Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | MEENAKSHI THAKUR | -- | 2131 | 2022-04-18 11:38:29 | | | |
| 2 | Rita Xu | Meta information modification | 2131 | 2022-04-18 11:50:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Thakur, M.; Raza, A.; , .; Prakash, C.S.; Anand, A. Respiratory Control of Crop Yield under High Temperature. Encyclopedia. Available online: https://encyclopedia.pub/entry/21872 (accessed on 13 January 2026).
Thakur M, Raza A, , Prakash CS, Anand A. Respiratory Control of Crop Yield under High Temperature. Encyclopedia. Available at: https://encyclopedia.pub/entry/21872. Accessed January 13, 2026.
Thakur, Meenakshi, Ali Raza, , Channapatna S. Prakash, Anjali Anand. "Respiratory Control of Crop Yield under High Temperature" Encyclopedia, https://encyclopedia.pub/entry/21872 (accessed January 13, 2026).
Thakur, M., Raza, A., , ., Prakash, C.S., & Anand, A. (2022, April 18). Respiratory Control of Crop Yield under High Temperature. In Encyclopedia. https://encyclopedia.pub/entry/21872
Thakur, Meenakshi, et al. "Respiratory Control of Crop Yield under High Temperature." Encyclopedia. Web. 18 April, 2022.
Copy Citation
Respiration and photosynthesis are indispensable plant metabolic processes that are affected by elevated temperatures leading to disruption of the carbon economy of the plants. Increasing global temperatures impose yield penalties in major staple crops that are attributed to increased respiratory carbon loss, through higher maintenance respiration resulting in a shortage of non-structural carbohydrates and an increase in metabolic processes like protein turnover and maintenance of ion concentration gradients.
acclimation
alternative oxidase
heat stress
mitochondria
1. Introduction
The rising temperature is an intrinsic component of global climate change that controls the carbon fluxes in all the crops. High temperature affects the major plant physiological processes, such as photosynthesis and respiration; therefore, it becomes important to estimate the plant carbon dioxide (CO2) balance that finally decides the crop productivity [1][2][3].Through these two pathways, the terrestrial ecosystems exchange about 120 Gt of carbon per year with the atmosphere [4]. A rough estimate states that half of the CO2 assimilated annually through photosynthesis is released back to the atmosphere by plant respiration [5][6][7], and merely 15–25% of the fixed carbon finally translates into yield [8][9]. The projected elevation in temperature beyond 2.0 °C by the end of the decade [10] may increase the magnitude of carbon loss exponentially in the physiological temperature range of 0 to 38 °C [11], which will further exacerbate in a species-and environment-dependent manner at higher temperatures between 48 and 60 °C [12][13][14][15].
The carbon lost through the ‘breathing out’ processes in plants can occur via two mechanisms, namely photorespiration and dark/mitochondrial respiration. These processes release CO2, but dark respiration occurs regardless of light in the plant cells [16][17]. Biochemically, dark respiration is an enzymatically regulated, multistep, amphibolic process that produces ATP by the oxidation of glucose formed during photosynthesis. Glucose is initially broken into pyruvate during glycolysis, which is oxidized to form acetyl-CoA, releasing a molecule of CO2. The acetyl-CoA then enters the tricarboxylic acid (TCA) cycle, where it is oxidized to CO2 and also produces reductants (nicotinamide adenine dinucleotide: NADH; dihydroflavine-adenine dinucleotide: FADH2) that pass through the mitochondrial electron transport chain (ETC). The oxidation of the reductants produces a proton gradient across the inner membrane of the mitochondria that drives the synthesis of ATP. High temperatures impact dark respiration in plants with an exponential increase [18], which can become detrimental due to irreversible damage to the enzymatic machinery [15]. Climate change prediction models have speculated a 3–20% decline in the yield of major crops like wheat, rice, maize, and soybean with every 1 °C increase in the global mean temperatures [19][20], which makes it pertinent to relate this loss to the waste of carbon due to respiration.
2. Respiratory Carbon Loss-A Constraint to Crop Yield
Respiration, rather than photosynthesis, may be the primary contributor to yield losses in a high temperature climate [11]. Low respiration rates are generally correlated with high crop yields [21][22]. Walker et al. [23] reported that photorespiration decreased soybean and wheat yields by 36% and 20%, respectively, in the United States. In another study, a 10–12% and 17–35% decrease in the yields of wheat and rice, respectively, was reported due to high temperatures [24]. The yield loss in wheat and rice due to high night temperature (HNT) is mainly ascribed to higher dark respiration, which increases the consumption of photoassimilates, thereby resulting in the reduction of non-structural carbohydrates (NSCs) in stem tissues [25][26]. Glaubitz et al. [27] reported that increasing night temperature from 25 °C to 35 °C resulted in increased leaf respiratory carbon losses in grapevines, as reflected by the decrease in NSCs of 0.025 and 0.041 mg g−1dry weight, respectively. Such losses are consistent with metabolite profiling studies in wheat and rice, which revealed an increase in TCA cycle intermediates in leaves exposed to HNT, supporting increased respiration in the photosynthesizing tissue [25][28]. Xu et al. [29] suggest that increased dark respiration restrains source availability under the combined stress of high day and night temperatures, leading to a considerably more severe yield penalty due to carbon loss.
3. Heat-Induced Changes in the Proportion of Maintenance Respiration
Dark respiration (Rd) is typically partitioned into two functional components, i.e., growth respiration (Rg) and maintenance respiration (Rm), which are impacted upon by environmental stresses [9][30][31]. Figure 1 illustrates the differences in these components under elevated temperatures. Growth respiration is a dominant component of respiration in younger tissues, while the latter contributes majorly to the older tissues [32]. Growth respiration is defined as the amount of photoassimilates respired to provide energy for the synthesis of additional biomass [33]. It also provides a carbon skeleton and reductants to facilitate nutrient uptake/assimilation followed by biosynthesis of cellular components to drive the growth of tissues. Thus, the relationship between the growth rates of a species and temperature is actually a measure of the rate of the growth respiration component [34]. A recent analysis of 101 evergreen species growing in different biomes (boreal to tropical) showed that respiration increased with an increase in growth temperatures in accordance with previous studies [35][36]. Leaf form accounted for the response ratio of Rg to warming, as species with needle-like leaves had a significantly higher response (25 ± 9%) than broad-leaved ones [36].
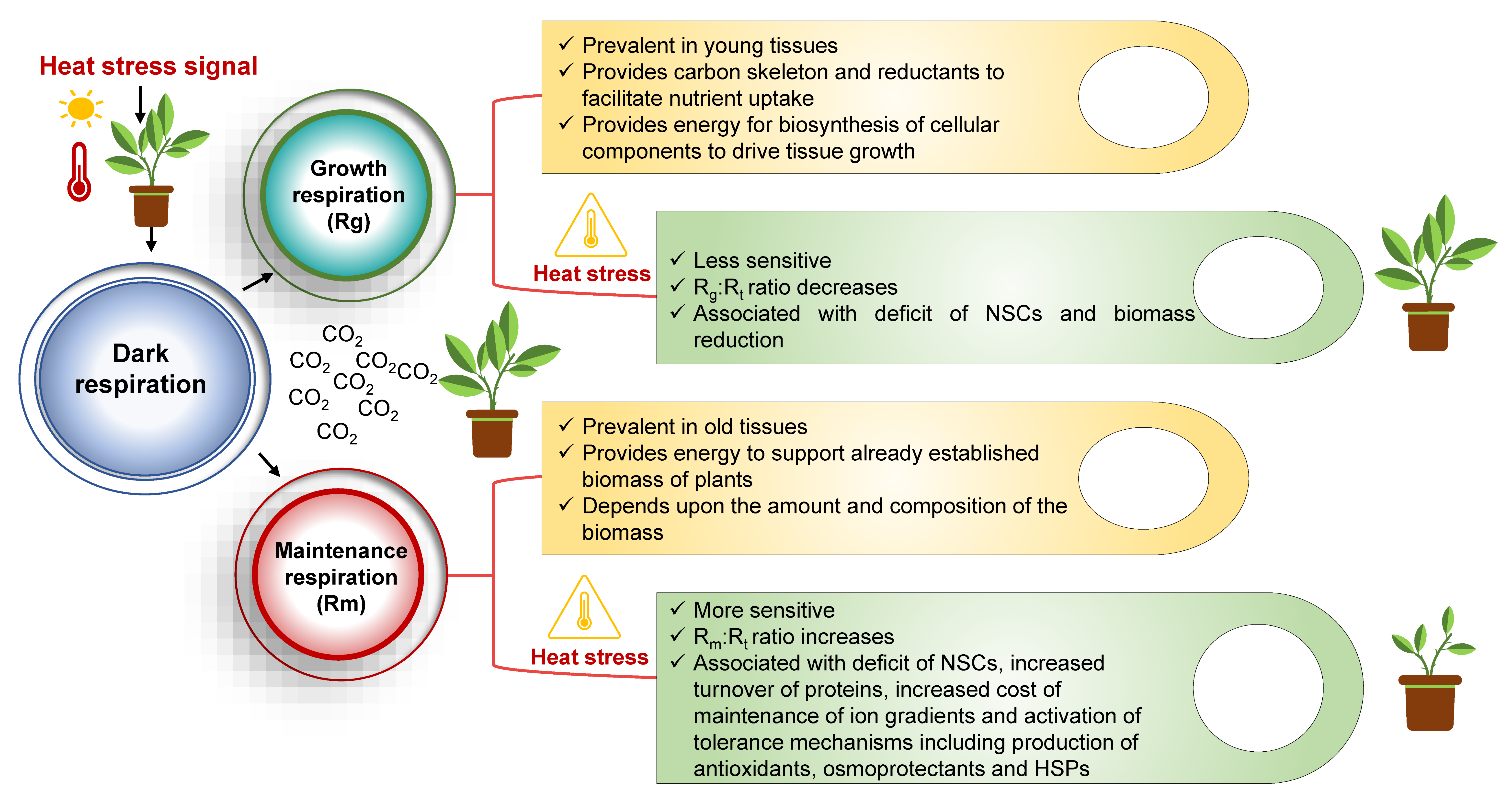
Figure 1. Growth respiration and maintenance respiration under elevated environmental temperature. HSPs: heat shock proteins; NSCs: non-structural carbohydrates; Rg: growth respiration; Rm: maintenance respiration; Rt: total respiration.
On the other hand, maintenance respiration comprises the respiratory processes that help in supporting the already established biomass of the plant [33]. It depends upon the amount and composition of the biomass, as both these factors undergo change depending on the environment and developmental stage of the plant. Although the role of both the components is integral to the life cycle of the plants, their estimation can only be done by employing physiological models [32][37][38][39]. The higher temperature responsiveness of Rm over Rg in mature tissues was concluded from various studies, e.g., Marigolds when exposed to a 10 °C increase in temperature resulted in a 43% to 55% increase in the proportion of maintenance respiration to total respiration (Rt) [40]. Additionally, a significant reduction in ATP content and total biomass was observed in rice plants subjected to 10 °C higher temperature at the reproductive stage than the ambient temperature (28 °C), thereby suggesting that energy produced by respiration under high temperature conditions was mainly attributed to maintenance respiration rather than growth respiration [32]. Mathematically, maintenance respiration is expressed as the product of maintenance respiration coefficient and plant size. The Q10 value (proportional increase in rate of respiration with a 10 °C rise in temperature) of the maintenance respiration coefficient varies between 1.35 and 3.0 depending upon the species, developmental stage, and environmental conditions as shown in the data compiled from various studies (Table 1). The sensitivity of Q10 to temperature indicates that the response of respiration to temperature cannot be represented by one value.
Table 1. Q10 values for maintenance respiration coefficient in various crops.
| Crop | Experimental Temperature | Q10 Value | Reference |
|---|---|---|---|
| Marigold (Tagetes patula) | 20 °C (Control) 30 °C (Elevated) |
1.35–1.55 | [40] |
| Barley (Hordeum vulgare) | 15 °C (Control) 28 °C (Elevated) |
3.00 | [41] |
| Subterranean clover (Trifolium subterraneum) | 10 °C (Control) 35 °C (Elevated) |
1.85 | [42] |
| Japanese knotweed (Reynoutria japonica) |
15 °C (Control) 25 °C (Elevated) |
1.90 | [43] |
| Wheat (Triticum aestivum) | 15 °C (Control) 20 °C (Elevated) |
1.80 | [44] |
| 10 °C (Control-Night temperature) 21 °C (Elevated-Night temperature) |
1.97 | [45] |
4. Substrate Availability for Respiration under High Temperature
The considerable variation observed in the Q10–temperature relationship is influenced by the supply of the respiratory substrate and the respiration capacity [4][46]. Environmental variables that affect the biosynthesis of the substrates [18][46] or increase the metabolism of energy consuming processes like turnover of proteins and maintenance of ion gradients [47], make Q10 values highly dynamic in response to temperature. Additional energy costs are incurred by mechanisms imparting heat tolerance in the crops, e.g., upregulation of the antioxidant defense system to counteract the upsurge in the level of reactive oxygen species (ROS), synthesis of osmoprotectants, and accretion of heat shock proteins (HSPs). The need for respiratory substrate in the plants is mainly met from the non-structural carbohydrates [25][26][27][48] and the protein turnover [11][32]. Studies on the effect of elevated night temperatures have shown that the high rate of nighttime respiration exerted pressure on the supply of NSCs, which subsequently reduced the biomass and yield of rice [25][26]. The concentration of sugars has been positively correlated with the rate of dark respiration in Pinus [49], Quercus rubra [50], and Spinacia oleracea [51]. The light control of carbohydrate synthesis affected the rate of dark respiration in Geum urbanum plants grown under 75% shade as it declined due to limited photosynthate supply, but Q10 declined only when the leaves experienced near darkness for long periods. It was concluded that intense shade for a prolonged period would cause a reduction in both respiration and Q10 due to adenylate restriction on respiration in addition to the substrate availability [4].
5. Regulation of Respiratory Flux at High Temperature
Adenylates (in particular the ratio of ATP to ADP and the concentration of ADP per se), are likely the most important in regulating respiratory flux at warm temperatures [52]. Adenylate control would indicate that the respiratory capacity at warmer temperatures exceeded the level required for cell processes to proceed [18], which in turn would lead to elevated ATP:ADP ratios or low ADP concentration, causing downregulation of respiration [53]. The increased leakiness of membranes at high temperatures could further contribute to substrate limitation because concentration gradients of TCA cycle intermediates are more difficult to maintain when mitochondrial membranes are excessively fluid [18].
6. Positive Correlation between Protein Turnover Cost and Respiratory Cost at High Temperature
Nitrogen (N) utilization processes, including nitrate reduction and ammonium assimilation, are thought to have high respiratory costs [54]. In fact, the estimates of construction respiration are greatly influenced by the form of N source, e.g., nitrate or ammonium [55]. The protein turnover rate increases with temperature, suggesting that the protein turnover cost is a major component of the N-utilization cost and dominates during maintenance respiration. Hachiya et al. [56] studied the protein turnover cost in Petunia x hybrida petals grown at three different temperatures (20, 25,and 35 °C) during the development of the petals. Most petals are non-photosynthetic; therefore, ATP and reducing equivalents are supplied mainly from the respiratory pathway. The integrated protein turnover cost on dry weight basis was similar between 20 and 25 °C but increased by more than four times at 35 °C, suggesting that the high temperature enhanced the cost of protein turnover, thereby increasing the total cost of N-utilization along with respiration in the petals.
7. Diurnal Dynamics of Respiration
The diurnal or diel cycle of plant growth interacts with the respiratory metabolism, which can be directly linked with the availability of respiratory metabolites regulating the process at different times of the day [57]. The photosynthate synthesized during the day supports carbon supply for the entire plant during the day, which is reduced to critical levels by the end of the night [58]. The strong coupling between carbon fixation through photosynthesis and loss due to respiration [59] indicates the diurnal fluctuation in rates of dark respiration as a result of changes in the concentration of various metabolites supporting the respiratory process [60]. In this case, the supply of sugars is stabilized over the day–night cycle, and the diel variation in respiration may be explained by changes in the availability of amino acids, proteins, organic acids, and/or lipids. These metabolites may drive respiration by supplying intermediates to the TCA cycle, reductants for ATP synthesis via oxidative phosphorylation, and carbon skeletons required for biosynthesis or nitrogen assimilation into amino acids [61].
Metabolomic studies have shown that warmer day (30 °C) and night (28 °C) temperatures lead to the accumulation of amino acids derived from shikimate pathways, such as phenylalanine, tyrosine, tryptophan, aspartic acid, lysine, proline, and ɣ-amino butyrate (GABA), in thermo-sensitive rice cultivars (DR2 and M202) but not in intermediate (IR64 and IRRI123) and temperature-tolerant cultivars (IR72 and Taipei 309) [28]. Similarly, in wheat, high night temperatures showed a prevalence of fumarate and alanine without any significant change in the level of glutamine, glutamate, and GABA [25]. The accumulation of TCA intermediates like malate and fumarate during the day, and citrate, aconitate, and succinate during the night [62][63], reiterates the circadian control of the TCA pathway, which is a hub for the process of respiration and can be markedly influenced by an increase in temperature [64]. Rashid et al. [64] assessed the influence of growth temperature and the diel cycle on the concentrations of metabolites involved in the respiratory network of rice. They raised the plants under 25 °C:20 °C, 30 °C:25 °C, and 40 °C:35 °C day:night cycles and measured the dark respiration and changes in metabolites at five time points spanning a single 24 h period and observed that shikimate pathway-derived aromatic amino acids were the only metabolites to interact in response to both the growth temperature and the day:night cycle. Cook et al. [65] reported increased concentrations of α-ketoglutarate, fumarate, malate, and citrate in Arabidopsis leaves when cooled from 20 °C to 4 °C. All these studies suggest that there are distinct respiratory metabolite adjustments to temperature and the diel cycle.Further, detailed experiments on the interaction of the diel cycle and temperature will generate a better understanding of the metabolites controlling dark respiration in plants. Therefore, the instantaneous measurement of respiration rates at a single point during the day can overlook the differential response prevalent during an extended period.
References
- Hüve, K.; Bichele, I.; Ivanova, H.; Keerberg, O.; Pärnik, T.; Rasulov, B.; Tobias, M.; Niinemets, U. Temperature responses of dark respiration in relation to leaf sugar concentration. Physiol. Plant. 2012, 144, 320–334.
- Heskel, M.A.; O’sullivan, O.S.; Reich, P.B.; Tjoelker, M.G.; Weerasinghe, L.K.; Penillard, A.; Atkin, O.K. Convergence in the temperature response of leaf respiration across biomes and plant functional types. Proc. Natl. Acad. Sci. USA 2016, 113, 3832–3837.
- Sadok, W.; Jagadish, S.K. The hidden costs of nighttime warming on yields. Trends Plant Sci. 2020, 25, 644–651.
- Schlesinger, W.H. Biogeochemistry: An Analysis of Global Change, 2nd ed.; Academic Press: San Diego, CA, USA, 1997.
- Amthor, J.S. Terrestrial higher-plant response to increasing atmospheric in relation to the global carbon cycle. Glob. Chang. Biol. 1995, 1, 243–274 .
- Waring, R.H.; Landsberg, J.J.; Williams, M. Net primary production of forests: A constant fraction of gross primary production? Tree Physiol. 1998, 18, 129–134.
- Gifford, R.M. The global carbon cycle: A viewpoint on the missing sink. Aust. J. Plant Physiol. 1994, 21, 1–15.
- Gifford, R.M.; Thorne, J.H.; Hitz, W.D.; Giaquinta, R.T. Crop productivity and photoassimilate partitioning. Science 1984, 225, 801–808.
- Amthor, J.S. Evolution and applicability of a whole plant respiration model. J. Theor. Biol. 1986, 122, 473–490 .
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Pean, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. 2022; Cambridge University Press: Cambridge, UK, 2021.
- Li, G.; Chen, T.; Feng, B.; Peng, S.; Tao, L.; Fu, G. Respiration, rather than photosynthesis, determines rice yield loss under moderate high-temperature conditions. Front. Plant Sci. 2021, 12, 1287.
- Tjoelker, M.G.; Oleksyn, J.; Reich, P.B. Modelling respiration of vegetation: Evidence for a general temperature-dependent Q10. Glob. Chang. Biol. 2001, 7, 223–230.
- Heskel, M.A.; Bitterman, D.; Atkin, O.K.; Turnbull, M.H.; Griffin, K.L. Seasonality of foliar respiration in two dominant plant species from the Arctic tundra: Response to long-term warming and short-term temperature variability. Funct. Plant Biol. 2014, 41, 287–300.
- Weerasinghe, L.K.; Creek, D.; Crous, K.Y.; Xiang, S.; Liddell, M.J.; Turnbull, M.H.; Atkin, O.K. Canopy position affects the relationships between leaf respiration and associated traits in a tropical rainforest in Far North Queensland. Tree Physiol. 2014, 34, 564–584.
- O’Sullivan, O.S.; Heskel, M.A.; Reich, P.B.; Tjoelker, M.G.; Weerasinghe, L.K.; Penillard, A.; Zhu, L.L.; Egerton, J.J.G.; Bloomfield, K.J.; Creek, D.; et al. Thermal limits of leaf metabolism across biomes. Glob. Chang. Biol. 2017, 23, 209–223.
- Kromer, S. Respiration during photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 45–70.
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2018, 221, 32–49.
- Atkin, O.K.; Tjoelker, M.G. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003, 8, 343–351.
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331.
- Cavanagh, A.P.; South, P.F.; Bernacchi, C.J.; Ort, D.R. Alternative pathway to photorespiration protects growth and productivity at elevated temperatures in a model crop. Plant Biotechnol. J. 2021.
- Nunes-Nesi, A.; Carrari, F.; Lytovchenko, A.; Smith, A.M.; Loureiro, M.E.; Ratcliffe, R.G.; Sweetlove, L.J.; Fernie, A.R. Enhanced photosynthetic performance and growth as a consequence of decreasing mitochondrial malate dehydrogenase activity in transgenic tomato plants. Plant Physiol. 2005, 137, 611–622.
- Hauben, M.; Haesendonckx, B.; Standaert, E.; Van Der Kelen, K.; Azmi, A.; Akpo, H.; Van Breusegem, F.; Guisez, Y.; Bots, M.; Lambert, B.; et al. Energy use efficiency is characterized by an epigenetic component that can be directed through artificial selection to increase yield. Proc. Natl. Acad. Sci. USA 2009, 106, 20109–20114.
- Walker, B.J.; VanLoocke, A.; Bernacchi, C.J.; Ort, D.R. The costs of photorespiration to food production now and in the future. Annu. Rev. Plant Biol. 2016, 67, 107–129.
- Cai, C.; Yin, X.; He, S.; Jiang, W.; Si, C.; Struik, P.C.; Luo, W.; Li, G.; Xie, Y.; Xiong, Y.; et al. Responses of wheat and rice to factorial combinations of ambient and elevated CO2 and temperature in FACE experiments. Glob. Chang. Biol. 2016, 22, 856–874.
- Impa, S.M.; Raju, B.; Hein, N.T.; Sandhu, J.; Prasad, P.V.V.; Walia, H.; Jagadish, S.K. High night temperature effects on wheat and rice: Current status and way forward. Plant Cell Environ. 2021, 44, 2049–2065.
- Xu, J.; Misra, G.; Sreenivasulu, N.; Henry, A. What happens at night? Physiological mechanisms related to maintaining grain yield under high night temperature in rice. Plant Cell Environ. 2021, 44, 2245–2261.
- Tombesi, S.; Cincera, I.; Frioni, T.; Ughini, V.; Gatti, M.; Palliotti, A.; Poni, S. Relationship among night temperature, carbohydrate translocation and inhibition of grapevine leaf photosynthesis. Environ. Exp. Bot. 2019, 157, 293–298 .
- Glaubitz, U.; Erban, A.; Kopka, J.; Hincha, D.K.; Zuther, E. High night temperature strongly impacts TCA cycle, amino acid and polyamine biosynthetic pathways in rice in a sensitivity-dependent manner. J. Exp. Bot. 2015, 66, 6385–6397.
- Xu, J.; Henry, A.; Sreenivasulu, N. Rice yield formation under high day and night temperatures-A prerequisite to ensure future food security. Plant Cell Environ. 2020, 43, 1595–1608.
- Lambers, H. Respiration in intact plants and tissues: Its regulation and dependence on environmental factors, metabolism and invaded organisms. In Higher Plant Cell Respiration; Douce, R., Day, D.A., Eds.; (Encyclopedia of Plant Physiology), New Series; Springer: Berlin, Germany, 1985; Volume 18.
- Slot, M.; Kitajima, K. General patterns of acclimation of leaf respiration to elevated temperatures across biomes and plant types. Oecologia 2015, 177, 885–900.
- Amthor, J.S.; Bar-Even, A.; Hanson, A.D.; Millar, A.H.; Stitt, M.; Sweetlove, L.J.; Tyerman, S.D. Engineering strategies to boost crop productivity by cutting respiratory carbon loss. Plant Cell 2019, 31, 297–314.
- Amthor, J.S. The McCree-de Wit-Penning de Vries-Thornley respiration paradigms: 30 years later. Ann. Bot. 2000, 86, 1–20 .
- Adu-Bredu, S.; Yokota, T.; Hagihara, A. Temperature effect on maintenance and growth respiration coefficients of young, field-grown hinoki cypress (Chamaecyparisobtusa). Ecol. Res. 1997, 12, 357–362.
- Atkin, O.K.; Bloomfield, K.J.; Reich, P.B.; Tjoelker, M.G.; Asner, G.P.; Bonal, D.; Bönisch, G.; Bradford, M.G.; Cernusak, L.; Cosio, E.; et al. Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytol. 2015, 206, 614–636.
- Crous, K.Y.; Uddling, J.; De Kauwe, M.G. Temperature responses of photosynthesis and respiration in evergreen trees from boreal to tropical latitudes. New Phytol. 2022, 234, 353–374.
- Thornley, J.H.M. Respiration, growth and maintenance in plants. Nature 1970, 227, 304–305.
- McCree, K.J. Maintenance requirements of white clover at high and low growth rates. Crop Sci. 1982, 22, 345–351.
- Thornley, J.H.M.; Johnson, I.R. Plant and Crop Modeling. Clarendon Press Oxford: Oxford, UK, 1990.
- Van Iersel, M.W. Respiratory Q10 of marigold (Tagetes patula) in response to long-term temperature differences and its relationship to growth and maintenance respiration. Physiol. Plant. 2006, 128, 289–301.
- Winzeler, H.; Hunt, L.A.; Mahon, J.D. Ontogenetic changes in respiration and photosynthesis in a uniculm barley. Crop Sci. 1976, 16, 786–790.
- McCree, K.J.; Silsbury, J.H. Growth and maintenance requirements of subterranean clover. Crop Sci. 1978, 18, 13–18.
- Mariko, S.; Koizumi, H. Respiration for maintenance and growth in Reynoutria japonica ecotypes from different altitudes on Mt Fuji. Ecol. Res. 1993, 8, 241–246.
- Gifford, R.M. Whole plant respiration and photosynthesis of wheat under increased CO2 concentration and temperature: Long-term vs. short-term distinctions for modelling. Glob. Chang. Biol. 1995, 1, 385–396.
- Tan, K.; Zhou, G.; Ren, S. Response of leaf dark respiration of winter wheat to changes in CO2 concentration and temperature. Chin. Sci. Bull. 2013, 58, 1795–1800.
- Atkin, O.K.; Bruhn, D.; Tjoelker, M.G. Response of plant respiration to changes in temperature: Mechanisms and consequences of variations in Q10 values and acclimation. In Advances in Photosynthesis and Respiration, Plant Respiration; Lambers, H., Ribas-Carbo, M., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 18.
- Atkin, O.K.; Macherel, D. The crucial role of plant mitochondria in orchestrating drought tolerance. Ann. Bot. 2009, 103, 581–597.
- Thakur, M.; Sharma, P.; Anand, A.; Pandita, V.K.; Bhatia, A.; Pushkar, S. Raffinose and hexose sugar content during germination are related to infrared thermal fingerprints of primed onion (Allium cepa L.) seeds. Front. Plant Sci. 2020, 11, 579037.
- Ögren, E. Maintenance respiration correlates with sugar but not nitrogen concentration in dormant plants. Physiol. Plant. 2000, 108, 295–299.
- Whitehead, D.; Griffin, K.L.; Turnbull, M.H.; Tissue, D.T.; Engel, V.C.; Brown, K.J.; Schuster, W.S.; Walcroft, A.S. Response of total night-time respiration to differences in total daily photosynthesis for leaves in a Quercus rubra L. canopy: Implications for modelling canopy CO2 exchange. Glob. Chang. Biol. 2004, 10, 925–938.
- Noguchi, K.; Terashima, I. Different regulation of leaf respiration between Spinacia oleracea, a sun species, and Alocasia odora, a shade species. Physiol. Plant. 1997, 101, 1–7.
- Hoefnagel, M.H. Interdependence between chloroplasts and mitochondria in the light and the dark. Biochim. Biophys. ActaBioenerg. 1998, 1366, 235–255.
- Douce, R.; Neuburger, M. The uniqueness of plant mitochondria. Annu. Rev. Plant Biol. 1980, 40, 371–414.
- Zerihun, A.; Mckenzie, B.A.; Morton, J.D. Photosynthate costs associated with the utilization of different nitrogen-forms: Influence on the carbon balance of plants and shoot-root biomass partitioning. New Phytol. 1998, 138, 1–11.
- Williams, K.; Percival, F.; Merino, J.; Mooney, H.A. Estimation of tissue construction cost from heat of combustion and organic nitrogen content. Plant Cell Environ. 1987, 10, 725–734.
- Hachiya, T.; Terashima, I.; Noguchi, K. Increase in respiratory cost at high growth temperature is attributed to high protein turnover cost in Petunia x hybrida petals. Plant Cell Environ. 2007, 30, 1269–1283.
- Lee, C.P.; Eubel, H.; Millar, A.H. Diurnal changes in mitochondrial function reveal daily optimization of light and dark respiratory metabolism in Arabidopsis. Mol. Cell. Proteom. 2010, 9, 2125–2139.
- Blasing, O.E.; Gibon, Y.; Günther, M.; Höhne, M.; Morcuende, R.; Osuna, D.; Thimm, O.; Usadel, B.; Scheible, W.R.; Stitt, M. Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 2005, 17, 3257–3281.
- Shameer, S.; Ratcliffe, R.G.; Sweetlove, L.J. Leaf energy balance requires mitochondrial respiration and export of chloroplast NADPH in the light. Plant Physiol. 2019, 180, 1947–1961 .
- Gibon, Y.; Pyl, E.-T.; Sulpice, R.; Lunn, J.E.; Hohne, M.; Gunther, M.; Stitt, M. Adjustment of growth, starch turnover, protein content and central metabolism to a decrease of the carbon supply when Arabidopsis is grown in very short photoperiods. Plant Cell Environ. 2009, 32, 859–874.
- O’Leary, B.M.; Lee, C.P.; Atkin, O.K.; Cheng, R.; Brown, T.B.; Millar, A.H. Variation in leaf respiration rates at night correlates with carbohydrate and amino acid supply. Plant Physiol. 2017, 174, 2261–2273.
- Gibon, Y.; Usadel, B.; Blaesing, O.E.; Kamlage, B.; Hoehne, M.; Trethewey, R.; Stitt, M. Integration of metabolite with transcript and enzyme activity profiling during diurnal cycles in Arabidopsis rosettes. Genome Biol. 2006, 7, R76.
- Watanabe, C.; Sato, S.; Yanagisawa, S.; Uesono, Y.; Terashima, I.; Noguchi, K. Effects of elevated CO2 on levels of primary metabolites and transcripts of genes encoding respiratory enzymes and their diurnal patterns in Arabidopsis thaliana: Possible relationships with respiratory rates. Plant Cell Physiol. 2014, 55, 341–357.
- Rashid, F.A.A.; Crisp, P.A.; Zhang, Y.; Berkowitz, O.; Pogson, B.J.; Day, D.A.; Masle, J.; Dewar, R.C.; Whelan, J.; Atkin, O.K. Molecular and physiological responses during thermal acclimation of leaf photosynthesis and respiration in rice. Plant Cell Environ. 2020, 43, 594–610.
- Cook, D.; Fowler, S.; Fiehn, O.; Thomashow, M. A prominent role for the CBF cold response pathway in configuring the low-temperature metabolome of Arabidopsis. Proc. Natl. Acad. Sci.USA 2004, 101, 15243–15248.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
18 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




