
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daniel Romaus-Sanjurjo | -- | 1885 | 2022-04-18 17:19:07 | | | |
| 2 | Lindsay Dong | Meta information modification | 1885 | 2022-04-19 03:30:48 | | |
Video Upload Options
Ischemic stroke is a leading cause of death and disability worldwide. Following an ischemic insult, cells undergo endoplasmic reticulum (ER) stress, which increases the ER’s protein-folding and degradative capacities and blocks the global synthesis of proteins by phosphorylating the eukaryotic translation initiation factor 2-alpha (eIF2α). Phosphorylation of eIF2α is directly related to the dynamics of stress granules (SGs), which are membraneless organelles composed of RNA-binding proteins and mRNA. SGs play a critical role in mRNA metabolism and translational control. Other translation factors are also linked to cellular pathways, including SG dynamics following a stroke. Because the formation of SGs is closely connected to mRNA translation, it is interesting to explore the relationship between SG dynamics and cellular outcome in cases of ischemic damage.
1. Introduction
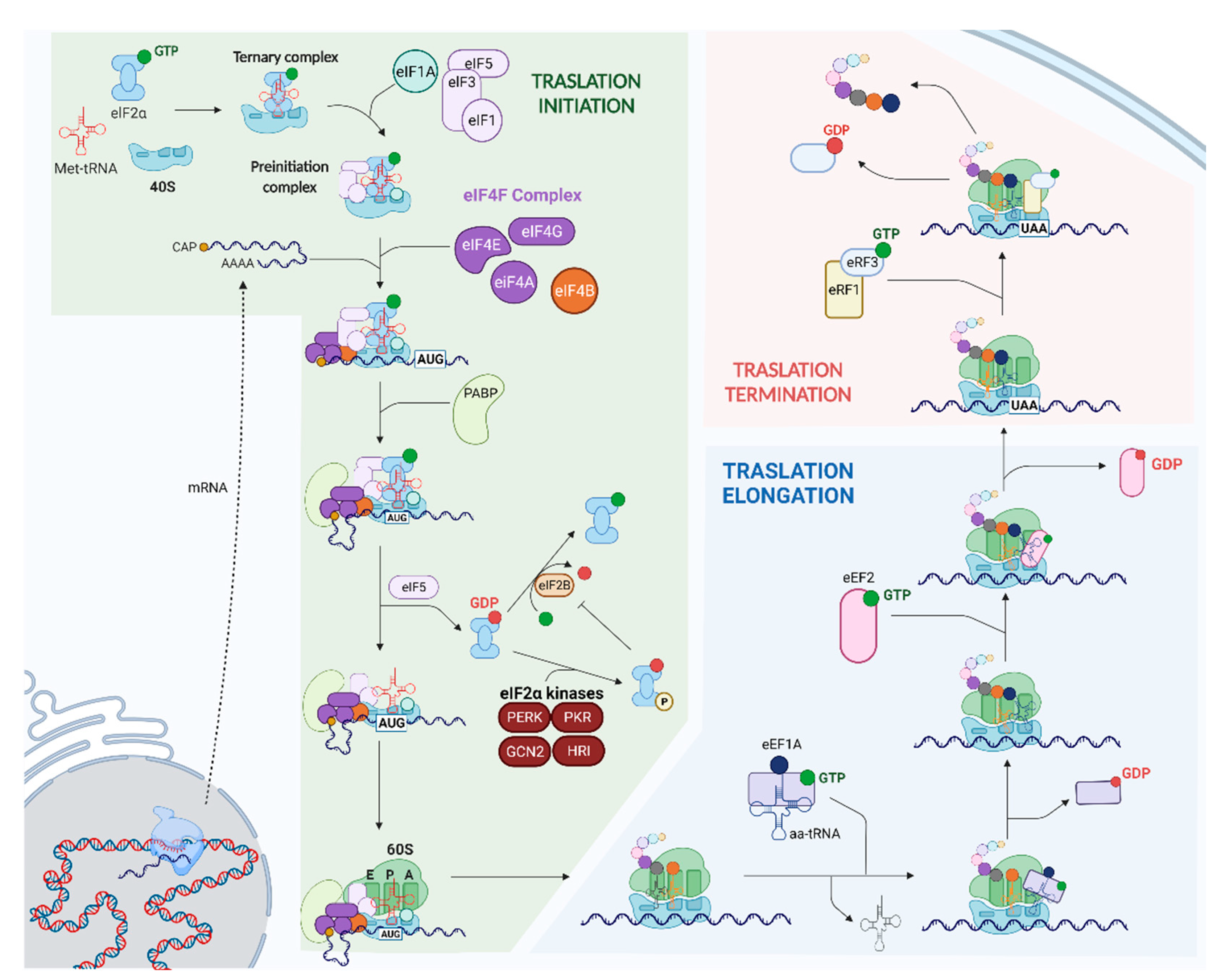
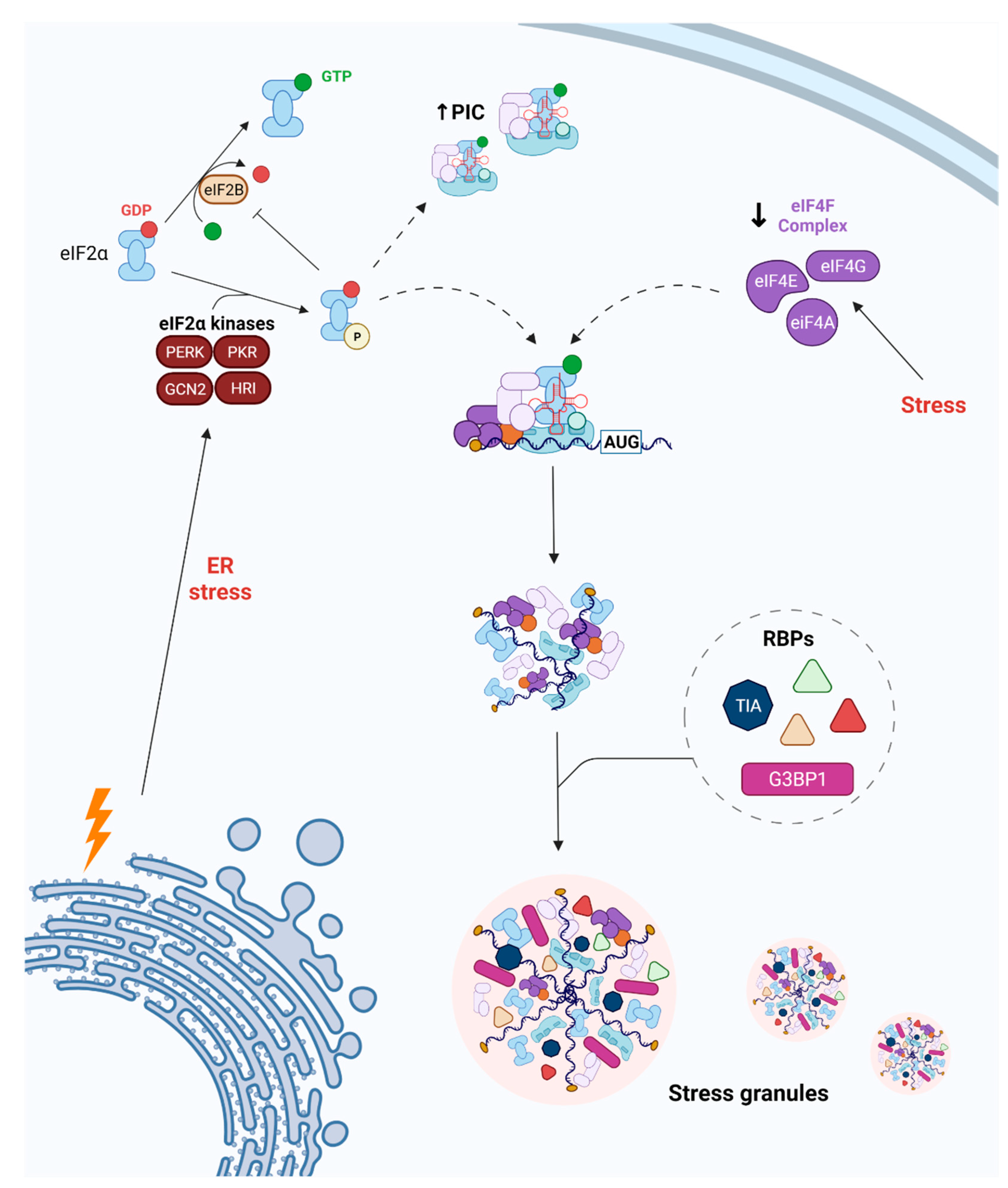
2. SG Dynamics following Cerebral Ischemia
2.1. Changes in RBP Expression
2.2. Modulation of RBP Function and Expression
2.3. Endothelial Cells and SG Dynamics
3. Two Different Ways SG Dynamics Act in Triggering Neuroprotection after Ischemia
References
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020, 19, 255–265.
- Ramos-Cabrer, P.; Campos, F.; Sobrino, T.; Castillo, J. Targeting the ischemic penumbra. Stroke 2011, 42, S7–S11.
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of Ischaemic Stroke: An Integrated View. Trends Neurosci. 1999, 22, 391–397.
- Fredrick, K.; Ibba, M. PROTEIN SYNTHESIS: Errors Rectified in Retrospect. Nature 2009, 457, 157.
- Kapur, M.; Monaghan, C.E.; Ackerman, S.L. Regulation of MRNA Translation in Neurons-A Matter of Life and Death. Neuron 2017, 96, 616–637.
- Han, Y.; Yuan, M.; Guo, Y.S.; Shen, X.Y.; Gao, Z.K.; Bi, X. Mechanism of Endoplasmic Reticulum Stress in Cerebral Ischemia. Front. Cell. Neurosci. 2021, 15, 294.
- Harding, H.P.; Novoa, I.; Zhang, Y.; Zeng, H.; Wek, R.; Schapira, M.; Ron, D. Regulated Translation Initiation Controls Stress-Induced Gene Expression in Mammalian Cells. Mol. Cell 2000, 6, 1099–1108.
- Wang, Y.C.; Li, X.; Shen, Y.; Lyu, J.; Sheng, H.; Paschen, W.; Yang, W. PERK (Protein Kinase RNA-Like ER Kinase) Branch of the Unfolded Protein Response Confers Neuroprotection in Ischemic Stroke by Suppressing Protein Synthesis. Stroke 2020, 51, 1570–1577.
- Donnelly, N.; Gorman, A.M.; Gupta, S.; Samali, A. The EIF2α Kinases: Their Structures and Functions. Cell. Mol. Life Sci. CMLS 2013, 70, 3493–3511.
- Wolozin, B.; Ivanov, P. Stress Granules and Neurodegeneration. Nat. Rev. Neurosci. 2019, 20, 649–666.
- Kahl, A.; Blanco, I.; Jackman, K.; Baskar, J.; Milaganur Mohan, H.; Rodney-Sandy, R.; Zhang, S.; Iadecola, C.; Hochrainer, K. Cerebral Ischemia Induces the Aggregation of Proteins Linked to Neurodegenerative Diseases. Sci. Rep. 2018, 8, 2701.
- Liu, X.; Yamashita, T.; Shi, X.; Bian, Y.; Bian, Z.; Omote, Y.; Takemoto, M.; Hishikawa, N.; Ohta, Y.; Abe, K. Dynamic Changes and Mislocalizations of Neurodegenerative Disease-Related Proteins in Mice Stroke Model. Brain Res. 2020, 1742, 146862.
- He, T.; Zuo, Y.; Ai-Zakwani, K.; Luo, J.; Zhu, H.; Yan, X.X.; Liu, F. Subarachnoid Hemorrhage Enhances the Expression of TDP-43 in the Brain of Experimental Rats and Human Subjects. Exp. Ther. Med. 2018, 16, 3363–3368.
- Thammisetty, S.S.; Pedragosa, J.; Weng, Y.C.; Calon, F.; Planas, A.; Kriz, J. Age-Related Deregulation of TDP-43 after Stroke Enhances NF-ΚB-Mediated Inflammation and Neuronal Damage. J. Neuroinflammation 2018, 15, 312.
- Kanazawa, M.; Kakita, A.; Igarashi, H.; Takahashi, T.; Kawamura, K.; Takahashi, H.; Nakada, T.; Nishizawa, M.; Shimohata, T. Biochemical and Histopathological Alterations in TAR DNA-Binding Protein-43 after Acute Ischemic Stroke in Rats. J. Neurochem. 2011, 116, 957–965.
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581.
- Ávila-Gómez, P.; Vieites-Prado, A.; Dopico-López, A.; Bashir, S.; Fernández-Susavila, H.; Gubern, C.; Pérez-Mato, M.; Correa-Paz, C.; Iglesias-Rey, R.; Sobrino, T.; et al. Cold Stress Protein RBM3 Responds to Hypothermia and Is Associated with Good Stroke Outcome. Brain Commun. 2020, 2, fcaa078.
- Ávila-Gómez, P.; Pérez-Mato, M.; Hervella, P.; Dopico-López, A.; da Silva-Candal, A.; Bugallo-Casal, A.; López-Amoedo, S.; Candamo-Lourido, M.; Sobrino, T.; Iglesias-Rey, R.; et al. Clinical Medicine Associations between RNA-Binding Motif Protein 3, Fibroblast Growth Factor 21, and Clinical Outcome in Patients with Stroke. J. Clin. Med 2022, 2022, 949.
- Si, W.; Li, Z.; Huang, Z.; Ye, S.; Li, X.; Li, Y.; Kuang, W.; Chen, D.; Zhu, M. RNA Binding Protein Motif 3 Inhibits Oxygen-Glucose Deprivation/Reoxygenation-Induced Apoptosis Through Promoting Stress Granules Formation in PC12 Cells and Rat Primary Cortical Neurons. Front. Cell. Neurosci. 2020, 14, 287.
- Voelz, C.; Habib, P.; Köberlein, S.; Beyer, C.; Slowik, A. Alteration of MiRNA Biogenesis Regulating Proteins in the Human Microglial Cell Line HMC-3 After Ischemic Stress. Mol. Neurobiol. 2021, 58, 1535–1549.
- Xu, X.; Zhang, C.; Jiang, J.; Xin, M.; Hao, J. The TDP43 CFTS Affect Brain Endothelial Cell Functions by Regulating YAP and Tight Junction Proteins in Cerebral Ischemic Injury. Res. Sq. 2021.
- Colombrita, C.; Zennaro, E.; Fallini, C.; Weber, M.; Sommacal, A.; Buratti, E.; Silani, V.; Ratti, A. TDP-43 Is Recruited to Stress Granules in Conditions of Oxidative Insult. J. Neurochem. 2009, 111, 1051–1061.
- Thilmann, R.; Xie, Y.; Kleihues, P.; Kiessling, M. Persistent Inhibition of Protein Synthesis Precedes Delayed Neuronal Death in Postischemic Gerbil Hippocampus. Acta Neuropathol. 1986, 71, 88–93.
- Dienel, G.A.; Pulsinelli, W.A.; Duffy, T.E. Regional Protein Synthesis in Rat Brain Following Acute Hemispheric Ischemia. J. Neurochem. 1980, 35, 1216–1226.
- Bodsch, W.; Takahashi, K.; Barbier, A.; Ophoff, B.G.; Hossmann, K.A. Cerebral Protein Synthesis and Ischemia. Prog. Brain Res. 1985, 63, 197–210.
- Ayuso, M.I.; Martínez-Alonso, E.; Regidor, I.; Alcázar, A. Stress Granule Induction after Brain Ischemia is Independent of Eukaryotic Translation Initiation Factor (EIF) 2α Phosphorylation and Is Correlated with a Decrease in EIF4B and EIF4E Proteins. J. Biol. Chem. 2016, 291, 27252–27264.
- DeGracia, D.J.; Rudolph, J.; Roberts, G.G.; Rafols, J.A.; Wang, J. Convergence of Stress Granules and Protein Aggregates in Hippocampal Cornu Ammonis 1 at Later Reperfusion Following Global Brain Ischemia. Neuroscience 2007, 146, 562–572.
- Kayali, F.; Montie, H.L.; Rafols, J.A.; DeGracia, D.J. Prolonged Translation Arrest in Reperfused Hippocampal Cornu Ammonis 1 Is Mediated by Stress Granules. Neuroscience 2005, 134, 1223–1245.
- Arimoto-Matsuzaki, K.; Saito, H.; Takekawa, M. TIA1 Oxidation Inhibits Stress Granule Assembly and Sensitizes Cells to Stress-Induced Apoptosis. Nat. Commun. 2016, 7, 10252.
- Roberts, G.G.; di Loreto, M.J.; Marshall, M.; Wang, J.; DeGracia, D.J. Hippocampal Cellular Stress Responses after Global Brain Ischemia and Reperfusion. Antioxid. Redox Signal. 2007, 9, 2265–2275.




