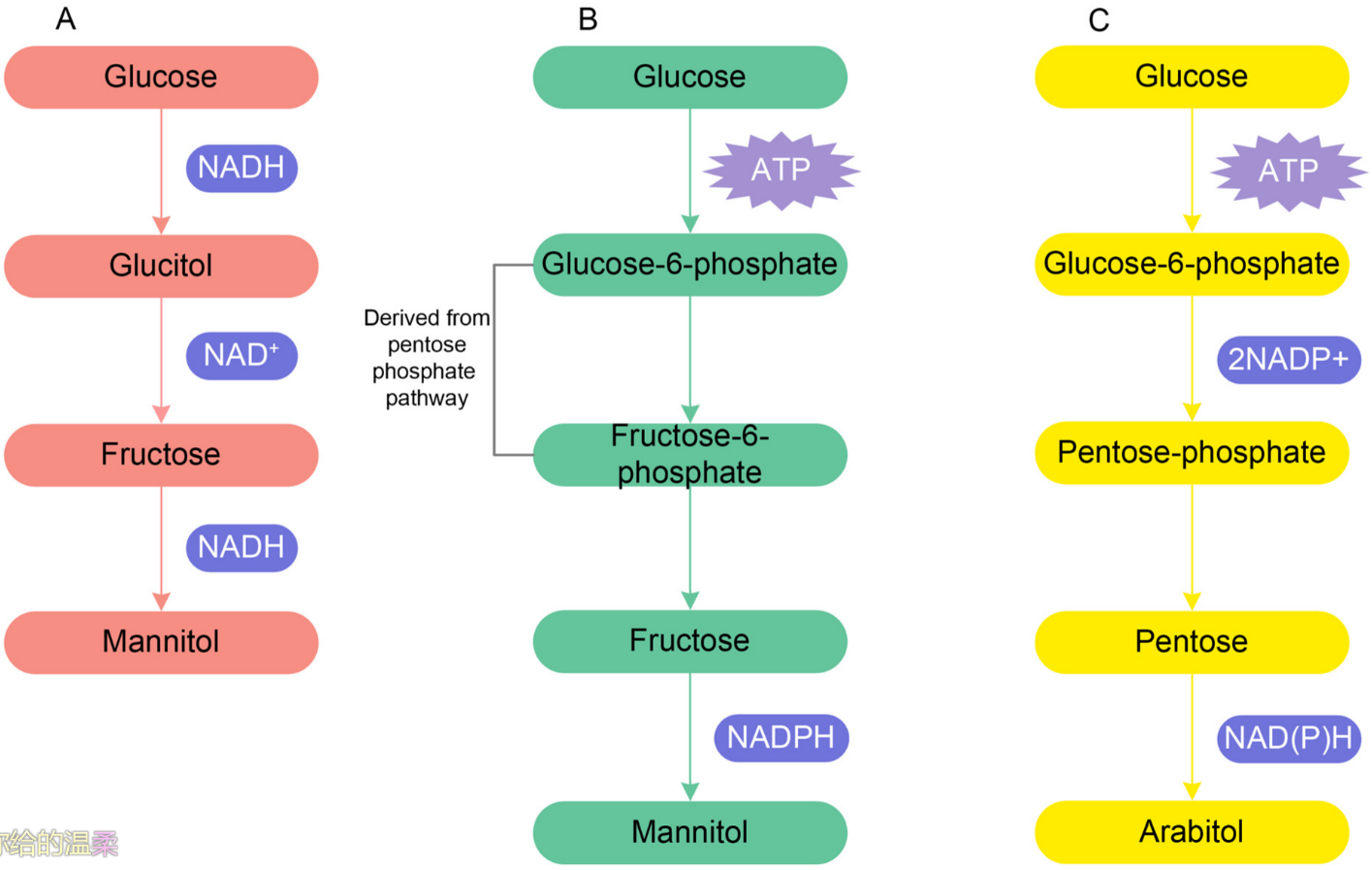
[58] proposed two pathways of mannitol synthesis; one is directly from glucose entering the hyphae and another is from hexose phosphate derived from pentose phosphate pathway with ATPase hydrolysis (
Figure 1A,B). Arabitol synthesis depends upon a stimulation of the pentose phosphate pathway and is derived from pentose sugar (xylulose and ribulose) via the pentose phosphate pathway (
Figure 1C). Jennings
[56] concluded that mannitol, arabitol, glycerol, and erythritol are the major polyols which are accumulated by mycelium, and variation in carbon sources, such as glucose and fructose, has an effect in the accumulation of various polyols.
Figure 1. (A). Mannitol synthesis from glucose. (B). Mannitol synthesis from the hexose phosphate derived from the pentose phosphate pathway. (C). Arabitol synthesis from pentose sugar via the pentose phosphate pathway.
In contrast to the mycelial fungi discussed above, there is evidence that zoosporic genera in the Thraustochytriales require sodium chloride for growth. Siegenthaler et al.
[65][66] suggested that phosphate uptake in
Thraustochytrium roseum required sodium chloride. Also, they demonstrated that the presence of the amino acid proline in their cells, as well as high levels of inorganic ions which contribute to the solute potential of the cells. Wethered and Jennings
[67] noted that proline concentrations in cells increased with the increased salinity of the medium.
Norkrans
[68] and Norkrans and Kylin
[69] drew attention to marine occurring yeasts that are halotolerant with growth in the range of 0–24% sodium chloride. Gustafsson and Norkrans
[70] and Adler and Gustafsson
[71] reported that polyols accumulated in the marine occurring yeast
Debaryomyces hansenii due to salt-stress, while Adler
[72] showed that the accumulation of glycerol in
D. hansenii played a role in osmoregulation.
Fungi in man-made salterns, soda lakes, coastal lagoons and the Dead Sea, tolerate very high environmental NaCl concentrations when compared to the marine fungi discussed here
[73][74]. Larsen
[73] characterised these fungi into four categories depending on tolerance to NaCl concentrations: non-tolerant up to 1%, slight 10%, moderate 20% and extreme 30% NaCl. These fungi too face similar physiological conditions to marine fungi, namely an external environment with relatively low water potential and high concentration of ions
[75]. It is speculated that cation transporters prevent intracellular accumulation of Na
+, which would be toxic but plays a role in maintaining the high K
+/Na
+ ratio required for growth in an environment with high salt content. The halophilic
Wallemia ichthyophaga accumulates glycerol, while
Hortaea werneckii also accumulates erythritol, arabitol and mannitol as solutes
[76][77]. The same mechanism applies to the growth of marine fungi in seawater
[53].
5. Marine Fungi and Climate Change
Kumar et al.
[78] opined on the ecology and evolution of marine fungi and their potential adaptation to climate change, but did not consider their physiology and tolerance of saline conditions. Marine fungi are unique with many characteristics that define their life in a saline environment. These include wide adaptability to saline conditions in mangroves/estuaries and salterns, mechanisms for maintaining accumulation of ions in the vacuoles, exclusion of high level of sodium chloride, maintaining turgor in the mycelium, optimal growth at alkaline pH, a broad temperature growth range from polar waters to higher temperatures in sand dunes/intertidal periods (0–40 °C), growth at depths and often under anoxic conditions
[79]. With these features, marine fungi may well positively respond to the challenges that climate change will bring. Key amongst these will be an increase in CO2 levels, the predicted rise in temperatures, changes (dilution due to melting of the ice caps) in the salinity of seawater and rising sea-levels which will affect the distribution of sea grasses and mangrove/salt marsh plants
[78].
1. An increase in CO2 levels will affect the acidity of seawater, which has implications for the growth of fungi with an alkaline pH requirement. Caldeira and Wickett
[80] noted that seawater pH has dropped by 0.1 units, and may decrease by a further 0.7 units within the next three centuries. Krause et al.
[81] carried out acidification experiments in microcosms with seawater from the Baltic Sea and recorded fungal abundance (as colony forming units). Their results suggested that even moderate acidification may lead to an increase in fungal abundance of almost an order of magnitude.
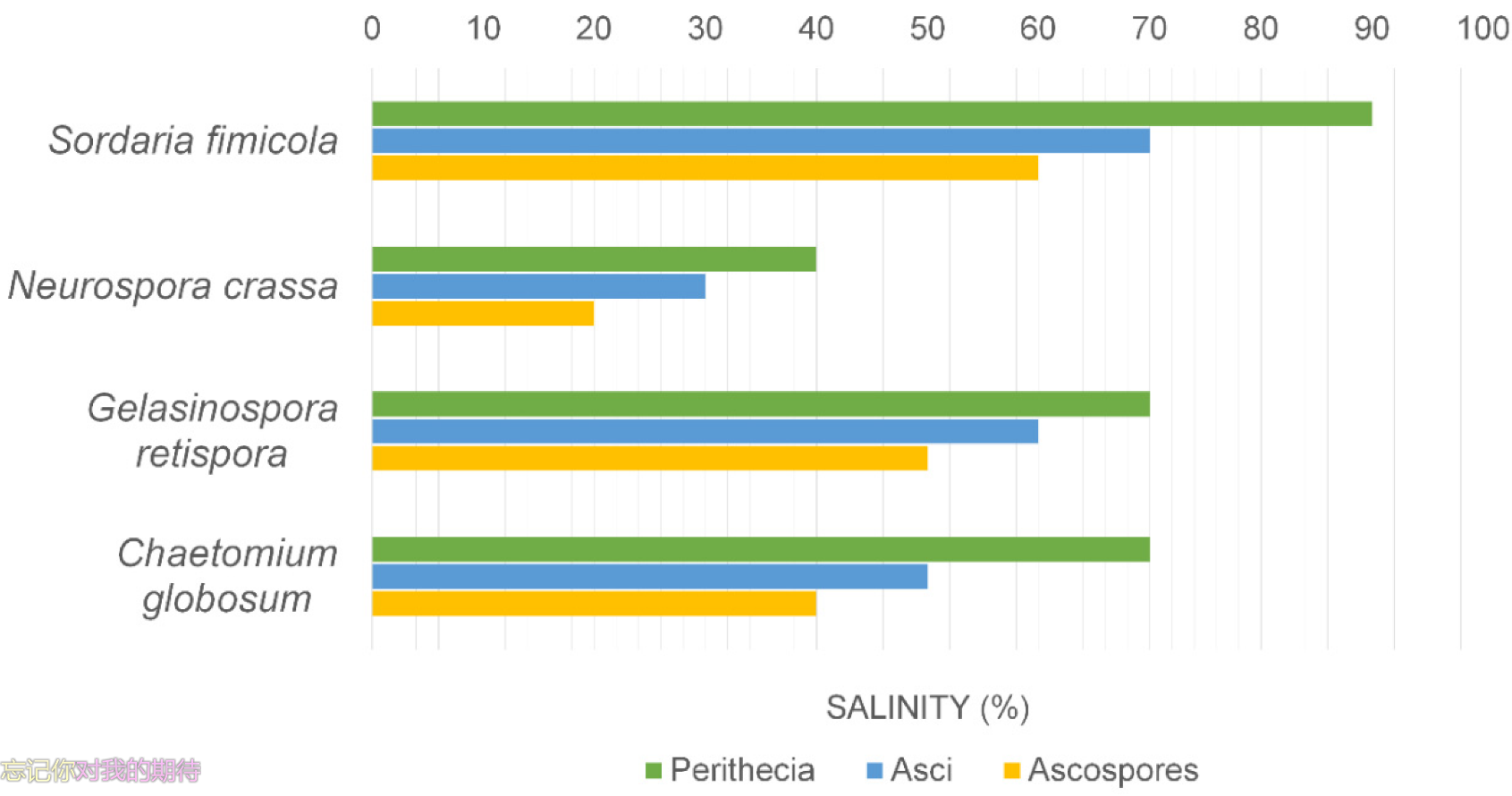
2. Marine fungi have a broad tolerance to variation in salinity in terms of mycelial growth, spore germination and sporulation, and therefore should adapt to changes in oceanic salinity (see Figure 2).
Figure 1. Effect of salinity on the production of perithecia, asci and ascospores of fungi.
3. Marine fungi appear to tolerate a wide range in seawater temperature (See
Table 1). Although marine fungi are worldwide in distribution, certain taxa may be restricted geographically to the tropics, subtropics, temperate or polar waters
[82][83][84][85][86]. However, there is little overlap in fungal species from tropical and temperate regions
[87]. Consequently, many marine fungi in temperate regions will have to adapt to increased temperature. Pang et al.
[88] have shown that an
Aspergillus terreus strain isolated from a shallow hydrothermal vent was able to grow at 45 °C, pH 3, and 30% salinity.