Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sonia Araceli Soto-Rodriguez | -- | 3321 | 2022-04-19 17:43:48 | | | |
| 2 | Amina Yu | -11 word(s) | 3310 | 2022-04-20 06:32:55 | | | | |
| 3 | Amina Yu | Meta information modification | 3310 | 2022-04-20 06:41:41 | | | | |
| 4 | Amina Yu | Meta information modification | 3310 | 2022-04-20 07:33:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Soto-Rodriguez, S.; Lozano-Olvera, R.; Ramos-Clamont Montfort, G.; Zenteno, E.; Sánchez Salgado, J.L.; , . PirAB from Vibrio parahaemolyticus and AHPND in Shrimp. Encyclopedia. Available online: https://encyclopedia.pub/entry/21956 (accessed on 07 February 2026).
Soto-Rodriguez S, Lozano-Olvera R, Ramos-Clamont Montfort G, Zenteno E, Sánchez Salgado JL, . PirAB from Vibrio parahaemolyticus and AHPND in Shrimp. Encyclopedia. Available at: https://encyclopedia.pub/entry/21956. Accessed February 07, 2026.
Soto-Rodriguez, Sonia, Rodolfo Lozano-Olvera, Gabriela Ramos-Clamont Montfort, Edgar Zenteno, Jose Luis Sánchez Salgado, . "PirAB from Vibrio parahaemolyticus and AHPND in Shrimp" Encyclopedia, https://encyclopedia.pub/entry/21956 (accessed February 07, 2026).
Soto-Rodriguez, S., Lozano-Olvera, R., Ramos-Clamont Montfort, G., Zenteno, E., Sánchez Salgado, J.L., & , . (2022, April 19). PirAB from Vibrio parahaemolyticus and AHPND in Shrimp. In Encyclopedia. https://encyclopedia.pub/entry/21956
Soto-Rodriguez, Sonia, et al. "PirAB from Vibrio parahaemolyticus and AHPND in Shrimp." Encyclopedia. Web. 19 April, 2022.
Copy Citation
PirAB is a potent toxin which can causes 100% mortality in shrimp, the toxin produce masive sloughing of the epithelial cells of the shrimp hepatopancreas, kown as acute hepatopancreatic necrosis diseaseas (AHPND) in diseased penaeid shirmp. This toxin is produced by specific strains of Vibrio parahaemolyticus, which harbors a plasmid of ~70 kbp (pVA1) containing the pirAVp and pirBVp genes that encode PirAB toxin.
PirAB
AHPND
shrimp
microbiota change
inhibitors
PirAB receptors
virulence plasmid
1. Virulence Plasmid of Vibrio parahaemolyticus AHPND
Acute hepatopancreatic necrosis disease (AHPND) is mainly caused by Vp, which harbors a plasmid of ~70 kbp (pVA1) containing the pirAVp and pirBVp genes that encode the delta-endotoxin responsible for the typical lesions in shrimp Hp [1][2]. The genomes of pVA1-harboring Vp revealed a large pan genome with high genetic diversity grouped into three main clades and specific structural differences, in addition to the instability of the pirABVp region of the pVA1 plasmid [3]. The structural differences found in pVA1 are likely due to the horizontal propagation of the plasmid to other Vibrio species [4], such as V. harveyi [5][6], V. campbellii [6], V. owensii [6], and V. punensis [7]. These processes might result in the appearance of new pathogenic AHPND strains, which would pose a major threat to the shrimp industry. Likewise, this variability in structural elements could eventually influence their niche adaptation ability, growth behavior, and virulence/pathogenesis.
Recently, Aguilar-Rendón et al. [8] found large variability of the plasmid copy number (7 to 121 copies) per bacterial cell of Vp AHPND strains analyzed by qPCR [9][10], although it has been reported that virulence does not depend on the copy number of pirAVp/pirBVp genes [11]. To date, no clear evidence of their role in AHPND has been found. It using a shotgun metagenomics approach on bottom seawater with P. vannamei inoculated with two Vp AHPND strains registered more than one copy of pVA1 per bacterial cell (1.9 to 13.5 copies per bacterial cell) throughout the experimental infection period [12]. The copy number of the virulent plasmid was not dependent on the degree of virulence of the Vp AHPND strain but rather on bacterial density (Figure 1). Nonetheless, it has been evaluated the variability in the plasmid copy number per bacterial cell in relation to the degree of virulence or bacterial density and how this may influence AHPND pathogenesis.
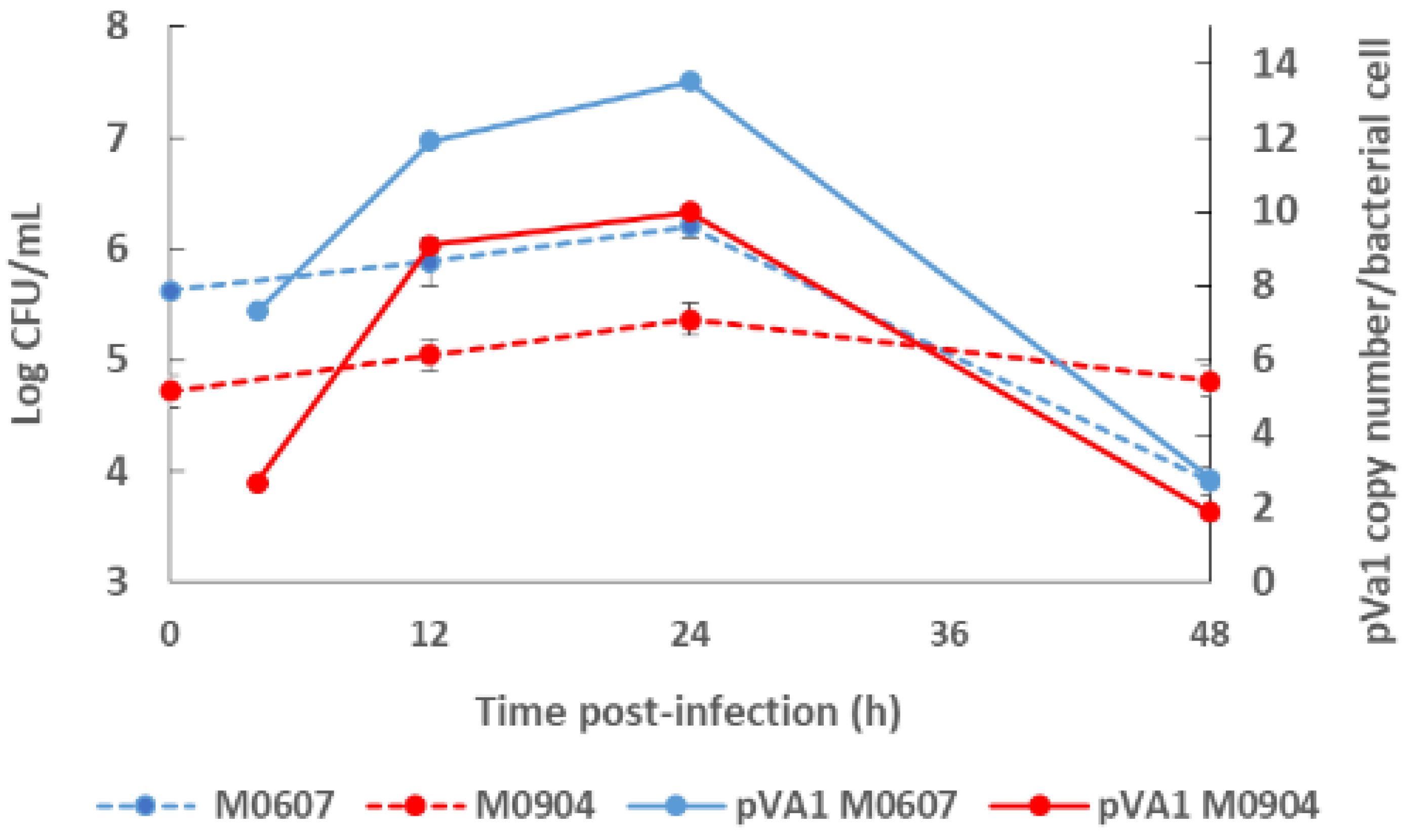
Figure 1. Bacterial density of the bottom seawater and virulent plasmid copy (pVA1) number of moderate virulence Vibrio parahaemolyticus strain M0607 and high virulent V. parahaemolyticus strain M0904 during experimental infections at 105 CFU mL−1.
2. Factors That Could Induce or Inhibit Toxin Production
2.1. Quorum Sensing
Quorum sensing (QS) is a cell-to-cell signaling mechanism in response to an increased bacterial cell population [13]. Bacterial QS produce, release, and recognize molecular autoinducers (AIs) that bind to surface bacterial receptors, triggering signal transduction cascades that alter the expression of genes related with survival and infection factors, such as sporulation, luminescence, biofilm formation, and virulence [14]. The QS mechanism is widely distributed in Vibrionaceae members, with the acyl-homoserine lactones (AHLs) being among the more common AIs. For example, AHLs have been implicated in the signaling mechanisms that activate the production of luciferase in V. fischeri [13]. In addition, V. harveyi produces and responds to three other AIs: (1) HAI-1, [N-(3- hydroxy butyryl)-homoserine lactone], an intra-species AI; (2) CAI-1, [(Z)-3-aminoundec-2- en-4one], which is restricted to the Vibrio genera; and (3) the inter-species AI-2 [(2S,4S)-2-methyl-2,3,3,4-tetrahydroxytetrahydrofuran-borate]. These three AIs act in parallel to regulate over 600 target genes through complex signaling cascades [13][15]. The capacity of Vibrionaceae for “sensing self” and “sensing others” allows for both competition and cooperation in complex microbial communities [16].
Virulence gene expression regulated by QS has been studied extensively in V. harveyi and may serve as a basis for understanding the QS mechanisms in Vp given that this pathogen contains the central conserved components of the QS pathway known in V. harveyi [17]. For example, a LuxT homolog of V. harveyi, SwrT, activates genes that encode for translocation across surfaces and swarming and is lateral-flagella-driven in Vp [18][19]. In addition, V. harveyi and presumably Vp produce three types of AIs, namely auto inducer 2 (AI-2), harveyi auto inducer 1 (HAI1), and cholerae auto inducer 1 (CAI1), which are recognized by the surface membrane receptors LuxP/LuxQ, LuxN, and CqsS, respectively [20]. In a preliminary one, [21] showed that the production of PirABVp binary toxin is regulated by the AI-2 QS process. They tested the effect of a cell-free supernatant from V. harveyi containing AI-2 (CFS-VH) on an AHPND-causing Vp strain. The AI-2-containing supernatant accelerated the production time and yield of both PirAVp and PirBVp toxins, whereas the application of the furanone [(5Z)-4-bromo5-(bromomethylene)-2(5H)-furanone] AI-2 antagonist delayed AHPND toxin production or secretion. It opens new perspectives on QS mechanisms in Vp and on possible treatments and management strategies to control AHPND infection in shrimp culture. Interestingly, AI-2 is synthetized by numerous bacterial species and can facilitate inter-species cell–cell signaling [22], resulting in changes of Vp behavior in complex microbial communities.
2.2. Environmental Factors
Bacterial adaptation and survival depend on the capacity to properly respond to changes in internal and external environments. The survival of Vibrio spp. in marine environments depends on carbon and energy sources, dissolved oxygen, water pH, salinity, temperature, and starvation [23]. In particular, changes in temperature due to global warming are a growing concern for aquaculture due to the increased risk of Vp-induced diseases. Environmental stress can increase horizontal gene transfer mechanisms in AHPND-causing Vp strains, promoting their growth [24][25] and increasing the risk of AHPND outbreaks and disease dispersion in tropical zones. Recently, the effect of temperature shifts on pirAVp and pirBVp gene expression of the AHPND-Vp AAHMRU04 strain isolated from white shrimp exhibiting clinical signs of AHPND was evaluated [26]. Bacteria were grown at 30 °C for 24 h and subsequently exposed to a set of different temperature trials for 4 days. The pirAVp and pirBVp genes were induced when the temperature shifted from high (26–32 °C) to low (22–28 °C) [26].
The relationship between salinity and AHPND in P. vannamei was studied by [27]. Pathogen-free shrimp cultures (5, 10, 15, and 20 g L−1 of NaCl) were challenged with a Vp AHPND broth. In all salinity treatments, Vp AHPND caused infection in shrimp as confirmed by histological damage and the presence of pirABVp toxin genes by PCR analysis. However, cumulative mortality was different, showing higher survival in shrimp maintained at lower salinities. Since Vp reproduces more efficiently in high salinity environments, it is likely that a greater amount of PirABVp toxin was produced, resulting in a higher cumulative mortality in P. vannamei when maintained under these conditions. However, different patterns were observed when challenging P. vannamei growing under different salinity conditions with the Vp AHPND strain E9 [28]. Mortality was higher at lower salinities and a positive correlation was present with the expression of the pirAVp gene. Although more experiments are needed to determine the influence of salinity on the expression of pirABVp, these experiments corroborate that the toxin can be expressed at different salinities [29] and that the management of salinity in shrimp culture can be an important factor to control Vp infectivity.
Another environmental factor that has been known with regard to the production of the PirABvp binary toxin is related to fluid shear and the hydrodynamic forces acting on Vp due to either natural influences or the use of aquaculture equipment to enhance shrimp productivity, such as blowers or aerators [30]. To this end, the effect of shaking conditions on the AHPND-causing Vp M0904 was known [31]. At a constant agitation of 110 rpm, bacteria developed cellular aggregates together with levan (branched polymeric fructans)-containing biofilm formations and acquired tolerance against antimicrobial agents (kanamycin, ampicillin, rifampicin, and tetracycline), possibly due to high biofilm production. In addition, a significant decrease was observed not only in PirAVp/PirBVp toxin production but also in the virulence of Vp M0904 to Artemia and Macrobrachium larvae. Increasing the shaking speed to 120 rpm produced an increase in PirAVp/PirBVp toxin production, the virulence of Vp M0904 to Artemia and Macrobrachium larvae, and the expression of polar flagellin (flaA), polar flagellin-specific chaperone (fliS), and chemotaxis protein (CheR). This type provides valuable information for understanding the behavior of Vp AHPND in aquaculture environments [31].
2.3. Biofilm Formation
The formation of bacterial biofilms represents one of the most important survival mechanisms, attachment, as well as host colonization strategies of bacteria [32]. This phenomenon is influenced by abiotic and biotic factors regulated by QS [33]. ToxR is an important virulence regulator implicated in the synthesis of Vp biofilms that also controls the expression of the virulence factors found in human pathogenic Vp, including thermostable direct hemolysin (TDH), TDH-related hemolysin (TRH), and T3SS [34][35]. The expression of these factors is regulated by QS through the production of and responses to AI-2 [33][36][37]. Under these conditions, biofilm and toxin production appear to be simultaneous activities.
Information on the relationship between biofilm formation and the production of PirABvp binary toxin in Vp AHPND is lacking. The only one to address this issue is that of [31], which observed an inverse relationship between the production of biofilms and that of the PirABVp toxin. This behavior refers to the formation of abiotic films in response to fluid shear and hydrodynamic forces. However, the regulation, growth kinetics, and characteristics of Vp AHPND biofilms in the host and their relationships with PirABvp toxin production remain uncharacterized.
3. Search for Membrane Receptors of PirAVp and PirBVp
3.1. Biological Activities of the PirAVp and PirBVp Subunits
Bacterial protein toxins, like PirABVp, are molecular self-governing virulence factors that target specific host cells, triggering different damaging processes involved in the disease of the infected organism. The binding of bacterial toxins to plasma cell surface receptors is an essential first step for shrimp intoxication. Knowing the structures of these receptors can further the understanding of the infection mechanisms with the aim of preventing host disease by blocking the toxin–receptor interaction using a mimetic antagonist [38]. The PirAVp/PirBVp toxin induces cell damage in the shrimp Hp, although it is not seen in other organs, and is considered a shrimp-specific toxin [39]. Moreover, it seems that PirAVp/PirBVp receptors will be found exclusively in this organ [40]. Recently, it has been observed that the B Subunit of the PirABVp toxin is an amino sugar-specific lectin-like, and it is able to recognize glycoproteins on the epithelium of the Hp, suggesting its participation in AHPND pathogenesis [41][42]. Nevertheless, the PirAVp/PirBVp binding model complex requires clarification and further information is needed.
It is known that PirAVp and PirBVp form a heterodimeric complex that binds to receptors located on the cells of the shrimp Hp [40][43]. However, the precise nature of the toxin receptors is still not known. Lee et al. [2] suggested that PirABVp structure is homologous to the insecticidal Photorhabdus insect-related (Pir) binary toxin, and in silico analysis showed that the PirAVp and PirBVp toxins possess similar structures to the functional domains of the pore-forming Bacillus thuringiensis Cry toxins [2][40]. The structural alignment of both toxins indicates that the PirAVp subunit is similar to the lectin-like recognition domain III of B. huringiensis toxin, whereas PirBVp corresponds to the pore-forming I and II domains [2][40][44][45]. In this context, the initial interaction of the PirAVp/PirBVp toxins would be through lectin-carbohydrate recognition between PirAVp and the glycans exposed on the surface of the plasma membrane of Hp cells [40]. Structural features and molecular docking of the PirAVp subunit show a potential sugar-binding cavity for glycans containing the N-Acetylgalactosamine (GalNac) molecule, whereas the PirBVp subunit structure contains a C-terminal receptor domain similar to Cry domain II for protein–protein ligand interactions and an N-terminal consistent with other membrane pore-forming toxins, including Cry domain I [2][44]. In addition, Hao et al. [39] analyzed the distribution and homology of PirABVp-like proteins in other bacterial species and showed that at least seven bacterial taxa harbor complete or partial pirAB genes, including Alcaligenes, Photorhabdus, Pectobacterium carotovorum, Vibrio, Xenorhabdus, Yersinia, and Shewanella violacea. All examined PirB proteins examined by Hao et al. [39] showed typical B. thuringiensis Cry structure formed by several α-helix bundles in the N-terminal of PirB and a coup of parallel or anti-parallel β-sheets in the C-terminal of PirBVp.
However, the protein structure in the receptor binding sites of compared PirB proposed by Lin et al. [40] reflected an evolutionary divergence in the amino acid sequences (for more details, see Hao et al. [39]). The conformation and the direction of Loop 2 of PirB are unique in V. parahaemolyticus, thus PirBVp might target a specific receptor in the cell membrane. In addition, the predicted structures of PirA toxins also showed remarkable differences in ligand-binding sites. These structural variations could largely influence the recognition events of PirABVp. It was proposed that PirABVp forms a heterotetrametric complex containing four PirAVp subunits and four PirBVp subunits [40] and that PirBVp first recognizes glycosaminoglycan molecules as mucin-like or beta-hexosaminidase where the Gal(β1–3/1–4)GlcNAc(α1–2) sequence is essential for PirBVp recognition in the hepatopancreatic membrane [42] (Figure 2). The role of the PirA subunit might be stabilizing the complex for a better binding to the possible receptor molecule on the shrimp hepatopancreatic epithelial cells [40]. A complete understanding of the receptor binding mechanisms of PirA/PirB toxins is essential in order to elucidate the toxin mechanism.
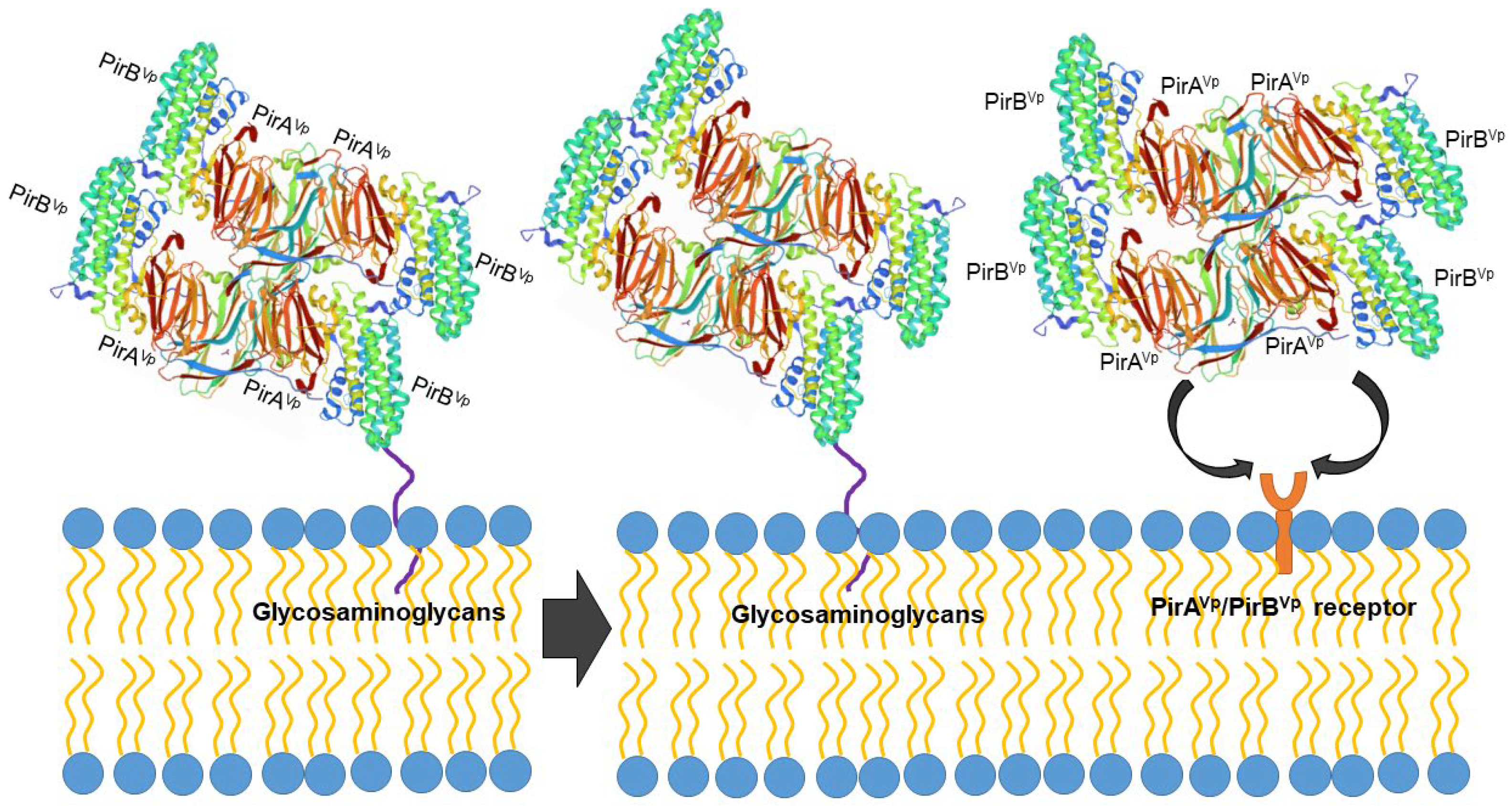
Figure 2. Proposed PirABVp binding scheme. The PirAVp/PirBVp heterotetrametric complex first uses PirBVp-lectin to recognize and bind with glycosaminoglycan molecules; meanwhile, PirAVp stabilizes the complex. Then the complex probably binds to the receptor molecules on the membrane of the hepatopancreatic epithelial cells of shrimp to trigger the massive sloughing of these cells.
Recent ones have suggested that the regions of interaction of PirABVp are different than those of insecticidal toxins. From the extracellular products (ECPs) of Vp, a heterotetrametric complex of 250 kDa has been purified, which contains four PirAVp and four PirBVp subunits. The PirBVp subunit was confirmed to show lectin-like activity and the recognition of mucin-like O-glycosidic structures in the shrimp Hp that may act as receptors for toxin binding, while PirAVp did not present this activity [41]. Lectin activity has been suggested due to its ability to interact specifically with oligosaccharides and glycoproteins such as mucin, but further structural assays will confirm the participation of the lectin effect in the pathogenesis of Vp AHPND.
The PirABVp complex seems to be necessary to induce AHPND signs. The mechanism of action of the entire toxin during the AHPND disease process remains to be determined. However, experiments conducted with the recombinant proteins rPirAVp and rPirBVp showed that only the PirABVp complex and rPirBVp displayed Mg2+ or Ca2+ independent hemagglutinating activity (HA) toward rat red cells, whereas rPirAVp was not able to agglutinate erythrocytes from several animal species [41].
In a first attempt to determine the sugar specificity of the putative PirBVp lectin-like, subsequent competition experiments were conducted using a wide battery of monosaccharides, disaccharides, and glycoproteins. The PirBVp subunit binds to a glycoconjugate glycan moiety containing amino sugars [41]. Further experiments conducted by the same group showed the existence of different glycan receptors for PirBVp, and in particular a mucin-like receptor located at the surface membrane of the cell Hp and an internal hexosaminidase glycoprotein receptor that is possibly involved in toxin-related cell damage to shrimp tissues [42]. Beta-hexosaminidase (β-N-acetyl hexosaminidase) is a ubiquitous lysosomal enzyme with multiple roles in protein glycosylation and synthesis and glycoconjugate metabolism [46]. This glycoprotein plays an important role in arthropod molting and chitin degradation and in the defense system of P. vannamei against parasites [47][48]. Extracellular beta-hexosaminidases secreted by eukaryotes occur as dimers and possess N-glycosidically-linked glycans with oligomannosidic and complex-type glycan structures [49][50]. The possibility that PirBVp could recognize N-linked oligosaccharides expressed by endosomal or secreted beta-hexosaminidase, which would allow for an increased pathogenesis of Vp in crustaceans, cannot be excluded.
Previous data suggest putative lectin-like PirBVp subunit activity [41][42] that contrasts with the functions of domains I and II proposed for the Cry toxin and with the proposed function of the PirAVp subunit given that it has not been possible to verify that this subunit can recognize carbohydrates in the experiments conducted to date. In light of this, the PirAVp subunit could play an initial stabilizing role, allowing PirBVp to bind with higher affinity to the glycan receptors located at the surface of Hp cells.
3.2. Expression of Mucin-like O-Glycosidic Structures in Shrimp
O-glycans are critical for the development and proper functioning of multicellular organisms. Mucin-type glycans are widely found on the cell surfaces and secreted glycoconjugates of invertebrates [51]. These O-glycans may serve as receptor-binding sites for a variety of pathogenic bacteria and their toxins [52]. A small unit of P. vannamei hemocyanin had O-glycans that were closely associated with agglutination activity toward Vibrio fluvialis, V. alginolyticus, and V. parahaemolyticus [53][54].
A mucin-like peritrophin-like gene from fleshy shrimp (Fenneropenaeus chinensis) is able to bind to Gram-negative bacteria [55], while another mucin-like peritrophin-like gene from the shrimp Exopalaemon carinicauda is involved in white spot syndrome viral infections [56]. In addition, a mucin-like peritrophin has been implicated in V. harveyi infection in the black tiger shrimp P. monodon [57]. Abiotic characteristics, such as decreases in temperature and changes in diet, increase the expression of several mucin-like proteins in P. vannamei [58][59][60]. These modifications could be related to the pathologic development of Vibrio infection, increasing the number of binding targets in the shrimp digestive system. Searching for possible receptors for the lectin-like PirBVp [42] has yielded evidence of some correspondence with a mucin-like protein expressed in the shrimp Hp of P. vannamei. These are the beginning of a better understanding of the infection mechanisms of Vp in shrimp.
3.3. Receptor on Shrimp Hemocytes
The PirABVp toxin is known to mainly target the epithelial cells of shrimp Hp tubules. In addition. Moreover, the dysregulation of apoptosis-related genes in Vp AHPND-challenged P. vannamei hemocytes suggests that Vp AHPND induces apoptosis in hemocytes [61]. In the transcriptome of Vp AHPND-challenged P. vannamei, an aminopeptidases N1 (LvAPN1) gene was identified [62]. DNA sequence analysis of the LvAPN1 gene showed a putative C-terminal transmembrane domain and various putative N- and O-glycosylation sites. The expression of LvAPN1 increases in hemocytes after challenging P. vannamei with either Vp AHPND or the partially purified Vp AHPND toxins. Silencing of LvAPN1 significantly reduced LvAPN1 transcription levels in the stomach, Hp, and hemocytes and increased the survival of adult P. vannamei that were challenged with the partially purified Vp AHPND toxins. These observations suggest the putative role of LvAPN1 as a PirABVp toxin receptor located on the hemocyte surface [62].
Other putative carbohydrate receptors for the PirABVp toxin could be located in the surface of P. vannamei hemocytes, as these cells express a plethora of glycoconjugates. Using commercial lectins with different carbohydrate specificities, the presence of carbohydrate moieties containing mainly N-acetyl-glucosamine (GlcNAc) and N-acetylneuraminic acid (sialic acid) was demonstrated [63]. In another, these carbohydrates were recognized by the rPirBVp subunit [41].
4. Search of Potential Inhibitors of the PirABVp Toxin
Understanding the structural biology of PirABVp is essential for finding or developing antiadhesive agents or receptor analogs that could prevent adhesion and subsequent cell entry of the toxin, thus inhibiting its activity. In particular, it is important to decipher the roles and structural features of complex carbohydrates that serve as toxin receptors. According to the group, the PirB subunit presents lectin-like activity, and its adhesion can be inhibited in the presence of fucosylated glycans and by those that contain N-acetyl glucosamine [41][42].
In addition to glycans, peptides that can interact with PirABVp are also needed. Computational tools like molecular docking can play an important role in the search for antiadhesive peptides or in the design of antiadhesive peptide analogs through the creation of precise structural models of peptide-toxin complexes and the calculation of binding free energies [64][65]. The search for bifunctional peptides that can be used to improve shrimp growth while at the same time protecting them from the PirABVp toxin is also important. For example, oilseed peptides have been found to contribute to improved shrimp health and growth performance when used as feed ingredients [66]. In silico one have revealed six dual-target peptides from different oilseed proteins capable of interfering with the formation of the PirAVp/PirBVp complex. Such peptides (1139–2977 Da in mass and 10–28 residues in length) are possible candidates for the future development of peptide-based anti-AHPND agents [65].
References
- Tran, L.H.; Nunan, L.; Redman, R.M.; Mohney, L.L.; Pantoja, C.R.; Fitzsimmons, K.; Lightner, D.V. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting Penaeid shrimp. Dis. Aquat. Org. 2013, 105, 45–55.
- Lee, C.T.; Chen, I.T.; Yang, Y.T.; Ko, T.P.; Huang, Y.T.; Huang, J.Y.; Huang, M.F.; Lin, S.J.; Chen, C.Y.; Lin, S.S.; et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc. Natl. Acad. Sci. USA 2015, 112, 10798–10803.
- González-Gómez, J.P.; Soto-Rodriguez, S.; López-Cuevas, O.; Castro-del Campo, N.; Chaidez, C.; Gomez-Gil, B. Phylogenomic Analysis Supports Two Possible Origins for Latin American Strains of Vibrio parahaemolyticus Associated with Acute Hepatopancreatic Necrosis Disease (AHPND). Curr. Microbiol. 2020, 77, 3851–3860.
- Dong, X.; Chen, J.; Song, J.; Wang, H.; Wang, W.; Ren, Y.; Guo, C.; Wang, X.; Tang, K.F.J.; Huang, J. Evidence of the horizontal transfer of pVA1-type plasmid from AHPND-causing V. campbellii to non-AHPND V. owensii. Aquaculture 2019, 503, 396–402.
- Kondo, H.; Van, P.T.; Dang, L.T.; Hirono, I. Draft genome sequence of non-Vibrio parahaemolyticus acute hepatopancreatic necrosis disease strain KC13.17.5, isolated from diseased shrimp in Vietnam. Genome Announc. 2015, 3, e00978-15.
- Xiao, J.; Liu, L.; Ke, Y.; Li, X.; Liu, Y.; Pan, Y.; Yan, S.; Wang, Y. Shrimp AHPND-causing plasmids encoding the PirAB toxins as mediated by pirAB-Tn903 are prevalent in various Vibrio species. Sci. Rep. 2017, 7, 42177.
- Restrepo, L.; Bayot, B.; Arciniegas, S.; Bajaña, L.; Betancourt, I.; Panchana, F.; Reyes Muñoz, A. PirVP genes causing AHPND identified in a new Vibrio species (Vibrio punensis) within the commensal Orientalis clade. Sci. Rep. 2018, 8, 13080.
- Aguilar-Rendón, K.G.; Lozano-Olvera, R.; Yáñez-Rivera, B.; Soto-Rodriguez, S.A. Bacteriological and histopathological analysis of Penaeus vannamei experimentally infected with Vibrio parahaemolyticus-AHPND strains. Dis. Aquat. Org. 2020, 140, 167–177.
- Han, J.E.; Tang, K.F.J.; Tran, L.H.; Lightner, D.V. Photorhabdus insect-related (Pir) toxin-like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Dis. Aquat. Org. 2015, 113, 33–40.
- Han, J.E.; Tang, K.F.J.; Lightner, D.V. Genotyping of virulence plasmid from Vibrio parahaemolyticus isolates causing acute hepatopancreatic necrosis disease in shrimp. Dis. Aquat. Org. 2015, 115, 245–251.
- Tinwongger, S.; Nochiri, Y.; Thawonsuwan, J.; Nozaki, R.; Kondo, H.; Awasthi, S.P.; Hinenoya, A.; Yamasaki, S.; Hirono, I. Virulence of acute hepatopancreatic necrosis disease PirAB-like relies on secreted proteins not on gene copy number. J. Appl. Microbiol. 2016, 121, 1755–1765.
- Aguilar-Rendón, K.G.; Soto-Rodriguez, S.A.; Gomez-Gil, B.; Lozano-Olvera, R.; Yáñez-Rivera, B. Water microbiome dynamics of Pacific white shrimp Penaeus vannamei infected with Vibrio parahaemolyticus strains responsible for acute hepatopancreatic necrosis disease. Aquaculture 2022, 551, 737871.
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588.
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427.
- Federle, M.J.; Bassler, B.L. Interspecies communication in bacteria. J. Clin. Investig. 2003, 112, 1291–1299.
- Wellington, S.; Greenberg, E.P. Quorum sensing signal selectivity and the potential for interspecies cross talk. mBio 2019, 10, e00146-19.
- Makino, K.; Oshima, K.; Kurokawa, K.; Yokoyama, K.; Uda, T.; Tagomori, K.; Iijima, Y.; Najima, M.; Nakano, M.; Yamashita, A.; et al. Genome sequence of Vibrio parahaemolyticus: A pathogenic mechanism distinct from that of V. cholerae. Lancet 2003, 361, 743–749.
- Jaques, S.; McCarter, L.L. Three new regulators of swarming in Vibrio parahaemolyticus. J. Bacteriol. 2006, 188, 2625–2635.
- Eickhoff, M.J.; Fei, C.; Huang, X.; Bassler, B.L. LuxT controls specific quorum-sensing-regulated behaviors in Vibrionaceae spp. via repression of qrr1, encoding a small regulatory RNA. PLoS Genet. 2021, 17, e1009336.
- Zhang, Y.; Qiu, Y.; Tan, Y.; Guo, Z.; Yang, R.; Zhou, D. Transcriptional regulation of opaR, qrr2-4 and aphA by the master quorum-sensing regulator OpaR in Vibrio parahaemolyticus. PLoS ONE 2012, 7, e34622.
- Pumkaew, M.; Taengchaiyaphum, S.; Powtongsook, S.; Pungrasmi, W.; Sritunyalucksana, K. Production of acute hepatopancreatic necrosis disease toxin is affected by addition of cell-free supernatant prepared from Al-2-producing Vibrio harveyi mutant. J. World Aquac. Soc. 2019, 50, 878–886.
- Federle, M.J. Autoinducer-2-based chemical communication in bacteria: Complexities of interspecies signaling. Contrib. Microbiol. 2009, 16, 18–32.
- Takemura, A.F.; Chien, D.M.; Polz, M.F. Associations and dynamics of Vibrionaceae in the environment, from the genus to the population level. Front. Microbiol. 2014, 5, 38.
- Williams, S.L.; Jensen, R.V.; Kuhn, D.D.; Stevens, A.M. Analyzing the metabolic capabilities of a Vibrio parahaemolyticus strain that causes Early Mortality Syndrome in shrimp. Aquaculture 2017, 476, 44–48.
- Fu, S.; Wei, D.; Yang, Q.; Xie, G.; Pang, B.; Wang, Y.; Lan, R.; Wang, Q.; Dong, X.; Zhang, X.; et al. Horizontal plasmid transfer promotes the dissemination of Asian acute hepatopancreatic necrosis disease and provides a novel mechanism for genetic exchange and environmental adaptation. mSystems 2020, 5, e00799.
- Pragthong, P.; Chirapongsatonkul, N. Temperature-dependent expression of virulence genes in Vibrio parahaemolyticus AHPND strain (VpAHPND). Int. J. Agric. Technol. 2020, 16, 1185–1198.
- Schofield, P.J.; Noble, B.L.; Caro, L.F.A.; Mai, H.N.; Padilla, T.J.; Millabas, J.; Dhar, A.K. Pathogenicity of Acute Hepatopancreatic Necrosis Disease (AHPND) on the freshwater prawn, Macrobrachium rosenbergii, and Pacific White Shrimp, Penaeus vannamei, at various salinities. Aquac. Res. 2021, 52, 1480–1489.
- López-Cervantes, G.; Álvarez-Ruiz, P.; Luna-Suárez, S.; Luna-González, A.; Esparza-Leal, H.M.; Castro-Martínez, C.; Gámez-Jiménez, C.; Soto-Alcalá, J. Temperature and salinity modulate virulence and PirA gene expression of Vibrio parahaemolyticus, the causative agent of AHPND. Aquac. Int. 2021, 29, 743–756.
- Soto-Rodriguez, S.; Lozano Olvera, R.; Palacios-Gonzalez, D.; Bolan-Mejía, M.; Aguilar-Rendon, K. Characterization and growth conditions of Vibrio parahaemolyticus strains with different virulence degrees that cause acute hepatopancreatic necrosis disease in Litopenaeus vannamei. J. World Aquac. Soc. 2019, 50, 1002–1015.
- Sultana, T.; Haque, M.; Salam, M.; Alam, M. Effect of aeration on growth and production of fish in intensive aquaculture system in earthen ponds. J. Bangladesh Agric. Univ. 2017, 15, 113–122.
- Kumar, V.; Roy, S.; Baruah, K.; Van Haver, D.; Impens, F.; Bossier, P. Environmental conditions steer phenotypic switching in acute hepatopancreatic necrosis disease-causing Vibrio parahaemolyticus, affecting PirAVP/PirBVP toxins production. Environ. Microbiol. 2020, 22, 4212–4230.
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microb. 2019, 17, 247–260.
- Mizan, M.F.; Jahid, I.K.; Kim, M.; Lee, K.H.; Kim, T.J.; Ha, S.D. Variability in biofilm formation correlates with hydrophobicity and quorum sensing among Vibrio parahaemolyticus isolates from food contact surfaces and the distribution of the genes involved in biofilm formation. Biofouling 2016, 32, 497–509.
- Zhang, Y.; Hu, L.; Osei-Adjei, G.; Zhang, Y.; Yang, W.; Yin, Z.; Lu, R.; Sheng, X.; Yang, R.; Huang, X.; et al. Autoregulation of ToxR and Its Regulatory Actions on Major Virulence Gene Loci in Vibrio parahaemolyticus. Front. Cell. Infect. Microbiol. 2018, 8, 291.
- Yildiz, F.H.; Visick, K.L. Vibrio biofilms: So much the same yet so different. Trends Microbiol. 2009, 17, 109–118.
- Henke, J.M.; Bassler, B.L. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J. Bacteriol. 2004, 186, 3794–3805.
- Zhu, J.; Miller, M.B.; Vance, R.E.; Dziejman, M.; Bassler, B.L.; Mekalanos, J.J. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 2002, 99, 3129–3134.
- Sharon, N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 527–537.
- Hao, J.; Zhang, Y.; Fu, S.; Lu, Y.; Hua, X.; Liu, Y. Pathogenicity and protein analysis of photorhabdus insect-related (Pir) toxin PirAB revealed PirABvp is a host-specific toxin. Aquaculture 2019, 500, 290–299.
- Lin, S.J.; Hsu, K.C.; Wang, H.C. Structural insights into the cytotoxic mechanism of Vibrio parahaemolyticus PirAvp and PirBvp toxins. Mar. Drugs 2017, 15, 373.
- Victorio-De Los Santos, M.; Vibanco-Pérez, N.; Soto-Rodriguez, S.; Pereyra, A.; Zenteno, E.; Cano-Sánchez, P. The B Subunit of PirAB(vp) Toxin Secreted from Vibrio parahaemolyticus Causing AHPND Is an Amino Sugar Specific Lectin. Pathogens 2020, 9, 182.
- De Los Santos, M.V.; Sánchez-Salgado, J.L.; Pereyra, A.; Zenteno, E.; Vibanco-Pérez, N.; Ramos-Clamont Montfort, G.; Soto-Rodriguez, S.A. The Vibrio parahaemolyticus subunit toxin PirB(vp) recognizes glycoproteins on the epithelium of the Penaeus vannamei hepatopancreas. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2022, 257, 110673.
- Lin, S.J.; Chen, Y.F.; Hsu, K.C.; Chen, Y.L.; Ko, T.P.; Lo, C.F.; Wang, H.C.; Wang, H.C. Structural insights to the heterotetrameric interaction between the Vibrio parahaemolyticus PirAvp and PirBvp toxins and activation of the cry-like pore-forming domain. Toxins 2019, 11, 233.
- Sengupta, A.; Sarkar, A.; Priya, P.; Ghosh Dastidar, S.; Das, S. New insight to structure-function relationship of GalNAc mediated primary interaction between insecticidal Cry1Ac toxin and HaALP receptor of Helicoverpa armigera. PLoS ONE 2013, 8, e78249.
- Kitami, M.; Kadotani, T.; Nakanishi, K.; Atsumi, S.; Higurashi, S.; Ishizaka, T.; Watanabe, A.; Sato, R. Bacillus thuringiensis cry toxins bund specifically to various proteins via domain III, which had a galactose-binding domain-like fold. Biosci. Biotechnol. Biochem. 2011, 75, 305–312.
- Fantus, I.G.; Goldberg, H.J.; Whiteside, C.I. The Hexosamine Biosynthesis Pathway, 1st ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 117–133.
- Xie, X.-L.; Huang, Q.-S.; Wang, Y.; Ke, C.-H.; Chen, Q.-X. Modification and Modificatory Kinetics of the Active Center of Prawn β-N-Acetyl-D-glucosaminidase. J. Biomol. Struct. Dyn. 2009, 26, 781–786.
- Song, Y.; Evenseth, L.M.; Iguchi, T.; Tollefsen, K.E. Release of chitobiase as an indicator of potential molting disruption in juvenile Daphnia magna exposed to the ecdysone receptor agonist 20-hydroxyecdysone. J. Toxicol. Environ. Health Part A 2017, 80, 954–962.
- Ettrich, R.; Kopecký, V., Jr.; Hofbauerová, K.; Baumruk, V.; Novák, P.; Pompach, P.; Man, P.; Plíhal, O.; Kutý, M.; Kulik, N.; et al. Structure of the dimeric N-glycosylated form of fungal beta-N-acetyl hexosaminidase revealed by computer modeling, vibrational spectroscopy, and biochemical studies. BMC Struct. Biol. 2007, 7, 32.
- Weitz, G.; Proia, R.L. Analysis of the glycosylation and phosphorylation of the alpha-subunit of the lysosomal enzyme, beta- hexosaminidase A, by site-directed mutagenesis. J. Biol. Chem. 1992, 267, 10039–10044.
- Zhu, F.; Li, D.; Chen, K. Structures and functions of invertebrate glycosylation. Open Biol. 2019, 9, 180232.
- Erlandson, M.A.; Toprak, U.; Hegedus, D.D. Role of the peritrophic matrix in insect-pathogen interactions. J. Insect Physiol. 2019, 117, 103894.
- Zhang, Z.; Wang, F.; Chen, C.; Zheng, Z.; Aweya, J.J.; Zhang, Y. Glycosylation of hemocyanin in Litopenaeus vannamei is an antibacterial response feature. Immunol. Lett. 2017, 192, 42–47.
- Zhang, Z.; Li, R.; Aweya, J.J.; Wang, F.; Zhong, M.; Zhang, Y. Identification and characterization of glycosylation sites on Litopenaeus vannamei hemocyanin. FEBS Lett. 2019, 593, 820–830.
- Du, X.-J.; Wang, J.-X.; Liu, N.; Zhao, X.-F.; Li, F.; Xiang, J. Identification and molecular characterization of a peritrophin-like protein from fleshy prawn (Fenneropenaeus chinensis). Mol. Immunol. 2006, 43, 1633–1644.
- Wang, L.; Li, F.; Xiang, J. A new shrimp peritrophin-like gene from Exopalaemon carinicauda involved in white spot syndrome virus (WSSV) infection. Fish Shellfish Immunol. 2013, 35, 840–846.
- Soonthornchai, W.; Rungrassamee, W.; Karoonuthaisiri, N.; Jarayabhand, P.; Klinbunga, S.; Söderhäll, K.; Jiravanichpaisal, P. Expression of immune-related genes in the digestive organ of shrimp, Penaeus monodon, after an oral infection by Vibrio harveyi. Dev. Comp. Immunol. 2010, 34, 19–28.
- Duan, Y.; Wang, Y.; Liu, Q.; Dong, H.; Li, H.; Xiong, D.; Zhang, J. Changes in the intestine microbial, digestion and immunity of Litopenaeus vannamei in response to dietary resistant starch. Sci. Rep. 2019, 9, 6464.
- Duan, Y.; Yun, W.; Ding, X.; Xiong, D.; Zhang, J. Response of intestine microbiota, digestion, and immunity in Pacific white shrimp Litopenaeus vannamei to dietary succinate. Aquaculture 2019, 517, 734762.
- Wang, Z.; Zhou, J.; Li, J.; Zou, J.; Fan, L. The immune defense response of Pacific white shrimp (Litopenaeus vannamei) to temperature fluctuation. Fish Shellfish Immunol. 2020, 103, 103–110.
- Zheng, Z.; Wang, F.; Aweya, J.J.; Li, R.; Yao, D.; Zhong, M.; Li, S.; Zhang, Y. Comparative transcriptomic analysis of shrimp hemocytes in response to acute hepatopancreas necrosis disease (AHPND) causing Vibrio parahaemolyticus infection. Fish Shellfish Immunol. 2018, 74, 10–18.
- Luangtrakul, W.; Boonchuen, P.; Jaree, P.; Kumar, R.; Wang, H.-C.; Somboonwiwat, K. Cytotoxicity of Vibrio parahaemolyticus AHPND toxin on shrimp hemocytes, a newly identified target tissue, involves binding of toxin to aminopeptidase N1 receptor. PLoS Pathog. 2021, 17, e1009463.
- Estrada, N.; Velázquez, E.; Rodríguez-Jaramillo, C.; Ascencio, F. Carbohydrate moieties and cytoenzymatic characterization of hemocytes in white leg shrimp Litopenaeus vannamei. Int. J. Cell Biol. 2016, 2016, 9032181.
- Kuyucak, S.; Norton, R.S. Computational approaches for designing potent and selective analogs of peptide toxins as novel therapeutics. Future Med. Chem. 2014, 6, 1645–1658.
- Ong, J.H.; Wong, W.L.; Wong, F.C.; Chai, T.T. Targeting PirAvp and PirBvp toxins of Vibrio parahaemolyticus with oilseed peptides: An in silico approach. Antibiotics 2021, 10, 1211.
- Shao, J.; Zhao, W.; Han, S.; Chen, Y.; Wang, B.; Wang, L. Partial replacement of fishmeal by fermented soybean meal in diets for juvenile white shrimp (Litopenaeus vannamei). Aquac. Nutr. 2019, 25, 145–153.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
4 times
(View History)
Update Date:
20 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




