Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Thiago Soares | -- | 1790 | 2022-04-18 16:54:36 | | | |
| 2 | Camila Xu | Meta information modification | 1790 | 2022-04-19 05:30:32 | | | | |
| 3 | Camila Xu | Meta information modification | 1790 | 2022-04-19 05:31:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Soares, T.; Oliveira, B. Cocoa By-Products. Encyclopedia. Available online: https://encyclopedia.pub/entry/21895 (accessed on 07 February 2026).
Soares T, Oliveira B. Cocoa By-Products. Encyclopedia. Available at: https://encyclopedia.pub/entry/21895. Accessed February 07, 2026.
Soares, Thiago, Beatriz Oliveira. "Cocoa By-Products" Encyclopedia, https://encyclopedia.pub/entry/21895 (accessed February 07, 2026).
Soares, T., & Oliveira, B. (2022, April 18). Cocoa By-Products. In Encyclopedia. https://encyclopedia.pub/entry/21895
Soares, Thiago and Beatriz Oliveira. "Cocoa By-Products." Encyclopedia. Web. 18 April, 2022.
Copy Citation
Theobroma cacao L., the cocoa-producing tree, belongs to the order of Malvales, family Malvaceae and genus Theobroma. Its name has Greek origins, Theos and Bromos, meaning “food of the gods”.
cocoa beans
cocoa by-products
circular economy
1. Introduction
Theobroma cacao L., the cocoa-producing tree, belongs to the order of Malvales, family Malvaceae and genus Theobroma. Its name has Greek origins, Theos and Bromos, meaning “food of the gods” [1]. It is the only plant with commercial use in the production of chocolate. Despite having a pulp with a pleasant flavour, cocoa seed is the part of cocoa mostly used in the food industry, generating several products (Figure 1) with the greatest focus on chocolate [1].
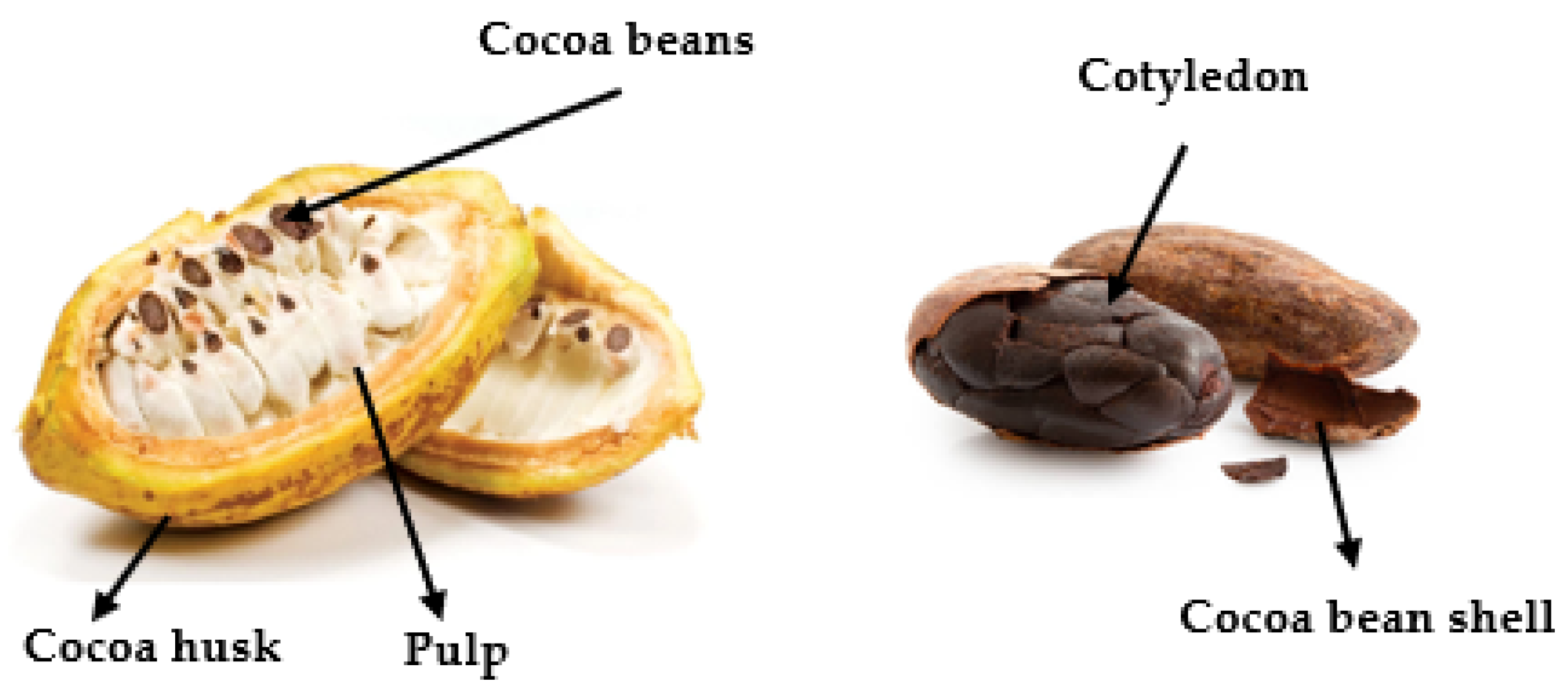 Figure 1. Constituents of cocoa fruit [2].
Figure 1. Constituents of cocoa fruit [2].There are some hypotheses for the origin and dispersion of cocoa, one of them that it comes from the upper region of the Orinoco and Amazon basins [3]. Cristovão Colombo was responsible for the knowledge and management of cocoa in the world since his arrival in America [3]. With the growing popularity of cocoa and its products, in 1824, the portuguese transported some Forastero cocoa seedlings from Brazil to São Tomé e Príncipe. Around 1850, other seedlings went to Equatorial Guinea and, finally, in the mid-1900s, the seedlings spread to Côte d’Ivoire, Ghana and Nigeria, countries that became the world’s largest cocoa producers [3].
There are four main varieties of Theobroma cacao L.: Nacional, Criollo, Forastero and Trinitário [4]. Forastero is the most representative variety in world, with about 80% of production, as it is more resistant to diseases and has higher productivity [5]. Nacional is the rarest among the four varieties and is characterized by its more refined taste, being less bitter and more aromatic, thus having greater economic value [6].
The annual production of the cocoa industry is about 4.7 million tons of cocoa seeds [7], as shown in Figure 2A. According to data from the International Cocoa Organization (ICCO), Côte d’Ivoire was the largest cocoa producer in the 2018/2019 harvest with 2154 thousand tons, followed by Ghana (812 thousand tons) and Ecuador (322 thousand tons), as shown in Figure 2B. African countries are responsible for nearly 80% of the world’s cocoa seed production, although Nacional cocoa is found mainly in Ecuador and nearby regions.
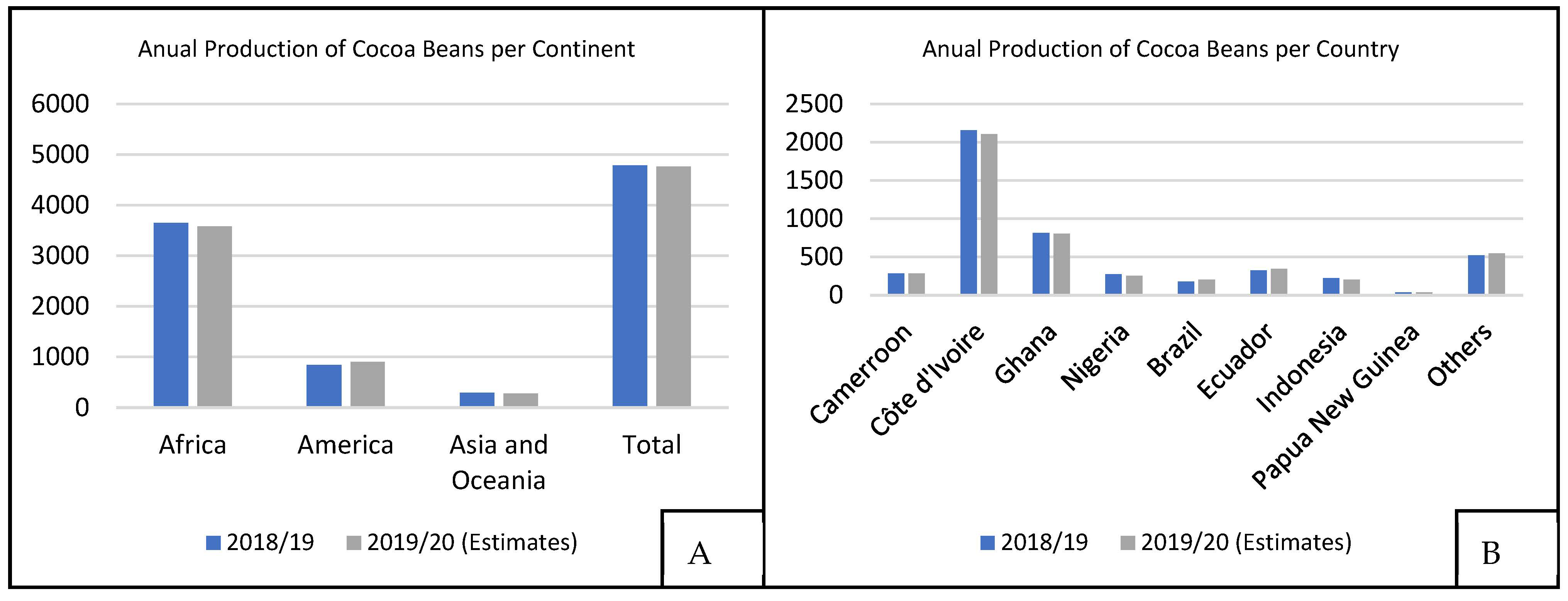 Figure 2. World cocoa production, at 103 t, for 2018/2019 and 2019/2020 harvests, (A) Annual production of cocoa beans divided by each continent and (B) Annual production of cocoa beans divided by each country [7].
Figure 2. World cocoa production, at 103 t, for 2018/2019 and 2019/2020 harvests, (A) Annual production of cocoa beans divided by each continent and (B) Annual production of cocoa beans divided by each country [7].2. Cocoa Transformation
The main activity of the cocoa industry is the transformation of cocoa fruits into cocoa mass. This process is carried out in two consecutive steps: pre-processing followed by processing [4]. The quality of the final product is closely related to these steps. An inadequate fermentation in the pre-processing step, for example, can negatively influence the chemical constituents and metabolites of the final product [4].
2.1. Cocoa Pre-Processing
After harvesting, the fruits are washed and opened manually, and the placenta separated from the seeds. This step allows a more adequate fermentation, generating seeds with better sensory characteristics. After removing the pulp, the seeds have about 65% moisture [8]. To avoid undesirable chemical reactions and the development of spoilage microorganisms, the interval between opening the fruit and fermentation should not exceed 24 h [9][10].
Fermentation lasts between 2 and 8 days, due to the type of cocoa and the environmental characteristics [1]. During this process, the seeds are stirred frequently to optimize air penetration and improve product homogeneity [11]. The pulp pH of around 3.6 and the low level of available oxygen create the ideal conditions for alcoholic fermentation in the presence of yeasts [8]. With penetration of oxygen in the fermentation process, over time, acetic bacteria convert the alcohol into acetic acid, reducing the pH and increasing the temperature (45–50 °C) [8]. The diffusion of acids in the seed and the increase in temperature kill the germ. Subsequently, seeds become cocoa beans and taste precursors, amino acids, peptides, and reducing sugars (the precursors of the Maillard reaction in the roasting phase) are also formed [12].
Finally, the cocoa beans go into drying and storage. There are two techniques for this step: natural or artificial. The natural technique is quite simple, requiring only the space available to perform it. The artificial one uses dryers in a warehouse [8]. One of the most important parameters to control is the water removal rate. If high, the inside of the cocoa beans will remain moist, promoting the proliferation of fungi/microorganisms and reducing the quality of the product. If slow, the product will lose weight, increasing the fragility of cocoa beans [13]. The drying stage is intended not only to eliminate the water in excess (final moisture close to 7%) [14], but also to reduce the acidity of the cocoa beans, following the biochemical changes initiated in the fermentation, which will give the characteristic flavour and aroma of chocolate [14]. This whole process takes place near the fruit harvest site.
2.2. Cocoa Processing
The processing stage already takes place at the company that purchases the raw material. The first step in this process is cleaning the dry cocoa beans, using sieves and magnets, to separate foreign materials (stones, metals and plant pieces). At the same time, shelling may occur [9]. The industry can use two different procedures in series: heat pre-treatment and the roasting of cocoa beans with subsequent shelling. The advantage of heat pre-treatment before roasting is the production of cocoa beans of similar sizes [9]. In this phase, equipment such as fluidized bed dryers, infrared, continuous air roasters, among others, can be used. Cocoa beans are usually broken due to impact at high speeds against obstacles [12]. After breaking, the product is transferred to vibrating sieves to separate the husks with the aid of a blow of air, due to the difference in weight [9]. The last by-product of cocoa, as shown in Figure 3, is obtained at this stage.
In the roasting process, the control of time and temperature (110–140 °C) is essential, as they are responsible for the characteristic flavour and taste of chocolate [11]. Roasting, as a heat treatment, reduces the water content and inactivates the enzymes in the product. Its main function is the development of the aroma, through Maillard reactions, initiated earlier in the fermentation step and responsible for the characteristic brown colour [10][14]. Finally, the roasted product undergoes a grinding process to reduce particle size, generating the mass of cocoa which is the precursor of other cocoa products [9].
3. Cocoa By-Products
Cocoa husk is the first and main residue of the cocoa industry, representing about 80% of the fruit in dry weight (d.w.). This by-product has a composition rich in lignin and non-starch polysaccharides (cellulose, hemicelluloses and pectin) (Table 1), terpenoids (chrysophanol), phenolic and carboxylic acids (protocatechuic, salicylic, citric and tartaric acids) and some free amino acids (glutamine, asparagine, serine and lysine) [15]. Serra Bonvehí and Ventura Coll (1999) evaluated fruits grown in Côte d’Ivoire, Nigeria, Cameroon, Colombia, Ecuador, Guinea and Brazil [16], verifying that geographic origin affects the protein amount (from 12.50 to 17.60 g/100 g of dry cocoa husk) [16]. The same authors also evaluated the free amino acids (315 mg/100g d.w.) lipids (3.0 g/100g d.w.), total free sugars and starch (2.80 g/100g d.w.), concluding that this by-product is a source of dietary fiber and has acceptable protein quality [16]. Vriesmann et al. (2011) analysed the composition of cocoa husks from Northeast Brazil, focusing on minerals; iron, calcium, potassium and sodium were present in mg/100 g d.w. and copper, magnesium, selenium and zinc in mg/kg [17].
Table 1. Chemical composition of cocoa by-products (g/100 g d.w.).
| Compounds | Cocoa Husk | Cocoa Pulp | Cocoa Bean Shell | References |
|---|---|---|---|---|
| Carbohydrates | 29.04–32.30 | 10.70–68.35 | 17.80–23.17 | [18][19] |
| Cellulose | 24.24–35.00 | 20.80–57.50 | 15.10 | [20] |
| Hemicellulose | 8.72–11.00 | 7.00–17.00 | - | [21] |
| Lignin | 14.60–26.38 | 12.00-14.60 | 32.41 | [22] |
| Pectin | 6.10–9.20 | 0.57–1.50 | 0.57–1.50 | [23][24] |
| Total dietary fibre | 36.60–56.10 | 16.89 | 18.60–60.60 | [17][25] |
| Total proteins | 4.21–10.74 | 0.41–5.56 | 15.79–18.10 | [26][27] |
| Lipids | 1.50–2.24 | 1.91–3.54 | 2.02–6.87 | [18][28] |
| Ash | 6.70–10.02 | 3.70–7.68 | 5.96–11.42 | [21][27] |
| Minerals (mg/100 g) | 3230.85 | 1297.07 | 56.75–312.57 | [17] |
| Total organic acids | - | 17.52 | - | [18] |
| Total phenolics * | 4.60–6.90 | - | 1.32–5.78 | [29] |
| Anthocyanins ** | - | - | 0.40 | [30] |
| Theobromine | 0.34 | - | 1.30 | [20][24] |
| Caffeine | - | - | 0.10 | [28] |
| Tannins | 5.20 | - | 3.30–4.46 | [22][29] |
| Flavonols ** | - | - | 1.50 | [30] |
* (g gallic acid equivalent/100 g) and ** (µg quercetin/100 g).
The large amount of biomass generated is scattered in the soil or burned by farmers. In nature, the decomposition of cocoa husks depends on physical, chemical and biological factors [31]. Physical factors are usually temperature and sun exposure. An important chemical factor is the biomass moisture [31]. Biological factors are microorganisms, bacteria and fungi [31]. Fungi are the main biological representatives, due to their ability to decompose the complex structures of the cocoa husk as a nutritional source. Under certain climatic conditions, this biomass allows the proliferation of undesirable fungi, including potential pathogens such as Marasmius perniciosus, Phytophthora palmivora and P. megakarya [32]. A demonstrative example of the importance of the handling and disposing of this by-product is what happened in Brazil, in the 1980s. The fungus Moniliophthora perniciosa, commonly known as witch’s broom, attacked and destroyed thousands of trees, substantially reducing the production of cocoa beans for nearly twenty years [33]. Due to this fungus, there was a significant economic deficit in the region’s cocoa industry.
Cocoa pulp, also known as mucilage, is a white mass that surrounds the cocoa beans [34]. During the pre-treatment process, this white mass releases a cloudy liquid called cocoa pulp juice, produced by the action of microorganisms (mainly yeasts and acidophilic bacteria) naturally present in the fruit and in processing sites [32]. Approximately 100–150 L of cocoa pulp juice per ton of wet cocoa beans are produced during the fermentation process [32][35]. This by-product has high potential as a culture medium for microorganisms at an industrial level. Its composition is rich in sugars and minerals, without alkaloids and other toxic substances (Table 1). The production of alcoholic beverages with standardized levels of volatile compounds (higher in alcohols, esters and aldehydes) is a possible application of this product, characterized by a low concentration of methanol. Due to its aroma, this product has high acceptability and a general acceptability compared to other fruit alcoholic beverages [23][26][36][37]. Other applications in this field were tested, such as the production of soft drinks, kefir and jams. These products had a nutritional value equivalent to commercial brands and high acceptability [18][38][39][40].
Cocoa bean shell is obtained after separation of the seed, in the heat pre-treatment process or in roasting. This by-product is a lignin cellulosic complex particularly rich in dietary fiber (ranging from 18 to 60%) and other compounds (Table 1). This by-product has been studied in recent years due to its chemical composition and high potential in different industries (chemical, food, environmental) and in human health. Cocoa bean shell has adsorption capacity, high porosity and low ash content, according to studies conducted for the environmental sector. These characteristics and good mechanical performance are attractive as lignocellulosic precursors of unbound carbon monoliths [41][42]. It is also effective as a low-cost raw material for polluting adsorbents (industrial dyes, gases and heavy metals) [28][43]. In terms of human health, fractions and extracts of this by-product seem to reduce the incidence of chronic diseases (obesity, diabetes and cancer) [44][45], control eating disorders [46][47] and protect human cells against ischemic damage [24].
References
- Afoakwa, E.O. Chocolate Science and Technology, 2nd ed.; Wiley-Blackwell Publishers: Chichester, UK, 2016; ISBN 978-1-118-91378-9.
- Campos-Vega, R.; Nieto-Figueroa, K.H.; Oomahb, B.D. Cocoa (Theobroma cacao L.) pod husk: Renewable source of bioactive compounds. Trends Food Sci. Technol. 2018, 81, 172–184.
- Queiroga, V.P.; Gomes, J.P.; Melo, B.A.; Alburquerque, E.M.B. Cacau (Theobroma cacao L.) Orgânico Sombreado Tecnologias de Plantio e Produção da Amêndoa Fina, 1st ed.; AREPB: Campina Grande, Brazil, 2021; ISBN 978-65-87070-08-7.
- Castro-Alayo, E.M.; Idrogo-Vásquez, G.; Siche, R.; Cardenas-Toro, F.P. Formation of Aromatic Compounds Precursors during Fermentation of Criollo and Forastero Cocoa. Heliyon 2019, 5, e01157.
- Torres-Moreno, M.; Torrescasana, E.; Salas-Salvadó, J.; Blanch, C. Nutritional composition and fatty acids profile in cocoa beans and chocolates with different geographical origin and processing conditions. Food Chem. 2015, 166, 125–132.
- Rusconi, M.; Conti, A. Theobroma cacao L., the food of the Gods: A scientific approach beyond myths and claims. Pharmacol. Res. 2010, 61, 5–13.
- ICCO—International Cocoa Organization Annual Report 2018/2019. Available online: https://www.icco.org/statistics/#production (accessed on 28 July 2021).
- Cruz, C.L.C.V. Melhoramento do Sabor da Amêndoa de Cacau Através de Tratamento Térmico em Forno Convencional e Microondas. Ph.D. Thesis, Universidade Federal de Campinas, Campinhas, Brazil, 2002.
- Beckett, S.T. The Science of Chocolate, 2nd ed.; RSC Publishing: Cambridge, UK, 2008; ISBN 978-0-85404-970-7.
- Efraim, P.; Alves, A.B.; Jardim, D.C.P. Polifenóis em cacau e derivados: Teores, fatores de variação e efeitos na saúde. Braz. J. Food Technol. 2011, 14, 181–201.
- Owusu, M.; Petersen, M.A.; Heimdal, H. Relationship of sensory and instrumental aroma measurements of dark chocolate as influenced by fermentation method, roasting and conching conditions. J. Food Sci. Technol. 2013, 50, 909–917.
- Beckett, S.T.; Fowler, M.S.; Ziegler, G.R. Industrial Chocolate Manufacture and Use, 4th ed.; John Willey & Sons: Chichester, UK, 2009; ISBN 978-1405139496.
- Lopes, A.S. Estudo Químico e Nutricional de Amêndoa de Cacau (Theobroma cacao L.) e Cupuaçu (Theobroma grandiflorum Schum) em Função do Processamento. Ph.D. Thesis, Universidade Federal de Campinas, Campinhas, Brazil, 2000.
- Lopes, A.S.; Garcia, N.H.P.; Vasconcelos, M.A.M. Avaliação das condições de torração após a fermentação da amêndoas de cupuaçu (Theobroma grandiflorum Schum) e cacau (Theobroma cacao L.). Braz. J. Food Technol. 2003, 6, 309–316.
- Silva, R.O. Utilização dos Resíduos Sólidos da Indústria Cacaueira para a Produção de Etanol. Ph.D. Thesis, Universidade Federal do Espírito Santo, Vitória, Brazil, 2018.
- Bonvehí, J.S.; Coll, F.V. Protein quality assessment in cocoa husk. Food Res. Int. 1999, 32, 201–208.
- Vriesmann, L.C.; de Mello Castanho Amboni, R.D.; de Oliveira Petkowicz, C.L. Cacao pod husks (Theobroma cacao L.): Composition and hot-water-soluble pectins. Ind. Crops Prod. 2011, 34, 1173–1181.
- Anvoh, K.Y.B.; Bi, A.Z.; Gnakri, D. Production and characterization of juice from mucilage of cocoa beans and its transformation into marmalade. Pak. J. Nutr. 2009, 8, 129–133.
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Analytical dataset of Ecuadorian cocoa shells and beans. Data Br. 2019, 22, 56–64.
- Ofori-Boateng, C.; Lee, K.T. The potential of using cocoa pod husks as green solid base catalysts for the transesterification of soybean oil into biodiesel: Effects of biodiesel on engine performance. Chem. Eng. J. 2013, 220, 395–401.
- Mansur, D.; Tago, T.; Masuda, T.; Abimanyu, H. Conversion of cacao pod husks by pyrolysis and catalytic reaction to produce useful chemicals. Biomass Bioenergy 2014, 66, 275–285.
- Lecumberri, E.; Mateos, R.; Izquierdo-Pulido, M.; Rupérez, P.; Goya, L.; Bravo, L. Dietary fibre composition, antioxidant capacity and physico-chemical properties of a fibre-rich product from cocoa (Theobroma cacao L.). Food Chem. 2007, 104, 948–954.
- Dias, D.R.; Schwan, R.F.; Freire, E.S.; Dos Santos Serôdio, R. Elaboration of a fruit wine from cocoa (Theobroma cacao L.) pulp. Int. J. Food Sci. Technol. 2007, 42, 319–329.
- Arlorio, M.; Coisson, J.; Restani, P.; Martelli, A. Characterization of pectins and some secondary compounds from Theobroma cacao hulls. J. Food Sci. 2001, 66, 653–656.
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of cocoa (Theobroma cacao L.) co-products. Food Res. Int. 2012, 49, 39–45.
- Solieri, L.; Giudici, P. Vinegars of the World, 1st ed.; Springer: Milan, Italy, 2009; ISBN 978-88-470-0866-3.
- Nsor-Atindana, J.; Zhong, F.; Mothibe, K.J.; Bangoura, M.L.; Lagnika, C. Quantification of total polyphenolic content and antimicrobial activity of cocoa (Theobroma cacao L.) bean shells. Pak. J. Nutr. 2012, 11, 574–579.
- Prabhakaran Nair, K.P. The Agronomy and Economy of Important Tree Crops of the Developing World, 1st ed.; Elsevier: London, UK, 2010; ISBN 978-0123846778.
- Yapo, B.M.; Besson, V.; Koubala, B.B.; Koffi, K.L. Adding value to cacao pod husks as a potential antioxidant-dietary fiber source. Am. J. Food Nutr. 2013, 1, 38–46.
- Lessa, O.A.; dos Santos Reis, N.; Leite, S.G.F.; Gutarra, M.L.E.; Souza, A.O.; Gualberto, S.A.; de Oliveira, J.R.; Aguiar-Oliveira, E.; Franco, M. Effect of the solid state fermentation of cocoa shell on the secondary metabolites, antioxidant activity, and fatty acids. Food Sci. Biotechnol. 2018, 27, 107–113.
- Rahim, I.; Kuswinanti, T.; Asrul, L.; Rasyid, B. Screening of fungal rot isolates from cocoa as phosphate-dissolving and their growth ability on three types of media. Procedia Food Sci. 2015, 3, 104–111.
- Watson, R.R.; Preedy, V.R.; Zibadi, S. Chocolate in Health and Nutrition; Humana Press: Heidelberg, Germany, 2013; ISBN 978-1-61779-802-3.
- Guest, D. Black pod: Diverse pathogens with a global impact on cocoa yield. Phytopathology 2007, 97, 1650–1653.
- Endraiyani, V.; Ludescher, R.D.; Di, R.; Karwe, M.V. Total phenolics and antioxidant capacity of cocoa pulp: Processing and storage study. J. Food Process. Preserv. J. 2017, 41, e13029.
- Dwapanyin, A.O.; Adomako, D.; Tetteh, J.P. The sugar content of cocoa sweatings and the effect of pressing the sweatings prior to fermentation on bean quality. J. Biochem. Mol. Biol. 1991, 1, 109–120.
- Duarte, W.F.; Dias, D.R.; Oliveira, J.M.; Teixeira, J.A.; de Almeida e Silva, J.B.; Schwan, R.F. Characterization of different fruit wines made from cacao, cupuassu, gabiroba, jaboticaba and umbu. LWT Food Sci. Technol. 2010, 43, 1564–1572.
- Takrama, J.F.; Kumi, W.O.; Otoo, G.; Addo, K.; Camu, N. Optimization of cocoa pulp juice fermentation with yeast starter cultures of cocoa heap fermentations. J. Agric. Sci. Food Technol. 2015, 1, 22–33.
- Puerari, C.; Magalhães, K.T.; Schwan, R.F. New cocoa pulp-based kefir beverages: Microbiological, chemical composition and sensory analysis. Food Res. Int. 2012, 48, 634–640.
- Escalante, M.; Badrie, N.; Bekele, F.L. Production and quality characterization of pulp from cocoa beans from Trinidad: Effects of varying levels of pulp on value-added carbonated cocoa beverages. In Proceedings of the 49th Annual Meeting, Port of Spain, Trinidad and Tobago, 30 June–6 July 2013; Caribbean Food Crops Society: San Juan, Puerto Rico, 2013. ISSN 95-07-0410.
- Afolabi, M.O.; Ibitoye, W.O.; Agbaje, A.F. Evaluation of nutritional and sensory properties of cocoa pulp beverage supplemented with pineapple juice. J. Food Res. 2015, 4, 58–61.
- Adegoke, K.A.; Bello, O.S. Dye sequestration using agricultural wastes as adsorbents. Water Resour. Ind. 2015, 12, 8–24.
- Plaza-Recobert, M.; Trautwein, G.; Pérez-Cadenas, M.; Alcañiz-Monge, J. Preparation of binderless activated carbon monoliths from cocoa bean husk. Microporous Mesoporous Mater. 2017, 243, 28–38.
- Fioresi, F.; Vieillard, J.; Bargougui, R.; Bouazizi, N.; Fotsing, P.N.; Woumfo, E.D.; Brun, N.; Mofaddel, N.; Le Derf, F. Chemical modification of the cocoa shell surface using diazonium salts. J. Colloid Interface Sci. 2017, 494, 92–97.
- DeVries, J.W. On defining dietary fibre. Proc. Nutr. Soc. 2003, 62, 37–43.
- Kris-Etherton, P.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71S–88S.
- Bessesen, D.H. The Role of Carbohydrates in Insulin Resistance. J. Nutr. 2001, 131, 2782S–2786S.
- Johnson, I.T. New approaches to the role of diet in the prevention of cancers of the alimentary tract. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2004, 551, 9–28.
More
Information
Subjects:
Green & Sustainable Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
6.3K
Revisions:
3 times
(View History)
Update Date:
19 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No


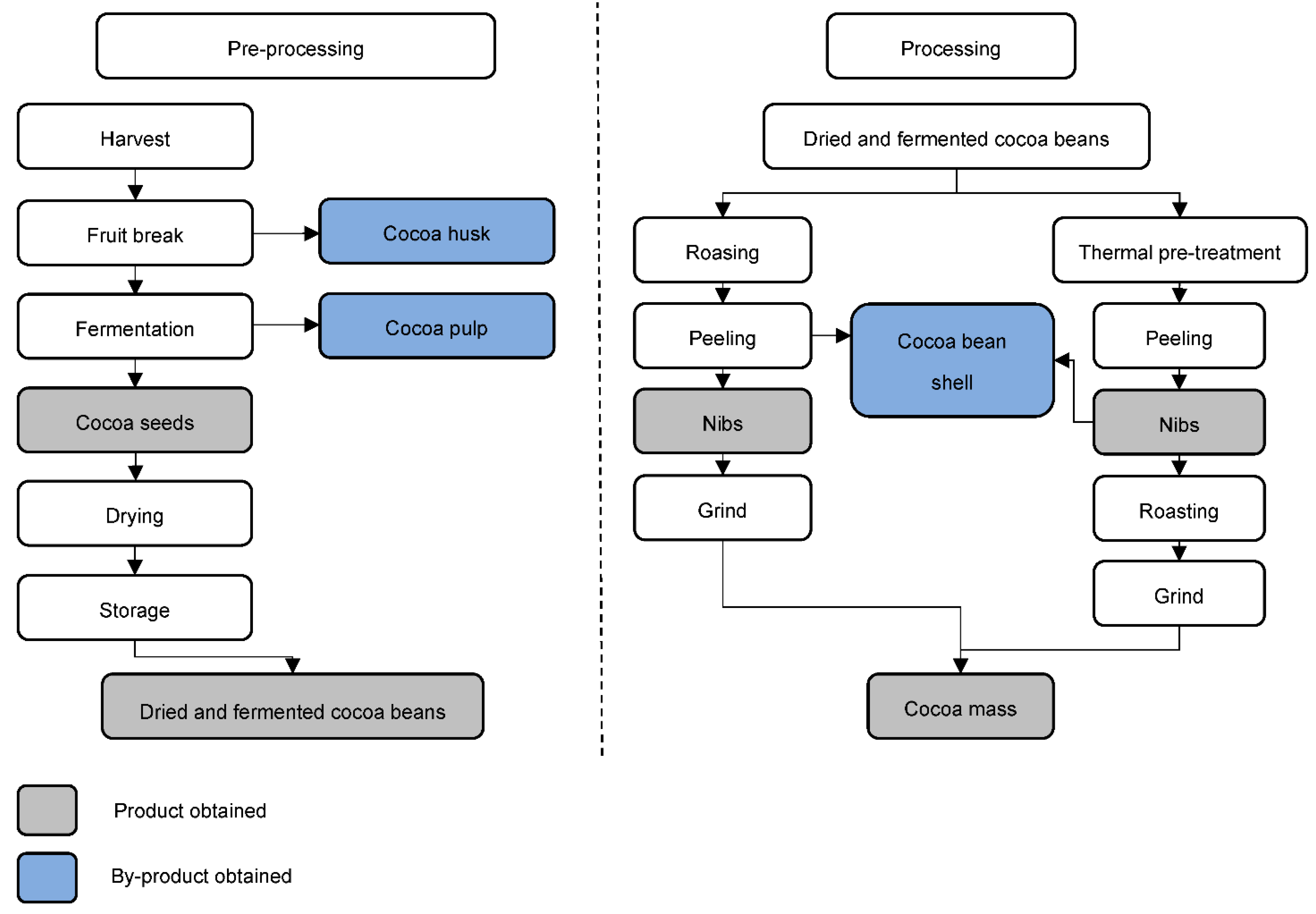 Figure 3. Pre-processing and processing steps for the transformation of cocoa fruit to cocoa mass [
Figure 3. Pre-processing and processing steps for the transformation of cocoa fruit to cocoa mass [

