Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Abraham Wall-Medrano | -- | 2084 | 2022-04-15 15:51:57 | | | |
| 2 | Beatrix Zheng | + 1 word(s) | 2085 | 2022-04-18 04:39:49 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wall-Medrano, A.; , .; Palomo, I.; Fuentes, E.; Villegas-Ochoa, M.A.; González-Aguilar, G.; Olivas-Aguirre, F. Health Effects of Sweet Potato (Ipomoea batatas L.). Encyclopedia. Available online: https://encyclopedia.pub/entry/21822 (accessed on 07 February 2026).
Wall-Medrano A, , Palomo I, Fuentes E, Villegas-Ochoa MA, González-Aguilar G, et al. Health Effects of Sweet Potato (Ipomoea batatas L.). Encyclopedia. Available at: https://encyclopedia.pub/entry/21822. Accessed February 07, 2026.
Wall-Medrano, Abraham, , Ivan Palomo, Eduardo Fuentes, Mónica Alejandra Villegas-Ochoa, Gustavo González-Aguilar, Francisco Olivas-Aguirre. "Health Effects of Sweet Potato (Ipomoea batatas L.)" Encyclopedia, https://encyclopedia.pub/entry/21822 (accessed February 07, 2026).
Wall-Medrano, A., , ., Palomo, I., Fuentes, E., Villegas-Ochoa, M.A., González-Aguilar, G., & Olivas-Aguirre, F. (2022, April 15). Health Effects of Sweet Potato (Ipomoea batatas L.). In Encyclopedia. https://encyclopedia.pub/entry/21822
Wall-Medrano, Abraham, et al. "Health Effects of Sweet Potato (Ipomoea batatas L.)." Encyclopedia. Web. 15 April, 2022.
Copy Citation
Sweet potato (SP; Ipomoea batatas (L.) Lam) is an edible tuber native to America and the sixth most important food crop worldwide. China leads its production in a global market of USD 45 trillion. SP domesticated varieties differ in specific phenotypic/genotypic traits, yet all of them are rich in sugars, slow digestible/resistant starch, vitamins, minerals, bioactive proteins and lipids, carotenoids, polyphenols, ascorbic acid, alkaloids, coumarins, and saponins, in a genotype-dependent manner. Individually or synergistically, SP’s phytochemicals help to prevent many illnesses, including certain types of cancers and cardiovascular disorders.
antioxidants
sweet potato
Ipomoea batatas
cancer
carotenoids
phenolic compounds
1. Introduction
The research of edible roots and tubers (R&T) has attracted the attention of researchers worldwide. Research published to date ranges from their economic and cultural dimensions to their nutritional/functional value as staple foods for certain countries [1][2][3][4]. Among R&T, sweet potato (SP; I. batatas (L.) Lam; also known as ‘boniato’, ‘moniato’, ‘caiapo’, ‘kumara’ or ‘kumera’) with its >1600 species, has been a major staple food for certain ancient populations for centuries [1][2]. In fact, archaeobotanical and epigraphic evidence allows the researchers to affirm that SP was and continues to be an ingredient widely used to make different drinks and foods, both sweet and salty, in populations of diverse cultures [3][4].
The genus Ipomoea belongs to the Convolvulaceae family, and 600–800 species have been identified by cytogenetics [5][6]. Most of them exhibit health-promoting bioactivities, such as those related to their phytochemical profile: anti-inflammatory (I. cairica), anti-constipation (I. digitata), analgesic (I. stans), antidiabetic and hypotensive (I. aquatica, I. batatas), hemostatic and vasoconstrictor (I. tricolor), psychotomimetic (I. muelleri, I. violacea) and anticancer (I. horsfalliae, I. turpethum) activities [7]. Sweet potato (SP; I. batatas (L.) Lam), contains a wide range of nutrients and xenobiotic phytochemicals with antioxidant, anti-nyctalopia/xerophthalmia, hepatoprotective/spasmolytic, anticoagulant/anti-HIV antibacterial, and antidiabetic potential. Particularly, specific anticancer bioactives (e.g., phenolic acids, carotenoids, and peptides) present in the aerial (leaves, steams, talks) and non-aerial (storage roots) parts of SP suggest phenotype/varietal-specific benefits [8][9][10][11][12]. It is noteworthy that certain phenotypic traits of SP genotypes are closely related to their functional/nutraceutical value: the peel and flesh (central parenchyma) pigmentation, going from white-creamy to dark purple (Figure 1) is related to their phenolic and carotenoid content [13][14][15][16].
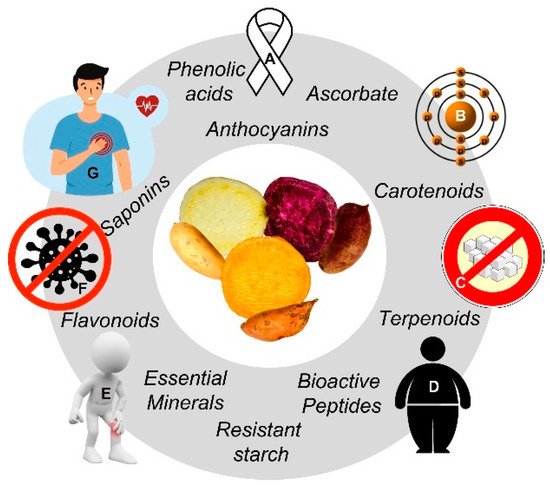 Figure 1. Sweet potato (SP; Ipomoea batatas L.) group of phytochemicals with associated health-promoting effects. Preventive actions (clockwise): Immunocompromise (A), prooxidant (B), diabetes (C), adiposity (D), inflammatory (E), infection (F), cardiovascular (G) diseases/metabolic rearrangements. Source: The authors (CC (by/nc/sa)-licensed clip art).
Figure 1. Sweet potato (SP; Ipomoea batatas L.) group of phytochemicals with associated health-promoting effects. Preventive actions (clockwise): Immunocompromise (A), prooxidant (B), diabetes (C), adiposity (D), inflammatory (E), infection (F), cardiovascular (G) diseases/metabolic rearrangements. Source: The authors (CC (by/nc/sa)-licensed clip art).However, food processing and preservation [13][14][17][18][19] and the gastrointestinal fate of its phytochemicals [20][21][22] may hinder the health-promoting potential of SP. The aim of this narrative research is to provide an update on SP’s botany/molecular phylogeny, agroindustry, and product commercialization/technological diversification, as well as the nutritional/functional value of SP’s major genotypes (by flesh color) and certain health effects (cancer chemoprevention and cardiovascular health promotion). Certain physiological considerations to ensure SP’s health benefits are discussed shortly.
2. Health Effects and Metabolic Fate of SP’s Phytochemicals
Once the diversity of nutrient and non-nutrient compounds has been examined, it is not surprising to find multiple reviews in the literature highlighting the biological activities attributable to SP in the maintenance of optimal nutritional states, and in the prevention of various diseases [8][9][10][11][12][13][14][15][16]. SP’s antioxidant, antimicrobial, anti-diabetic, anti-cancer, anti-inflammatory, hepatoprotective, neuroprotective, anti-obesity, and GI-health-promoting properties have been extensively reviewed [23][24][25]. However, the subsequent section focuses on relevant information on the anticancer and anti-cardiovascular disease (CVD), pathologies with the highest morbidity/mortality rates worldwide, in which SP can contribute to conventional clinical treatments as alternative adjuvants.
2.1. SP and Cancer
Plant bioactives exert many benefits in cancer chemoprevention; cyto/genotoxicity, cell cycle arrest, pro-apoptosis, intracellular signaling, immunomodulation, and anti-angiogenesis are probably the most studied mechanisms. Specifically, a robust body of evidence indicates that certain antioxidant phytochemicals, such as phenolic compounds, carotenoids, ascorbate, and antioxidant dietary fiber and RS, can halt the progression of certain types of cancer cells in vitro and ex vivo, although their effectiveness under clinical conditions remains uncertain. Personalized nutrition for cancer patients demands a continuous search for newer sources of phytochemicals to be used in complementary and alternative medicine. Several studies carried out in recent years [26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44] have reported multiple control points determining the process of initiation, promotion, or the spread of cancer (Table 1).
Table 1. Bioactive compounds in SP and their role against cancer.
| Variety | Phytochemical | Mechanism | Action |
|---|---|---|---|
| Initiation | |||
| Tainong 57 | Trypsin inhibitor |
DNA damage reparation |
↑ P53 leukemic cells |
| -- | Polyphenols | ↓ ROS | ↓ Oxidative damage induced by H2O2 in HepG2 cells. |
| Mixuan No. 1 | Protein hydrolysate |
↓ ROS | ↑ antioxidant activity, ↓ oxidative damage to DNA |
| Ayamurasaki | Anthocyanins | ↓ROS | ↓ Oxidative damage induced by radiation in thymocytes |
| Tainong 57 | Trypsin inhibitor | Cell cycle arrest | Phase G1 arrest |
| TU-155 | Polyphenols | Cell cycle arrest | ↓ciclin D1, A y E, ↑ Cip1/p21 |
| Promotion | |||
| NING No. 1 | Polysaccharides | Anti-inflammatory | ↓ IL-1β, IL-6 y TNF-α |
| TNG 73 | Anthocyanins | Anti-inflammatory | ↓ activation of NF-κβ in RAW 264.7 cells induced by LPS |
| -- | Caffeic acid and derivates | Inhibition in cell proliferation |
β-catenin and Tcf-4 pathway suppression |
| Progression | |||
| Bhu Krishna | Anthocyanins | Cell death induction | Apoptosis—↑ caspases |
| Diverse | Anthocyanins | Cell death induction | ↑ caspase 3 in colonic cells |
| -- | Polyphenols | Angiogenesis inhibition | ↓ VEGF165 in a dose-dependent manner |
| -- | BSG | Invasion inhibition | PI3K-Akt signaling pathway suppression |
| Zhongshu-1 | SPG-56 Glycoprotein |
Invasion inhibition | Regulation in the expression of proteins (MMP2, MMP9, VEGF, ocludin, and claudin) related with metastasis. |
| TNG 73 | Anthocyanins | Invasion inhibition | Cell migration suppression (MCF-7 cells) |
In the early stages, the cellular integrity, and in particular the genetic material, can be preserved by various compounds, such as trypsin inhibitors, anthocyanins, protein hydrolysates, or hydroxycinnamic acids present in the various varieties of SP. Damage to genetic material caused by reactive oxygen species (●OH o H2O2) o UV/gamma-irradiation can be decreased (if not avoided) after exposure to cells with functional compounds that have a high antioxidant capacity [26]. Additionally, it has been shown that the trypsin inhibitor present in the SP variety Tainong 57 can increase the expression of the protein p53, a nuclear protein known as “the guardian of the genome” due to its role in limiting abnormal cell formation, thus preserving the integrity of the genetic material [27].
If cell integrity is affected, this cell becomes abnormal and must multiply to promote a cancerous process. Cyclins are molecular mediators of the cell cycle; in normal conditions, they require binding with kinases to promote the different phases of cell division. Phenolic extracts of the SP variety Whatle/Loretan can decrease the expression of these proteins and limit complexing with their respective kinases [28]. According to the event and in the case of SP phytochemicals, Huang et al., have demonstrated the potential of trypsin inhibitors present in the Tainong 57 variety to limit cell division after arrest in the early phases (G1 phase) of altered cells [27].
As might be expected, the regulation of signaling pathways is a common and functional mechanism for the modulation of different tumors. The anthocyanins present in SP have been shown to be effective in negatively regulating the signaling pathway of β-catenin, a protein widely recognized for acting as a permanent coactivator of events, such as cell proliferation and differentiation [29][30]. In addition to the role of anthocyanins, phytosterols such as β-Sitosterol-d-glucoside play important roles in the regulation of additional pathways. β-Sitosterol-d-glucoside has been shown to be efficient in negatively regulating the PI3K/AKT/mTOR signaling pathway, a key pathway in processes, such as cell proliferation, apoptosis, metabolism, and angiogenesis [31][32]. The adverse systemic effects associated with the cancer process largely depend on tumor formation, its ability to survive, nutrient acquisition, and location, among other issues. Phenomena such as angiogenesis have been related to the capacity present in transformed cells to produce chemical mediators that promote vascularization and, therefore, the growth of tumor cells and their dissemination throughout the body (metastasis). Therefore, bioactives combinations with anti-angiogenic capacity seem essential in limiting processes such as the promotion and spread of cancer [33].
Chen et al. [34] demonstrated the ability of SP polyphenols to reduce the expression of the Vascular Endothelial Growth Factor (VEGF165) in a dose-dependent manner. Moreover, it has been shown that the glycoprotein SPG-56 present in the SP variety Zhongshu-1 can modulate the expression of essential proteins in cell attachment and adhesion. Dysregulation in the production of proteins, such as claudins or occludins (essential for the formation of tight junctions between cells), has been reported under in vitro conditions [35]. Beyond cancer promotion or progression stages, cell cytotoxicity on its own deserves attention. In all stages, the induction of cell death by apoptosis is a key tool to stop the number of viable cells in a programmed way. This event has already been reported for SP polyphenols. Particularly, the anthocyanin fractions from SP P40, O’Henry, NC Japanese as well as Bhu Krishna seem to be effective modulators in cell models [36][37].
The multi-target nature of SP’s phytochemicals helps to tackle cancer at many stages; however, future research on this matter should consider the SP varietal richness and plant part [23], gastrointestinal bioavailability [20][21][22][45][46], and pharmacokinetics of a given SP’s bioactivity to guarantee the effects observed in vitro/ex vivo conditions.
2.2. SP and Cardiovascular Diseases (CVD)
CVD are the leading cause of worldwide adult mortality. Prevalent cases of total CVD nearly doubled from 271 (CI95% 257–285) to 523 (CI95% 497–550) million deaths and 17.7 to 34.4 million disability-adjusted life years (DALYs) between 1990–2019 [47]. Since CVD and other non-communicable chronic diseases are closely related to lifestyle factors (e.g., unhealthy diet and sedentarism), it is necessary to promote the healthy intake of fruits, nuts, seeds, beans, vegetables, whole grains, and R&T [48], including the aerial/non-aerial parts of SP plant [23]. Numerous investigations indicate that the dietary intake of flavonoids (e.g., quercetin) from plant foods such as purple SP, can reduce the risk for CVD [49] while SP’s tannins, flavonoids, alkaloids reducing sugars, anthraquinones, and cardiac glycosides reduces serum creatinine and lactate-dehydrogenase activity, favoring cardiovascular health [23].
Consuming SP leaves reduces the risk for CVD by synergistically reducing lipid peroxidation and DNA damage, and regulating blood glucose, insulin, and lipid levels [50][51]. Such metabolic effects are partially explained by the 1: 2 ratio of linoleic/α-linolenic fatty acids [52], compounds that can protect the cardiovascular system from excessive inflammation and oxidative damage [53]. Moreover, Zhao et al. [51] showed that flavones from an SP leaf powder decreased total cholesterol and triglyceride levels in a dose-dependent manner while its insoluble dietary fiber increased fecal bile acids and cholesterol, reducing serum cholesterol levels [54]. In support of this, a randomized controlled clinical study carried out on 58 humans showed a decrease in circulating cholesterol (7 mg/dL) and triglycerides (2 mg/dL) after the consumption of 132 g of white SP as a meal replacement [55]. Moreover, it has been demonstrated in hamsters that consuming SP leaves increases the presence of favorable biomarkers to reduce the risks for CVD [55] by inducing vascular (aortic) relaxation [56] mediated by nitric oxide (NO) as an inhibitor, in the presence of N ω -nitro-l-arginine (NOLA), an inhibitor nitric oxide synthase (NOS), or by eliminating it from the endothelium [57]. As for SP root, <3 kDa hydrolyzed peptides (VSAIW, AIWGA, FVIKP, VVMPSTF, and FHDPMLR) from sporomin A and B, display a strong anti-ACE (angiotensin-converting enzyme) activity [23][58], while lactic acid bacteria (LAB)-based fermentation of white (Murasaki), orange (Evangeline) and purple (NIC-413)-fleshed SP varieties increases their anti-ACE/antioxidant activity [59]. Additional evidence on the cardioprotective effects of extracts of SP and/or its pure phytochemicals previously identified by chromatographic techniques is summarized in Table 2.
Table 2. SP phytochemicals in cardiovascular diseases (CVD).
| Phytochemical | Mechanism | Action |
|---|---|---|
| Heart | ||
| Anthocyanins | ↓ Malondialdehyde | Antioxidant ↓ Lipid peroxidation |
| Flavonoids/ anthocyanin |
Vasodilation induction/ ↓ endothelin—1 |
Antihypertensive |
| Tannins/saponins/ Flavonoids/terpenoids |
↓ Creatine kinase ↓ Lactate dehydrogenase |
Prevention in ischemic damage |
| Vascular | ||
| Aqueous extracts | ↑ Telomerase activity preventing cell senescence |
Prevention of coronary artery disease |
| Anthocyanins | Inhibition of PDGF receptor-β |
Regulation of platelet aggregation |
| Chlorogenic acid | ACE Inhibition | Antihypertensive |
| Anthocyanins/ethanolic extract | ↓ VCAM | Prevention of atherosclerosis |
| SP leaves | Elongate arterial occlusion time | Prevention of thrombotic events |
| Purple SP extract | ↓ cyclooxygenase-2, ↓ inducible nitric oxide synthase ↓ tumor necrosis factor-α |
↓Inflammation |
| Brain and Kidney | ||
| Anthocyanins | ↑ BDNF | Neuroprotection after ischemic stroke |
| Flavonoids/ acetylated anthocyanins |
Blocking VEGFR2/ROS/NLRP3 signaling | ↓ Kidney damage |
Angiotensin-converting enzyme (ACE), brain-Derived Nuclear Factor (BDNF), NLR family pyrin domain containing 3 (NLRP3), reactive oxygen species (ROS), platelet-derived growth factor (PDGF), sweet potato (SP), vascular cell adhesion molecule (VCAM), vascular endothelial growth factors receptor 2 (VEGFR2). Data source: [60][61][62][63][64][65][66][67][68][69][70][71].
In conclusion, this evidence suggests that several SP bioactives (leaves/root) may individually and synergistically prevent CVD by exerting many cardioprotective mechanisms. Further investigations on the associated molecular events are needed to support the epidemiological and in vivo and in vitro evidence discussed above.
References
- Guadarrama, L.M.; López, J.H. Patrones culinarios asociados al camote (Ipomoea batatas) y la yuca (Manihot esculenta) entre los mayas yucatecos, ch’oles y huastecos. Estud. Cult. Maya 2018, 52, 193–226.
- Motsa, N.M.; Modi, A.T.; Mabhaudhi, T. Sweet potato (Ipomoea batatas L.) as a drought tolerant and food security crop. South Afr. J. Sci. 2015, 111, 1–8.
- Rodriguez-Bonilla, L.; Cuevas, H.E.; Montero-Rojas, M.; Bird-Pico, F.; Luciano-Rosario, D.; Siritunga, D. Assessment of genetic diversity of sweet potato in Puerto Rico. PLoS ONE 2014, 9, e116184.
- Cartabiano-Leite, C.E.; Porcu, O.M.; de Casas, A.F. Sweet potato (Ipomoea batatas L. Lam) nutritional potential and social relevance: A review. History 2020, 11, 23–40.
- Mwanga, R.O.; Andrade, M.I.; Carey, E.E.; Low, J.W.; Yencho, G.C.; Grüneberg, W.J. Sweetpotato (Ipomoea batatas L.). In Genetic Improvement of Tropical Crops; Campos, H., Caligari, P.D.S., Eds.; Springer: Cham, Switzerland, 2017; pp. 181–218.
- Xiao, S.; Xu, P.; Deng, Y.; Dai, X.; Zhao, L.; Heider, B.; Zhang, A.; Zhou, Z.; Cao, Q. Comparative analysis of chloroplast genomes of cultivars and wild species of sweetpotato (Ipomoea batatas Lam). BMC Genom. 2021, 22, 262.
- Meira, M.; Da Silva, E.P.; David, J.M.; David, J.P. Review of the genus Ipomoea: Traditional uses, chemistry, and biological activities. Rev. Bras. Farmacogn. 2012, 22, 682–713.
- de Albuquerque, T.M.R.; Sampaio, K.B.; de Souza, E.L. Sweet potato roots: Unrevealing an old food as a source of health promoting bioactive compounds–A review. Trends Food Sci. Technol. 2019, 85, 277–286.
- de Albuquerque, T.M.R.; Borges, C.W.P.; Cavalcanti, M.T.; Lima, M.; Magnani, M.; de Souza, E.L. Potential prebiotic properties of flours from different varieties of sweet potato (Ipomoea batatas L.) roots cultivated in Northeastern Brazil. Food Biosci. 2020, 36, 100614.
- Wang, S.; Nie, S.; Zhu, F. Chemical constituents and health effects of sweet potato. Food Res. Int. 2016, 89, 90–116.
- Khoury, C.K.; Heider, B.; Castañeda-Álvarez, N.P.; Achicanoy, H.A.; Sosa, C.C.; Miller, R.E.; Scotland, R.W.; Wood, J.R.I.; Rossel, G.; Eserman, L.A.; et al. Distributions, ex situ conservation priorities, and genetic resource potential of crop wild relatives of sweetpotato . Front. Plant Sci. 2015, 6, 251.
- Mohanraj, R.; Sivasankar, S. Sweet Potato (Ipomoea batatas Lam)—A valuable medicinal food: A review. J. Med. Food 2014, 17, 733–741.
- Mu, T.H.; Singh, J. Sweet potato: Chemistry, processing, and nutrition—An introduction. In Sweet Potato; Mu, T.H., Singh, J., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–4.
- Truong, V.D.; Avula, R.Y.; Pecota KYencho, G. Handbook of Vegetables and Vegetable Processing. In Handbook of Vegetables and Vegetable Processing; Siddiq, M., Uebersax, M.A., Eds.; Wiley-Blackwell Publishing Co.: Ames, IA, USA, 2018. Available online: https://www.ars.usda.gov/ARSUserFiles/60701000/Sweetpotato%20Publications/s158.pdf (accessed on 5 March 2022).
- Cartier, A.; Woods, J.; Sismour, E.; Allen, J.; Ford, E.; Githinji, L.; Xu, Y. Physiochemical, nutritional and antioxidant properties of fourteen Virginia-grown sweet potato varieties. J. Food Meas. Charact. 2017, 11, 1333–1341.
- Kim, J.M.; Park, S.J.; Lee, C.S.; Ren, C.; Kim, S.S.; Shin, M. Functional properties of different Korean sweet potato varieties. Food Sci. Biotechnol. 2011, 20, 1501–1507.
- Anchundia, M.Á.; Pérez, E.; Torres, F. Composición química, perfil de aminoácidos y contenido de vitaminas de harinas de batata tratadas térmicamente. Rev. Chil. Nutr. 2019, 46, 137–143.
- Kourouma, V.; Mu, T.H.; Zhang, M.; Sun, H.N. Effects of cooking process on carotenoids and antioxidant activity of orange-fleshed sweet potato. LWT 2019, 104, 134–141.
- Failla, M.L.; Thakkar, S.K.; Kim, J.Y. In vitro bioaccessibility of β-carotene in orange fleshed sweet potato (Ipomoea batatas, Lam.). J. Agric. Food Chem. 2009, 57, 10922–10927.
- Rios-Romero, E.A.; Ochoa-Martínez, L.A.; Bello-Pérez, L.A.; Morales-Castro, J.; Quintero-Ramos, A.; Gallegos-Infante, J.A. Effect of ultrasound and steam treatments on bioaccessibility of β-carotene and physicochemical parameters in orange-fleshed sweet potato juice. Heliyon 2021, 7, e06632.
- Mbogo, D.; Muzhingi, T.; Janaswamy, S. Starch digestibility and β-carotene bioaccessibility in the orange-fleshed sweet potato puree-wheat bread. J. Food Sci. 2021, 86, 901–906.
- Yang, Z.W.; Tang, C.E.; Zhang, J.L.; Zhou, Q.; Zhang, Z.C. Stability and antioxidant activity of anthocyanins from purple sweet potato (Ipomoea batatas L. cultivar Eshu No. 8) subjected to simulated in vitro gastrointestinal digestion. Int. J. Food Sci. Technol. 2019, 54, 2604–2614.
- Alam, M.K. A comprehensive review of sweet potato (Ipomoea batatas Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529.
- Nguyen, H.C.; Chen, C.C.; Lin, K.H.; Chao, P.Y.; Lin, H.H.; Huang, M.Y. Bioactive compounds, antioxidants, and health benefits of sweet potato leaves. Molecules 2021, 26, 1820.
- Laurie, S.M.; Faber, M.; Claasen, N. Incorporating orange-fleshed sweet potato into the food system as a strategy for improved nutrition: The context of South Africa. Food Res. Int. 2018, 104, 77–85.
- Kocyigit, A.; Guler, E.M.; Dikilitas, M. Role of antioxidant phytochemicals in prevention, formation, and treatment of cancer in Reactive Oxygen Species (ROS) in Living Cells. In Reactive Oxygen Species (ROS) in Living Cells; InTech Open: Rijeka, Croatia, 2018; pp. 21–45.
- Huang, G.J.; Sheu, M.J.; Chen, H.J.; Chang, Y.S.; Lin, Y.H. Growth inhibition and induction of apoptosis in NB4 promyelocytic leukemia cells by trypsin inhibitor from sweet potato storage roots. J. Agric. Food Chem. 2007, 55, 2548–2553.
- Karna, P.; Gundala, S.R.; Gupta, M.V.; Shamsi, S.A.; Pace, R.D.; Yates, C.; Narayan, S.; Aneja, R. Polyphenol-rich sweet potato greens extract inhibits proliferation and induces apoptosis in prostate cancer cells in vitro and in vivo. Carcinogenesis 2011, 32, 1872–1880.
- Taira, J.; Uehara, M.; Tsuchida, E.; Ohmine, W. Inhibition of the β-catenin/Tcf signaling by caffeoylquinic acids in sweet potato leaf through down regulation of the Tcf-4 transcription. J. Agric. Food Chem. 2014, 62, 167–172.
- Mantilla, C.; Suárez Mellado, I.; Duque Jaramillo, A.; Navas, M.C. Mecanismos de señalización por β-catenina y su papel en la carcinogénesis. CES Med. 2015, 29, 109–127. Available online: http://www.scielo.org.co/pdf/cesm/v29n1/v29n1a10.pdf (accessed on 8 March 2022).
- Xu, H.; Li, Y.; Han, B.; Li, Z.; Wang, B.; Jiang, P.; Zhang, J.; Ma, W.; Zhou, D.; Li, X.; et al. Anti-breast-cancer activity exerted by β-sitosterol-D-glucoside from sweet potato via upregulation of microRNA-10a and via the PI3K–Akt signaling pathway. J. Agric. Food Chem. 2018, 66, 9704–9718.
- Patel, K.; Kumar, V.; Verma, A.; Rahman, M.; Patel, D.K. β-sitosterol: Bioactive compounds in foods, their role in health promotion and disease prevention “a concise report of its phytopharmaceutical importance”. Curr. Tradit. Med. 2017, 3, 168–177.
- Rajabi, M.; Mousa, S.A. The role of angiogenesis in cancer treatment. Biomedicines 2017, 5, 34.
- Chen, C.-M.; Li, S.-C.; Au, H.-K.; Shih, C.-K.; Hsu, C.-Y.; Liu, J.-F. Constituents in purple sweet potato leaves inhibit in vitro angiogenesis with opposite effects ex vivo. Nutrition 2011, 27, 1177–1182.
- Li, Z.; Yu, Y.; Wang, M.; Xu, H.; Han, B.; Jiang, P.; Ma, H.; Li, Y.; Tian, C.; Zhou, D.; et al. Anti-breast cancer activity of SPG-56 from sweet potato in MCF-7 bearing mice in situ through promoting apoptosis and inhibiting metastasis. Sci. Rep. 2019, 9, 146.
- Lim, S. Anthocyanin-Enriched Purple Sweet Potato for Colon Cancer Prevention; Kansas State University, ProQuest Dissertations Publishing: Ann Arbor, MI, USA, 2012; Available online: https://www.proquest.com/openview/aac0a1d3911587677e77c25f72859b42/1?pq-origsite=gscholar&cbl=18750 (accessed on 8 March 2022).
- Vishnu, V.R.; Renjith, R.S.; Mukherjee, A.; Anil, S.R.; Sreekumar, J.; Jyothi, A.N. Comparative study on the chemical structure and in vitro antiproliferative activity of anthocyanins in purple root tubers and leaves of sweet potato (Ipomoea batatas). J. Agric. Food Chem. 2019, 67, 2467–2475.
- Lee, J.H.; Woo, K.S.; Lee, H.-U.; Nam, S.S.; Lee, B.W.; Lee, Y.-Y.; Lee, B.; Kim, H.-J. Intracellular reactive oxygen species (ros) removal and cytoprotection effects of sweet potatoes of various flesh colors and their polyphenols, including anthocyanin. Prev. Nutr. Food Sci. 2019, 24, 293–298.
- Zhang, M.; Mu, T.H. Contribution of different molecular weight fractions to anticancer effect of sweet potato protein hydrolysates by six proteases on HT-29 colon cancer cells. Int. J. Food Sci. Technol. 2018, 53, 525–532.
- Han, Y.T.; Chen, X.H.; Xie, J.; Zhan, S.M.; Wang, C.B.; Wang, L.X. Purple sweet potato pigments scavenge ROS, reduce p53 and modulate Bcl-2/Bax to inhibit irradiation-induced apoptosis in murine thymocytes. Cell. Physiol. Biochem. 2011, 28, 865–872.
- Ayeleso, T.B.; Ramachela, K.; Mukwevho, E. A review of therapeutic potentials of sweet potato: Pharmacological activities and influence of the cultivar. Trop. J. Pharm. Res. 2016, 15, 2751–2761.
- Jiang, T.; Zhou, J.; Liu, W.; Tao, W.; He, J.; Jin, W.; Guo, H.; Yang, N.; Li, Y. The anti-inflammatory potential of protein-bound anthocyanin compounds from purple sweet potato in LPS-induced RAW264. 7 macrophages. Food Res. Int. 2020, 137, 109647.
- Sugata, M.; Lin, C.Y.; Shih, Y.C. Anti-inflammatory and anticancer activities of taiwanese purple-fleshed sweet potatoes (Ipomoea batatas L. Lam) extracts. BioMed Res. Int. 2015, 2015, 768093.
- Lim, S.; Xu, J.; Kim, J.; Chen, T.-Y.; Su, X.; Standard, J.; Carey, E.; Griffin, J.; Herndon, B.; Katz, B.; et al. Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol. Nutr. Food Res. 2013, 57, 1908–1917.
- Andre, C.M.; Burgos, G.; Ziebel, J.; Guignard, C.; Hausman, J.-F.; Felde, T.Z. In vitro iron bioaccessibility and uptake from orange-fleshed sweet potato (Ipomoea batatas (L.) Lam.) clones grown in Peru. J. Food Compos. Anal. 2018, 68, 79–86.
- Choquechambi, L.A.; Callisaya, I.R.; Ramos, A.; Bosque, H.; Mújica, A.; Jacobsen, S.E.; Sørensen, M.; Leidi, E.O. Assessing the nutritional value of root and tuber crops from Bolivia and Peru. Foods 2019, 8, 526.
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021.
- Lutz, M.; Fuentes, E.; Ávila, F.; Alarcón, M.; Palomo, I. Roles of phenolic compounds in the reduction of risk factors of cardiovascular diseases. Molecules 2019, 24, 366.
- Sesso, H.D.; Liu, S.; Gaziano, J.M.; Buring, J.E. Dietary lycopene, tomato-based food products and cardiovascular disease in women. J. Nutr. 2003, 133, 2336–2341.
- Chen, C.M.; Li, S.C.; Lin, Y.L.; Hsu, C.Y.; Shieh, M.J.; Liu, J.F. Consumption of purple sweet potato leaves modulates human immune response: T-lymphocyte functions, lytic activity of natural killer cell and antibody production. World J. Gastroenterol. 2005, 11, 5777–5781.
- Zhao, R.; Li, Q.; Long, L.; Li, J.; Yang, R.; Gao, D. Antidiabetic activity of flavone from Ipomoea batatas leaf in non-insulin dependent diabetic rats. Int. J. Food Sci. Technol. 2007, 42, 80–85.
- Johnson, M.; Pace, R.D. Sweet potato leaves: Properties and synergistic interactions that promote health and prevent disease. Nutr. Rev. 2010, 68, 604–615.
- Torres-Urrutia, C.; Guzmán, L.; Schmeda-Hirschmann, G.; Moore-Carrasco, R.; Alarcón, M.; Astudillo, L.; Gutierrez, M.; Carrasco, G.; Yuri, J.A.; Aranda, E.; et al. Antiplatelet, anticoagulant, and fibrinolytic activity in vitro of extracts from selected fruits and vegetables. Blood Coagul. Fibrinolysis 2011, 22, 197–205.
- Chen, C.-M.; Lin, Y.-L.; Hsu, C.-Y.; Shieh, M.-J.; Liu, J.-F. Consumption of purple sweet potato leaves decreases lipid peroxidation and DNA damage in humans. Asia Pac. J. Clin. Nutr. 2008, 17, 408–414. Available online: https://apjcn.nhri.org.tw/server/APJCN/17/3/408.pdf (accessed on 5 March 2022).
- Shih, C.K.; Chen, C.M.; Hsiao, T.J.; Liu, C.W.; Li, S.C. White sweet potato as meal replacement for overweight white-collar workers: A randomized controlled trial. Nutrients 2019, 11, 165.
- Yoon, S.; Pace, R.D.; Dawkins, N.L. The antioxidant potential of sweet potato greens in preventing cardiovascular disease risk in hamster. FASEB J. 2007, 21, A1091.
- Runnie, I.; Salleh, M.N.; Mohamed, S.; Head, R.J.; Abeywardena, M.Y. Vasorelaxation induced by common edible tropical plant extracts in isolated rat aorta and mesenteric vascular bed. J. Ethnopharmacol. 2004, 92, 311–316.
- Nazir, M.A.; Mu, T.H.; Zhang, M. Preparation and identification of angiotensin I-converting enzyme inhibitory peptides from sweet potato protein by enzymatic hydrolysis under high hydrostatic pressure. Int. J. Food Sci. Technol. 2020, 55, 482–489.
- Chintha, P.; Sarkar, D.; Pecota, K.; Dogramaci, M.; Shetty, K. Improving Phenolic Bioactive-Linked Functional Qualities of Sweet Potatoes Using Beneficial Lactic Acid Bacteria-Based Biotransformation Strategy. Horticulturae 2021, 7, 367.
- Jawi, I.M.; Mahendra, A.N.; Subawa, A.A.N.; Yasa, I.S.; Gunawan, W.G. Comparison of antihypertensive and antioxidative effect of Mahogany (Swietenia Mahagoni (L.) Jacq.) seed extract and purple sweet potato (Ipomoea batatas) tuber extract on rodent model of hypertension. Biomed. Pharmacol. J. 2017, 10, 577–582.
- Herawati, E.R.N.; Santosa, U.; Sentana, S.; Ariani, D. Protective effects of anthocyanin extract from purple sweet potato (Ipomoea batatas L.) on blood MDA levels, liver and renal activity, and blood pressure of hyperglycemic rats. Prev. Nutr. Food Sci. 2020, 25, 375–379.
- Suastika, L.; Oktaviono, Y.; Soemantri, D.; Sandra, F. Purple sweet potato extract and vitamin C increase the proliferation of endothelial progenitor cells from stable coronary artery disease patients. Bali Med. J. 2021, 10, 243–248.
- Choi, J.H.; Hwang, Y.P.; Park, B.H.; Choi, C.Y.; Chung, Y.C.; Jeong, H.G. Anthocyanins isolated from the purple-fleshed sweet potato attenuate the proliferation of hepatic stellate cells by blocking the PDGF receptor. Environ. Toxicol. Pharmacol. 2011, 31, 212–219.
- Satriyasa, B.K. Purple sweet potato ethanolic extract reduces aortic VCAM expression in rabbit with high-cholesterol diet. Bali Med. J. 2017, 6, S26–S28.
- Rahmawati, E.; Sasangka Prasetyawan, C.M.; Srihardyastutie, A.; Adnyana, M.O. Potential of Purple Sweet Potato (Ipomoea batatas L.) To Increase BDNF Level and VEGF Expression in The Cerebellum of Ischemic Stroke Rats. J. Pure Appl. Chem. Res. 2018, 7, 45–52.
- Rahmawati, I.S.; Soetjipto, S.; Adi, A.C.; Aulanni’am, A.A. The effectiveness of ethanol extract of purple sweet potato var. Ayamurasaki as a natural antihypertensive mitigator in deoxycorticosterone acetate-salt hypertensive rats. Drug Invent. Today 2019, 11, 3179–3193.
- Zheng, G.-H.; Shan, Q.; Mu, J.-J.; Wang, Y.-J.; Zhang, Z.-F.; Fan, S.-H.; Hu, B.; Li, M.-Q.; Xie, J.; Chen, P.; et al. Purple sweet potato color attenuates kidney damage by blocking VEGFR2/ROS/NLRP3 signaling in high-fat diet-treated mice. Oxidative Med. Cell. Longev. 2019, 2019, 5189819.
- Shafe, M.; Eze, E.; Ubhenin, A.; Tende, J. Effects of Aqueous Tuber Extract of Ipomea batatas on Cardiac Enzymes, Lipid Profile and Organ Weights in Wistar Rats. J. Basic Appl. Res. Biomed. 2016, 2, 414–417.
- Chang, H.H.; Lan, Y.C.; Chung, S.D.; Chien, C.T. Sweet potato leaf feeding decreases cholesterol, oxidative stress and thrombosis formation in syrian hamsters with a high-cholesterol diet. Life 2021, 11, 802.
- Osuntokun, O.T.; Yusuf-Babatunde, M.A.; Fasila, O.O. Components and Bioactivity of Ipomoea batatas (L.)(Sweet Potato) Ethanolic Leaf Extract. Asian J. Adv. Res. Rep. 2020, 10, 10–26.
- Li, J.; Shi, Z.; Mi, Y. Purple sweet potato color attenuates high fat-induced neuroinflammation in mouse brain by inhibiting MAPK and NF-κB activation. Mol. Med. Rep. 2018, 17, 4823–4831.
More
Information
Subjects:
Nutrition & Dietetics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.2K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
18 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




