Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sun, A.; , .; Yuan, J.; Xia, Y.; Liu, Y. TMDs in Aqueous Zinc Ion Batteries. Encyclopedia. Available online: https://encyclopedia.pub/entry/21890 (accessed on 07 February 2026).
Sun A, , Yuan J, Xia Y, Liu Y. TMDs in Aqueous Zinc Ion Batteries. Encyclopedia. Available at: https://encyclopedia.pub/entry/21890. Accessed February 07, 2026.
Sun, Aokui, , Jingchen Yuan, Yong Xia, Yuejun Liu. "TMDs in Aqueous Zinc Ion Batteries" Encyclopedia, https://encyclopedia.pub/entry/21890 (accessed February 07, 2026).
Sun, A., , ., Yuan, J., Xia, Y., & Liu, Y. (2022, April 18). TMDs in Aqueous Zinc Ion Batteries. In Encyclopedia. https://encyclopedia.pub/entry/21890
Sun, Aokui, et al. "TMDs in Aqueous Zinc Ion Batteries." Encyclopedia. Web. 18 April, 2022.
Copy Citation
Owing to the unique layered structure and more desirable layer spacing, transition metal dichalcogenide (TMD) materials are considered as the comparatively ideal cathode material of ZIBs which facilitate the intercalation/ deintercalation of hydrated Zn2+ between layers.
transition metal dichalcogenides
aqueous zinc ion batteries
modification strategy
1. Introduction
Due to the deterioration of the environment and the deficit of fossil energy, it is increasingly important to develop environmentally friendly, sustainable, and renewable energy [1][2][3]. At present, some renewable energy power generation can meet the requirements of environmental protection and sustainable development, such as solar power, wind power, and tidal power generation, but these energy sources suffer from regional limitations and instability, limiting their wider application. As an important part of energy for sustainable development, electrochemical energy storage has become an active research field in recent decades [4][5][6]. In the current energy market, lithium-ion batteries (LIBs) are dominant in automobile, medical equipment, portable wearable equipment, and other industries because of their excellent energy density and good environmental performance [7][8]. However, most lithium-ion batteries use organic solvents as electrolytes, which may cause safety problems and increase costs [9][10][11][12]. Compared with non-aqueous batteries, aqueous rechargeable batteries have the characteristics of low cost, non-toxicity, and non-flammability, which makes them safer, more environmentally friendly, and more economical [13][14][15].
In addition to LIBs, many other rechargeable aqueous metal ion batteries have been developed, including sodium ion batteries [16], potassium ion batteries [17], aluminum ion batteries [18], calcium ion batteries [19], and zinc ion batteries. Compared with other rechargeable aqueous metal ion batteries, zinc ion batteries have many advantages [20][21][22]: (1) Zinc ion batteries can be directly assembled in the air without inert environment, which can reduce the battery assembly cost. (2) Zinc ions can be electrodeposited reversibly in aqueous solution, so the zinc sheet can be directly used as the anode of the battery. (3) Zinc as anode has a higher theoretical capacity and lower oxidation/reduction potential (–0.76 V) than the standard hydrogen electrode, which indicates that there is a higher open circuit voltage when coupled with cathode. Therefore, in many rechargeable water-based metal ion batteries, the research on aqueous zinc ion batteries is increasingly concerned.
As shown in Figure 1a, the aqueous zinc ion battery (ZIB) is mainly composed of the battery shell, cathode material, anode material, electrolyte, and diaphragm. There are many kinds of cathode materials, including manganese-based materials [23], vanadium-based materials [24], and Prussian blue analogues [25], all of which possess a certain capacity of Zn2+ storage. Since the theoretical interlayer spacing of transition metal disulfide compounds is larger than the diameter of Zn2+, Zn2+ can be intercalated and deintercalated in TMD material, which also indicates that TMD material is feasible as the cathode of ZIB [26]. At present, few studies have been conducted on TMD as a cathode material of ZIB, and there is not a systematic exposition, which also shows from the aspect that TMD as cathode of ZIB is quite novel. Figure 1b displays the trend of the increasing number of publications, evidencing the increasing attention paid to TMD materials in ZIB. The latest research progress of TMD series materials as cathodes of ZIB in recent years is reviewed in this paper, and several modification methods which can improve the Zn2+ storage capacity and structural stability of TMD materials are expounded.
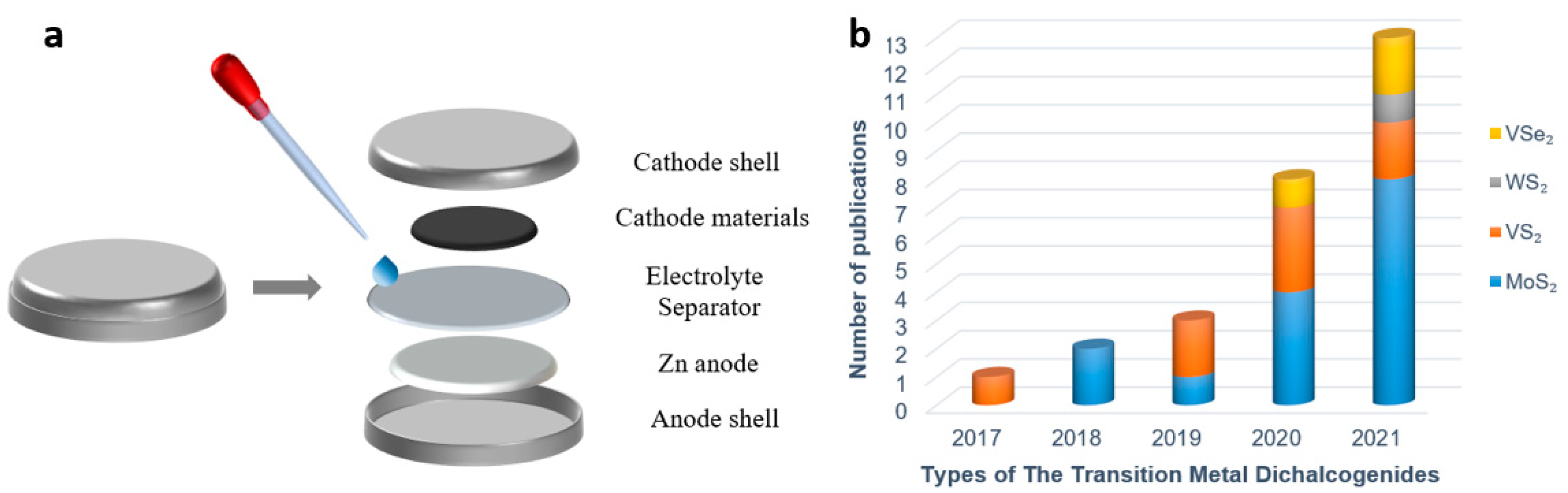 Figure 1. (a) Disassembly diagram of coin-type ZIBs; (b) The trend of publications on TMDs as ZIBs cathode materials.
Figure 1. (a) Disassembly diagram of coin-type ZIBs; (b) The trend of publications on TMDs as ZIBs cathode materials.2. Characterization and Synthetic Methods of TMD
Two-dimensional layered transition metal disulfides (TMDs) generally have X-M-X structure, where M is the transition metal, such as molybdenum, vanadium, tungsten, bismuth, and other metal elements, while X is generally sulfur, selenium, and other elements, as shown in Figure 2a. These layered structures facilitate the transport of various carriers and can also adapt to the volume change during ion insertion. Because of their different chemical composition and unique crystal structure, as well as the fact that the d-orbitals can be filled with different elements, TMDs can be used as functional materials for electronic insulation, semiconductors, and superconductivity [27].
Among all TMDs materials, molybdenum disulfide (MoS2) has received extensive attention as a typical representative. Molybdenum disulfide is composed of two layers of sulfur atoms and one layer of molybdenum atoms, with the metal molybdenum layer interposed between the two sulfur layers, alternately stacked to form a sandwich-like structure. The sulfur atoms are bound together by van der Waals force, while the S-Mo-S atoms are linked by strong covalent bonds [28][29]. MoS2 not only has a layered structure, but also has different phases (1T, 2H, and 3R), and each phase also has different physical properties and chemical characteristics [30]. As shown in Figure 2b, MoS2 in 2H and 3R phases both demonstrate the triangular prismatic coordination of Mo atoms, and 2H-MoS2 is very stable because of the two layers of units stacked in hexagon symmetry. Meanwhile, 3R-MoS2 has rhombus symmetry, and each unit has three layers. On the contrary, the Mo atoms of 1T-MoS2 (metal phase) are octahedral coordinated and most unstable [31][32]. VS2 is also a common material in TMDs, where the sulfur and vanadium layers are stacked together in a sandwich-like structure by van der Waals force interactions [33][34]. Due to the large interlayer spacing, VS2 has great potential in the intercalation/deintercalation of ions, such as Li+, Na+, Zn2+, Mg2+, and Al3+ [35][36][37].
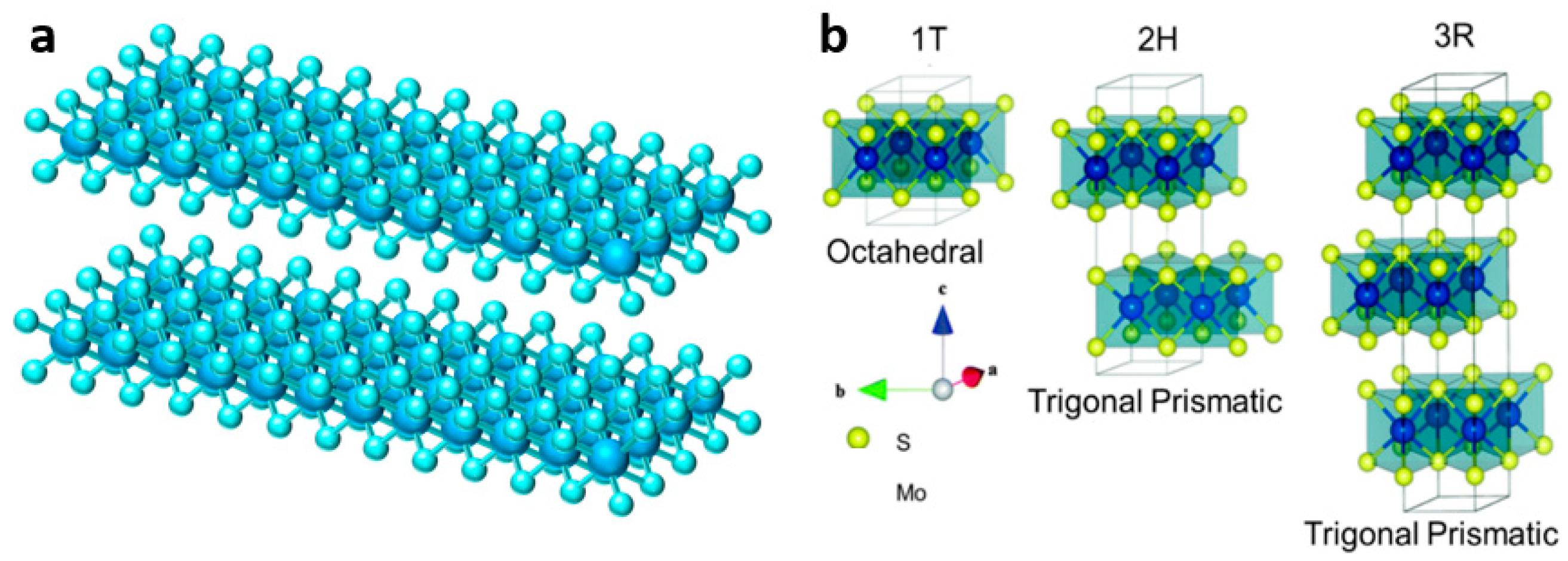 Figure 2. (a) Illustration of TMD structure; (b) Atomic structures of 1T-, 2H-, and 3R-MoS2 [31].
Figure 2. (a) Illustration of TMD structure; (b) Atomic structures of 1T-, 2H-, and 3R-MoS2 [31].In order to obtain layered and nanoflower-like sulfide materials, TMD materials are generally prepared by hydrothermal and solvothermal methods. The synthetic methods of TMD materials as ZIB cathode are summarized in Table 1.
Table 1. Fabrication methods, precursors and synthesis conditions of TMDs as ZIBs cathode.
|
Products |
Method | Precursors | Temperature | Duration | Ref |
|---|---|---|---|---|---|
| MoS2−x | Hydrothermal | (NH4)6Mo7O24·4H2O, TAA | 200 °C | 18 h | [38] |
| E- MoS2 | Hydrothermal | Na2MoO4, CS(NH2)2, carbon cloth, glucose, HCl | 190 °C | 24 h | [39] |
| MoS2-O | Hydrothermal | (NH4)6Mo7O24·4H2O, thiourea | 180 °C | 24 h | [40] |
| MoS2·nH2O | Hydrothermal | (NH4)6Mo7O24·4H2O, thiourea | 170 °C | 24 h | [41] |
| MoS2/PANI | Solvothermal | Na2MoO4, thiourea, C17H33CO2Na, ethanol, OA, HCl | 180 °C | 24 h | [42] |
| MoS2@CNTs | Hydrothermal | Na2MoO4·2H2O, thiourea, CNTs, glucose | 200 °C | 24 h | [43] |
| MoS2-160 | Hydrothermal | (NH4)6Mo7O24·4H2O, thiourea | 160 °C | 24 h | [44] |
| VS2 | Hydrothermal | NH4VO3, TAA, NH3·H2O | 180 °C | 20 h | [45] |
| VS2@SS | Hydrothermal | NH4VO3, TAA, NH3·H2O, stainless steel mesh | 180 °C | 10 h | [46] |
| rGO-VS2 | Solvothermal | VO(acac)2, cysteine, GO, NMP | 200 °C | 8 h | [47] |
| VS2@VOOH | Hydrothermal | V2O5, TAA, NH3·H2O | 180 °C | 18 h | [48] |
| VS2·NH3 | Solvothermal | VO(acac)2, TAA, NMP | 200 °C | 24 h | [49] |
| VS2/VOx | Solvothermal | Na3VO4·12H2O, TAA, ethylene | 180 °C | 20 h | [50] |
| 1T-WS2 | Solvothermal | WCl6, TAA, DMF | 200 °C | 24 h | [51] |
| VSe2 | Chemical Liquid Phase Synthesis | VO(acac)2, Se powder, OAm | 330 °C | 5 h | [52] |
References
- Yan, H.; Zhang, X.; Yang, Z.; Xia, M.; Xu, C.; Liu, Y.; Yu, H.; Zhang, L.; Shu, J. Insight into the electrolyte strategies for aqueous zinc ion batteries. Coord. Chem. Rev. 2021, 452, 214297.
- Yang, S.; Cheng, Y.; Xiao, X.; Pang, H. Development and application of carbon fiber in batteries. Chem. Eng. J. 2020, 384, 123294.
- Wang, J.; Yang, Y.; Zhang, Y.; Li, Y.; Sun, R.; Wang, Z.; Wang, H. Strategies towards the challenges of zinc metal anode in rechargeable aqueous zinc ion batteries. Energy Storage Mater. 2020, 35, 19.
- Zhou, H.; Li, X.; Li, Y.; Zheng, M.; Pang, H. Applications of MxSey (M = Fe, Co, Ni) and their composites in electrochemical energy storage and conversion. Nano-Micro Lett. 2019, 11, 40.
- Xiao, X.; Zou, L.; Pang, H.; Xu, Q. Synthesis of micro/nanoscaled metal–organic frameworks and their direct electrochemical applications. Chem. Soc. Rev. 2020, 49, 301.
- Hua, Y.; Li, X.; Chen, C.; Pang, H. Cobalt Based metal-organic frameworks and their derivatives for electrochemical energy conversion and storage. Chem. Eng. J. 2019, 370, 37.
- Zhang, N.; Xiao, X.; Pang, H. Transition metal (Fe, Co, Ni) fluoride-based materials for electrochemical energy storage. Nanoscale Horiz. 2019, 4, 99.
- Wang, F.; Liu, Y.; Zhao, Y.; Wang, Y.; Wang, Z.; Zhang, W.; Ren, F. Facile synthesis of two-dimensional porous MgCo2O4 nanosheets as anode for lithium-ion batteries. Appl. Sci. 2017, 8, 22.
- Qi, S.; Wu, D.; Dong, Y.; Liao, J.; Foster, C.W.; O’Dwyer, C.; Feng, Y.; Liu, C.; Ma, J. Cobalt-based electrode materials for sodium-ion batteries. Chem. Eng. J. 2019, 370, 185.
- Zheng, M.; Chi, Y.; Hu, Q.; Tang, H.; Jiang, X.; Zhang, L.; Zhang, S.; Pang, H.; Xu, Q. Carbon nanotube-based materials for lithium–sulfur batteries. J. Mater. Chem. A 2019, 7, 17204.
- Sharma, L.; Gond, R.; Senthilkumar, B.; Roy, A.; Barpanda, P. Fluorophosphates as efficient bifunctional electrocatalysts for metal air batteries. ACS Catal. 2020, 10, 43.
- Ru, Y.; Zheng, S.; Xue, H.; Pang, H. Potassium cobalt hexacyanoferrate nanocubic assemblies for high performance aqueous aluminum ion batteries. Chem. Eng. J. 2020, 382, 122853.
- Wrogemann, J.M.; Künne, S.; Heckmann, A.; Rodríguez-Pérez, I.A.; Siozios, V.; Yan, B.; Li, J.; Winter, M.; Beltrop, K.; Placke, T. Development of safe and sustainable dual ion batteries through hybrid aqueous/nonaqueous electrolytes. Adv. Energy Mater. 2020, 10, 1902709.
- Zeng, X.; Hao, J.; Wang, Z.; Mao, J.; Guo, Z. Recent progress and perspectives on aqueous zn-based rechargeable batteries with mild aqueous electrolytes. Energy Storage Mater. 2019, 20, 410.
- Kasiri, G.; Glenneberg, J.; Hashemi, A.B.; Kun, R.; Mantia, F.L. Mixed copper-zinc hexacya-noferrates as cathode materials for aqueous zinc-ion batteries. Energy Storage Mater. 2019, 19, 360.
- Jin, T.; Ji, X.; Wang, P.; Zhu, K.; Zhang, J.; Cao, L.; Chen, L.; Cui, C.; Deng, T.; Liu, S.; et al. High energy aqueous sodium-ion batteries. Angew. Chem. Int. Ed. 2021, 60, 11943–11948.
- Zhu, K.; Li, Z.; Jin, T.; Jiao, L. Low defects potassium cobalt hexacyanoferrate as a superior cathode for aqueous potassium ion batteries. J. Mater. Chem. A. 2020, 8, 21103–21109.
- Pang, Q.; Yang, S.; Yu, X.; He, W.; Zhang, S.; Tian, Y.; Xing, M.; Fu, Y.; Luo, X. Realizing reversible storage of trivalent aluminum ions using VOPO4·2H2O nanosheets as cathode material in aqueous aluminum metal batteries. J. Alloy. Compd. 2021, 885, 161008.
- Shi, Z.; Wu, J.; Ni, M.; Guo, Q.; Zan, F.; Xia, H. Superior performance of calcium birnessite by electrochemical conversion as cathode for aqueous calcium ion battery. Mater. Res. Bull. 2021, 144, 111475.
- Fang, G.; Zhou, J.; Pan, A.; Liang, S. Recent advances in aqueous zinc ion batteries. ACS Energy Lett. 2018, 3, 2480–2501.
- Konarov, A.; Voronina, N.; Jo, J.H.; Bakenov, Z.; Sun, Y.; Myung, S. Present and future perspective on electrode materials for rechargeable zinc-ion batteries. ACS Energy Lett. 2018, 3, 2620–2640.
- Ming, J.; Guo, J.; Xia, C.; Wang, W.; Alshareef, H.N. Zinc-ion batteries: Materials, mechanisms, and applications. Mater. Sci. Eng. R Rep. 2019, 135, 58–84.
- Zhang, T.; Tang, Y.; Fang, G.; Zhang, C.; Zhang, H.; Guo, X.; Cao, X.; Zhou, J.; Pan, A.; Liang, S. Electrochemical activation of manganese based cathode in aqueous Zinc-Ion electrolyte. Adv. Funct. Mater. 2020, 30, 2002711.
- Ding, J.; Gao, H.; Ji, D.; Zhao, K.; Wang, S.; Cheng, F. Vanadium-based cathodes for aqueous zinc-ion batteries: From crystal structures, diffusion channels to storage mechanisms. J. Mater. Chem. A 2021, 9, 5258–5275.
- Cao, T.; Zhang, F.; Chen, M.; Shao, T.; Li, Z.; Xu, Q.; Cheng, D.; Liu, H.; Xia, Y. Cubic manganese potassium hexacyanoferrate regulated by controlling of the water and fefects as a high capacity and stable cathode material for rechargeable aqueous zinc-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 26924–26935.
- Lee, W.S.V.; Xiong, T.; Wang, X.; Xue, J. Unraveling MoS2 and transition metal dichalcogenides as functional zinc-ion battery cathode: A perspective. Small Methods 2020, 5, 2000815.
- Wu, J.; Ciucci, F.; Kim, J.-K. Molybdenum disulfide based nanomaterials for rechargeable batteries. Chem. Eur. J. 2020, 26, 6296–6319.
- Zhao, X.; Sui, J.; Li, F.; Fang, H.; Wang, H.; Li, J.; Cai, W.; Cao, G. Lamellar MoSe2 nanosheets embedded with MoO2 nanoparticles: Novel hybrid nanostructures promoted excellent performances for lithium ion batteries. Nanoscale 2016, 8, 17902–17910.
- Wang, L.; Xu, Z.; Wang, W.; Bai, X. Atomic mechanism of dynamic electrochemical lithiation processes of MoS2 nanosheets. J. Am. Chem. Soc. 2014, 136, 6693–6697.
- Zhang, G.; Liu, H.; Qu, J.; Li, J. Two-dimensional layered MoS2: Rational design, properties and electrochemical applications. Energy Environ. Sci. 2016, 9, 1190.
- Kuc, A.; Heine, T. The electronic structure calculations of two-dimensional transition-metal dichalcogenides in the presence of external electric and magnetic fields. Chem. Soc. Rev. 2015, 44, 2603.
- Jiao, Y.; Hafez, A.M.; Cao, D.; Mukhopadhyay, A.; Ma, Y.; Zhu, H. Metallic MoS2 for high performance energy storage and energy conversion. Small 2018, 14, 1800640.
- Wu, D.; Wang, C.; Wu, M.; Chao, Y.; He, P.; Ma, J. Porous bowl-shaped VS2 nanosheets/graphene composite for high rate lithium-ion storage. J. Energy Chem. 2020, 43, 24–32.
- Jing, P.; Lu, H.; Yang, W.; Cao, Y. Interlayer-expanded and binder-free VS2 nanosheets assemblies for enhanced Mg2+ and Li+/Mg2+ hybrid ion storage. Electrochim. Acta 2020, 330, 135263–135272.
- Zhu, J.; Jian, T.; Wu, Y.; Ma, W.; Lu, Y.; Sun, L.; Meng, F.; Wang, B.; Cai, F.; Gao, J.; et al. A highly stable aqueous Zn/VS2 battery based on an intercalation reaction. Appl. Surf. Sci. 2021, 544, 148882.
- Kim, H.J.; Choi, B.K.; Lee, I.H.; Kim, M.J.; Chun, S.; Jozwiak, C.; Bostwick, A.; Rotenberg, E.; Chang, Y.J. Electronic structure and charge-density wave transition in monolayer VS2. Curr. Appl. Phys. 2021, 30, 8–13.
- Liu, J.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. A VS2@N-doped carbon hybrid with strong interfacial interaction for high-performance rechargeable aqueous Zn-ion batteries. J. Mater. Chem. C 2021, 9, 6308–6315.
- Xu, W.; Sun, C.; Zhao, K.; Cheng, X.; Rawal, S.; Xu, Y.; Wang, Y. Defect engineering activating (boosting) zinc storage capacity of MoS2. Energy Storage Mater. 2019, 16, 527–534.
- Li, H.; Yang, Q.; Mo, F.; Liang, G.; Liu, Z.; Tang, Z.; Ma, L.; Liu, J.; Shi, Z.; Zhi, C. MoS2 nanosheets with expanded interlayer spacing for rechargeable aqueous Zn-Ion batteries. Energy Storage Mater. 2019, 19, 94–101.
- Liang, H.; Cao, Z.; Ming, F.; Zhang, W.; Anjum, D.H.; Cui, Y.; Cavallo, L.; Alshareef, H.N. Aqueous Zinc-Ion storage in MoS2 by tuning the intercalation energy. Nano Lett. 2019, 19, 3199.
- Zhang, Z.; Li, W.; Wang, R.; Li, H.; Yan, J.; Jin, Q.; Feng, P.; Wang, K.; Jiang, K. Crystal water assisting MoS2 nanoflowers for reversible zinc storage. J. Alloy. Compd. 2021, 872, 159599.
- Huang, M.; Mai, Y.; Zhao, L.; Liang, X.; Fang, Z.; Jie, X. Tuning the kinetics of zinc ion in MoS2 by polyaniline intercalation. Electrochim. Acta 2021, 388, 138624.
- Huang, M.; Mai, Y.; Zhao, L.; Liang, X.; Fang, Z.; Jie, X. Hierarchical MoS2@CNTs hybrid as a long life and high rate cathode for aqueous rechargeable Zn-Ion batteries. ChemElectroChem 2020, 7, 4218–4223.
- Liu, J.; Xu, P.; Liang, J.; Liu, H.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Boosting aqueous zinc-ion storage in MoS2 via controllable phase. Chem. Eng. J. 2020, 389, 124405.
- He, P.; Yan, M.; Zhang, G.; Sun, R.; Chen, L.; An, Q.; Mai, L. Layered VS2 nanosheet based aqueous zn ion battery cathode. Adv. Energy Mater. 2017, 7, 1601920.
- Jiao, T.; Yang, Q.; Wu, S.; Wang, Z.; Chen, D.; Shen, D.; Liu, B.; Cheng, J.; Li, H.; Ma, L.; et al. Binder-free hierarchical VS2 electrodes for high-performance aqueous zn ion batteries towards commercial level mass loading. J. Mater. Chem. A 2019, 7, 16330.
- Chen, T.; Chen, X.; Zhang, Q.; Li, Y.; Peng, W.; Zhang, F.; Fan, X. VS2 nanosheets vertically grown on graphene as high-performance cathodes for aqueous zinc-ion batteries. J. Power Sources 2021, 477, 228652.
- Pu, X.; Song, T.; Tang, L.; Tao, Y.; Cao, T.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y. Rose-like vanadium disulfide coated by hydrophilic hydroxyvanadium oxide with improved electrochemical performance as cathode material for aqueous zinc-ion batteries. J. Power Sources 2019, 437, 226917.
- Yang, M.; Wang, Z.; Ben, H.; Zhao, M.; Luo, J.; Chen, D.; Lu, Z.; Wang, L.; Liu, C. Boosting the zinc ion storage capacity and cycling stability of interlayer-expanded vanadium disulfide through in-situ electrochemical oxidation strategy. J. Colloid Interface Sci. 2022, 607, 68–75.
- Yu, D.; Wei, Z.; Zhang, X.; Zeng, Y.; Wang, C.; Chen, G.; Shen, Z.X.; Du, F. Boosting Zn2+ and NH4+ storage in aqueous media via in-situ electrochemical induced VS2/VOx heterostructures. Adv. Funct. Mater. 2021, 31, 2008743.
- Tang, B.; Tian, N.; Jiang, J.; Li, Y.; Yang, J.; Zhu, Q. Investigation of zinc storage capacity of WS2 nanosheets for rechargeable aqueous zn-ion batteries. J. Alloy. Compd. 2022, 894, 162391.
- Wu, Z.; Lu, C.; Wang, Y.; Zhang, L.; Jiang, L.; Tian, W.; Cai, C.; Gu, Q.; Sun, Z.; Hu, L. Ultrathin VSe2 nanosheets with fast ion diffusion and robust structural stability for rechargeable zinc-ion battery cathode. Small 2020, 16, 2000698.
More
Information
Subjects:
Electrochemistry
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
19 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




