Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Akhil Varshney | -- | 2072 | 2022-04-15 08:28:35 | | | |
| 2 | Catherine Yang | Meta information modification | 2072 | 2022-04-15 09:00:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Varshney, A.; , .; Ramappa, M.; Basu, S.; Romano, V. AS-OCT in Corneal Disorders. Encyclopedia. Available online: https://encyclopedia.pub/entry/21809 (accessed on 08 February 2026).
Varshney A, , Ramappa M, Basu S, Romano V. AS-OCT in Corneal Disorders. Encyclopedia. Available at: https://encyclopedia.pub/entry/21809. Accessed February 08, 2026.
Varshney, Akhil, , Muralidhar Ramappa, Sayan Basu, Vito Romano. "AS-OCT in Corneal Disorders" Encyclopedia, https://encyclopedia.pub/entry/21809 (accessed February 08, 2026).
Varshney, A., , ., Ramappa, M., Basu, S., & Romano, V. (2022, April 15). AS-OCT in Corneal Disorders. In Encyclopedia. https://encyclopedia.pub/entry/21809
Varshney, Akhil, et al. "AS-OCT in Corneal Disorders." Encyclopedia. Web. 15 April, 2022.
Copy Citation
Anterior segment optical coherence tomography (AS-OCT) is a non-contact method of in vivo ocular imaging technology. It uses low coherence interferometry that measures the echo time delay of light backscattered from tissue structures and combines multiple axial scans into a composite B-scan image. Earlier ultrasound bio microscopy was used for anterior segment imaging, but with time AS-OCT has become a popular imaging tool for anterior segment imaging. AS-OCT is faster and provides more in-depth assessment of the anterior chamber and is repeatable and reproducible. For AS imaging, longer wavelengths (1050–1310 nm) are preferred than retinal OCT (800–900 nm) due to higher penetration and less scattering.
AS-OCT
cornea
ocular surface
1. Conjunctival Diseases
AS-OCT, also referred to as an optical biopsy, is an important non-invasive tool in diagnosis and monitoring of disease progression in various conjunctival diseases of the eye. Most notable of its uses is in differentiating malignant ocular surface squamous neoplasia (OSSN) from the benign lesions. The others uses are to differentiate a pterygium from a pseudopterygium and a pterygium from a pinguecula, to determine the precise measurement of a pterygium which helps to detect it’s growth and recurrence, and assess the risk of stromal scarring and astigmatism [1]. In a normal AS-OCT of the ocular surface, the tear film overlying the conjunctiva and the cornea is bright white. The corneal epithelium is thin and dark, while that of the conjunctiva and the limbus is thin but mildly hyper-reflective. The sub-epithelial tissue of the cornea is arranged in a regular linear pattern, whereas that of the conjunctiva is less regular and hyper-reflective.
Pterygium: A primary pterygium is seen as a hyper-reflective mass in the sub-epithelial conjunctival area extending into the cornea, causing elevation of the corneal epithelium and separating it from the underlying Bowman’s membrane. The pterygium tissue below the corneal epithelium extends beyond the corneal tip of the clinically evident pterygium mass, therefore, measuring the size of the pterygium more accurately.
Pinguecula: A Pinguecula is very similar to a pterygium but stops at the limbal region and doesn’t elevate the healthy corneal epithelium. There is also a clearly defined line of separation between the pinguecula and the underlying scleral tissue.
Pseudoterygium: A pseudopterygium is seen as an overgrowing membrane that is not attached to the underlying cornea, with a plane of cleavage between it and the underlying corneal epithelium [2][3]. Increased risk of stromal scarring can be associated with pterygia with flat cornea-scleral transition zone as compared to the nodular variety, in pterygia with reduced apical head thickness, and in pterygia with partially disrupted Bowman’s membrane [1][2].
Ocular surface tumors: OSSN is a lesion involving epithelium of both cornea and conjunctiva. On AS-OCT imaging, OSSN can have either or all of these three characteristic features: hyper-reflective epithelium, thickened epithelial layer, and an abrupt transition from normal to abnormal epithelium (Figure 1) [4][5]. Another feature that has been identified in AS-OCT of OSSN cases is presence of hypo-reflective or cystoid spaces. These cystoid spaces could be type 1, which are small, round with hypo-reflective contents or type 2, which are larger, irregular with hyper-reflective material inside [6]. AS-OCT findings have a 94% sensitivity and 100% specificity for diagnosing OSSN and differentiating it from pterygium [7]. For OSSN treated with topical chemotherapy, AS-OCT after treatment showed normalization of epithelium [4][8]. Since AS-OCT can diagnose microscopic subclinical disease, it also prevents premature termination of topical chemotherapy in patients with residual subclinical disease and thus prevents the risk of recurrence [8][9]. AS-OCT helps in non-invasive diagnosis of OSSN and can be used to determine the need for treatment initiation as well as monitoring of disease course [4][5][10]. Since involvement of sub epithelial tissue and sclera is difficult in AS-OCT due to posterior shadowing of epithelial lesion, histopathology continues to be gold standard for diagnosis of deeper infiltration of tumor [10]. Angiographic features of OSSN have also been described using FA, ICGA and recently OCT-angiography has been used to demonstrate vasculature patterns. FA demonstrates vascular leakage while ICGA shows conjunctival feeder vessels and sea fan shaped intra-tumoral vessels [11]. OCT-A shows blood flow in epithelium and sub-epithelial tissues and highest density of vessels is seen in conjunctival part of tumor. It is also able to show corneal vessels under the tumor which are clinically not visible. Although OCT-A has the advantage of getting images without contrast dyes, compared to FA and ICGA, it cannot evaluate vascular leakage and feeder vessels [12]. In patients treated with topical chemotherapy such as 5-FU, FA, and ICG also demonstrated regression of vascular leakage, feeder vessels and intratumoral vessels after completion of therapy [11].
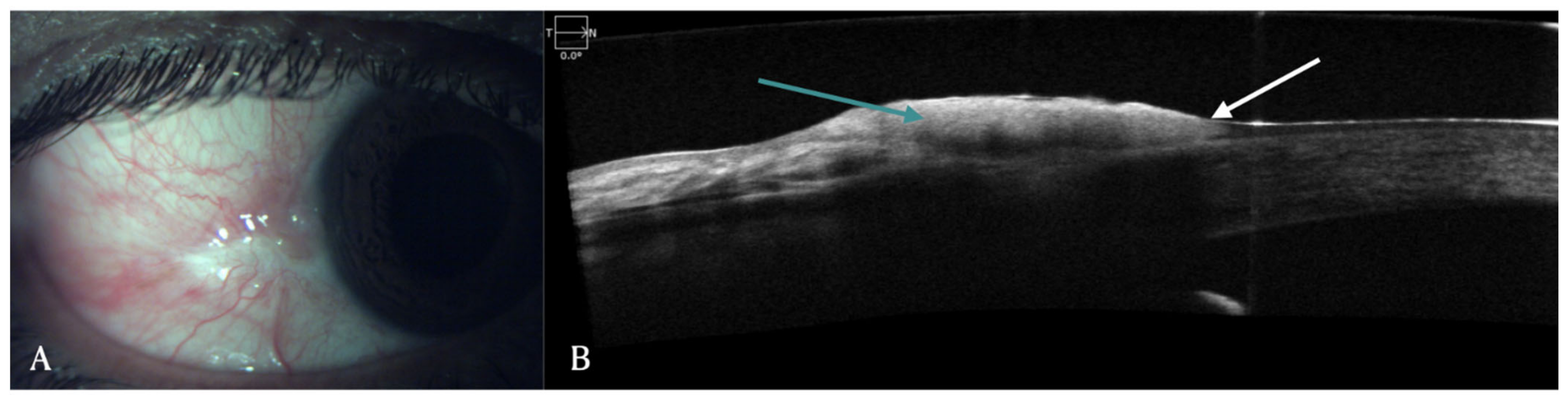
Figure 1. (A) Clinical picture of OSSN in a 51-year-old male patient. (B) Corresponding AS-OCT image showing thickened and hyper-reflective epithelial layer (green arrow) with abrupt transition from normal to abnormal epithelium (white arrow).
The conjunctival melanoma is seen as a hyper-reflective, sub epithelial lesion. A normal or slightly thickened epithelium is seen with hyper-reflective basal epithelium which suggests atypical melanocytes in the epithelium. This feature on AS-OCT helps in differentiating it from a pigmented OSSN [5][13]. The conjunctival naevus is seen as a well-circumscribed sub epithelial lesion underlying a normal epithelium. Cystic spaces are seen on AS-OCT, unlike melanomas. AS-OCT can also visualize cystic spaces in melanotic nevi seen in children, where cystic spaces are not clinically apparent [5]. AS-OCT images of conjunctival lymphomas typically show normal epithelium with homogenous, hypo-reflective (dark) sub epithelial lesions with smooth, well-defined borders. These can be differentiated from heterogenous, dark subepithelial lesions with irregular, diffuse borders seen in cases of conjunctival amyloidosis [5][13].
2. Limbal Stem Cell Deficiency (LSCD)
LSCD on OCT manifests as thinning of the limbal and corneal epithelium [14][15]. These changes are global in cases of total LSCD while in partial disease they are more profound in the affected areas. The unaffected areas may have normal epithelial thickness or it may be reduced, indicating a subclinical form of the disease [14]. Additionally, direct visualization of the disrupted palisades of Vogt is possible with the use of this device [16]. With the use of image processing software, Varma et al., have described a method of quantifying the reflectivity patterns of the line scans of OCT and both epithelial and stromal reflectivities were studied in eyes with LSCD. The authors devised a ratio between them (ES ratio) and proposed that values greater than 1.29 were diagnostic of LSCD. Several corneal disorders with vascularization may mimic LSCD. With the help of the segmentation feature of the OCTA, differentiating the true LSCD cases is possible as the vasculature in LSCD is usually restricted to the superficial layers [17]. A superficial vascular density value greater than 0.38 has good sensitivity and specificity for LSCD [17].
Limbal stem cell transplantation (LSCT) is perhaps one of the most popular treatment options in eyes with LSCD. Of the different types of LSCT, simple limbal epithelial transplantation (SLET) is preferred for both unilateral and bilateral LSCD in view of its single-step technique which does not require any additional laboratory support and yields stable results [18]. An important step of SLET is the dissection of the overlying fibrovascular pannus. However, the pannus may preclude assessment of the underlying stromal thickness and scarring on clinical examination and so perforation of the cornea may occur during this step in very thin corneas. Preoperative assessment of stromal thinning on OCT may help identifying such cases (Figure 2). The degree of stromal opacification may also be visible on these scans and the need for a secondary intervention for visual rehabilitation can be planned accordingly. The infra-red image of the cornea that accompanies the line scans also provides valuable information in the decision making in such cases (Figure 3). If the anterior chamber details are discernible, performing SLET in isolation is usually sufficient while a keratoplasty may be required if no structures are visible [18][19]. The postoperative monitoring of recovery of the corneal epithelium following SLET is also feasible with an AS-OCT (Figure 3) [20]. The ES ratio gradually decreases and returns to near normal values and this trend can be followed up with AS-OCT scans [20].
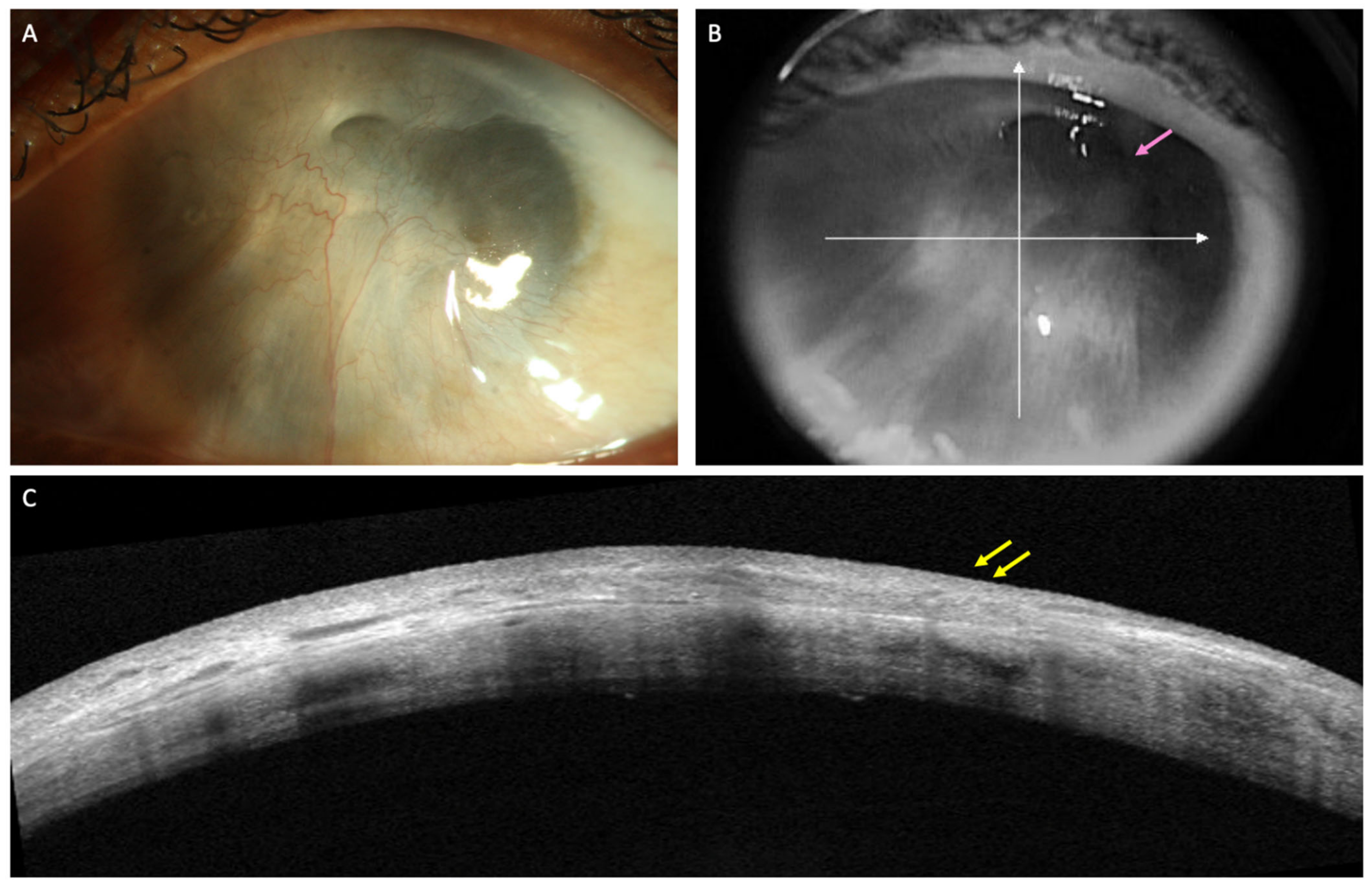
Figure 2. (A–C) Clinical images of the left eye of a patient with limbal stem cell deficiency (LSCD) due to chronic sequelae of ocular chemical burns. (A) Slit lamp image depicting total limbal stem cell deficiency with a thick fibrotic pannus covering the visual axis. (B) Details of the anterior chamber can be hazily visualized in the superotemporal quadrant (pink arrow) in the infrared image of optical coherence tomography (AS-OCT) scan. (C) AS-OCT line scan showing the hyperreflective pannus (yellow arrows) with sufficient corneal stromal thickness to permit pannus dissection.
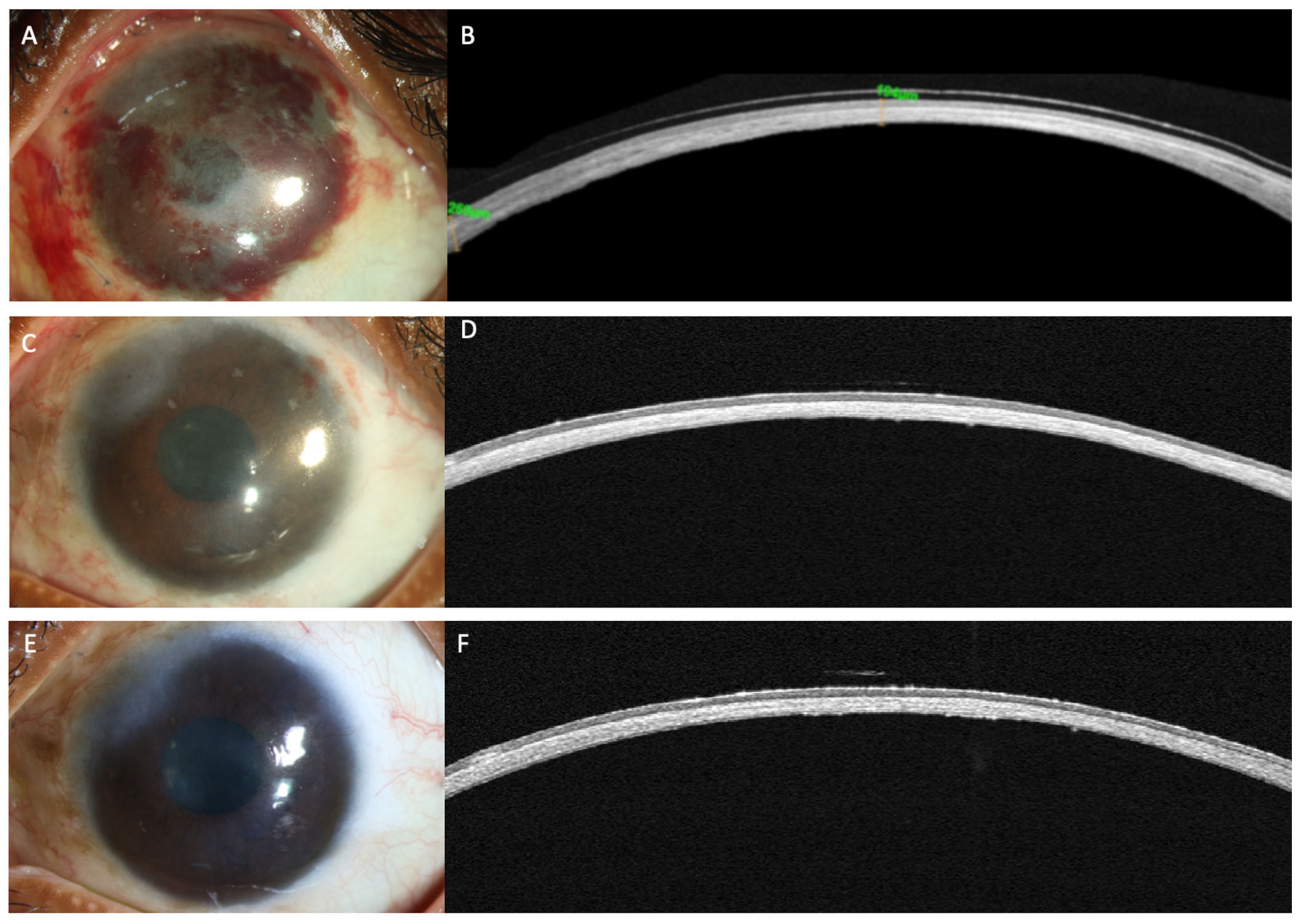
Figure 3. (A–F) This is a collage of images depicting recovery following simple limbal epithelial transplantation (SLET) in the patient from Figure 2. (A,B) Slit lamp image and the corresponding optical coherence tomography (OCT) image captured in the immediate postoperative period illustrating thinning of the corneal stroma with absence of the hyperreflective conjunctival epithelium. (C) The surface is well epithelialized, six weeks after SLET with stromal thinning evident on the OCT scan along with a normal hypo-reflective corneal epithelium (D) The stable ocular surface is maintained 5 months after the surgery (E,F).
3. Ocular Chemical Burns (OCBs)
The sequelae that ensue from OCBs usually depend on the severity of injury in the acute phase of the disease. One of the most important clinical findings that helps prognosticate these eyes is the presence of limbal ischemia. However, detection of this finding in the acute phase is subjectable and often incorrectly gauged [21]. OCTA provides an objective way of assessing the presence of and quantifying the extent of limbal ischemia (Figure 4). The extent of ischemia as measured by an OCTA is comparable to the ischemia assessed by standard angiography techniques [22]. Various indices have been described of which vessel density is most frequently used and correlates with the severity of the injury [23][24]. Incorporation of these parameters in the existing grading systems is being attempted to provide a more accurate method of predicting the prognosis of these cases [23]. Furthermore, the need for interventions in the acute phase, such as a tenonplasty, can also be determined by the severity and location of the ischemia. The degree of ischemia present may also predict the rate of epithelial healing and these eyes can be closely monitored for the need for procedures such an amniotic membrane grafting to augment the healing process. OCTA was used for serial monitoring of the suspect areas can help confirm the presence of ischemia, as vasospasm is reversible, and the normal vasculature becomes evident on the subsequent scans [25].
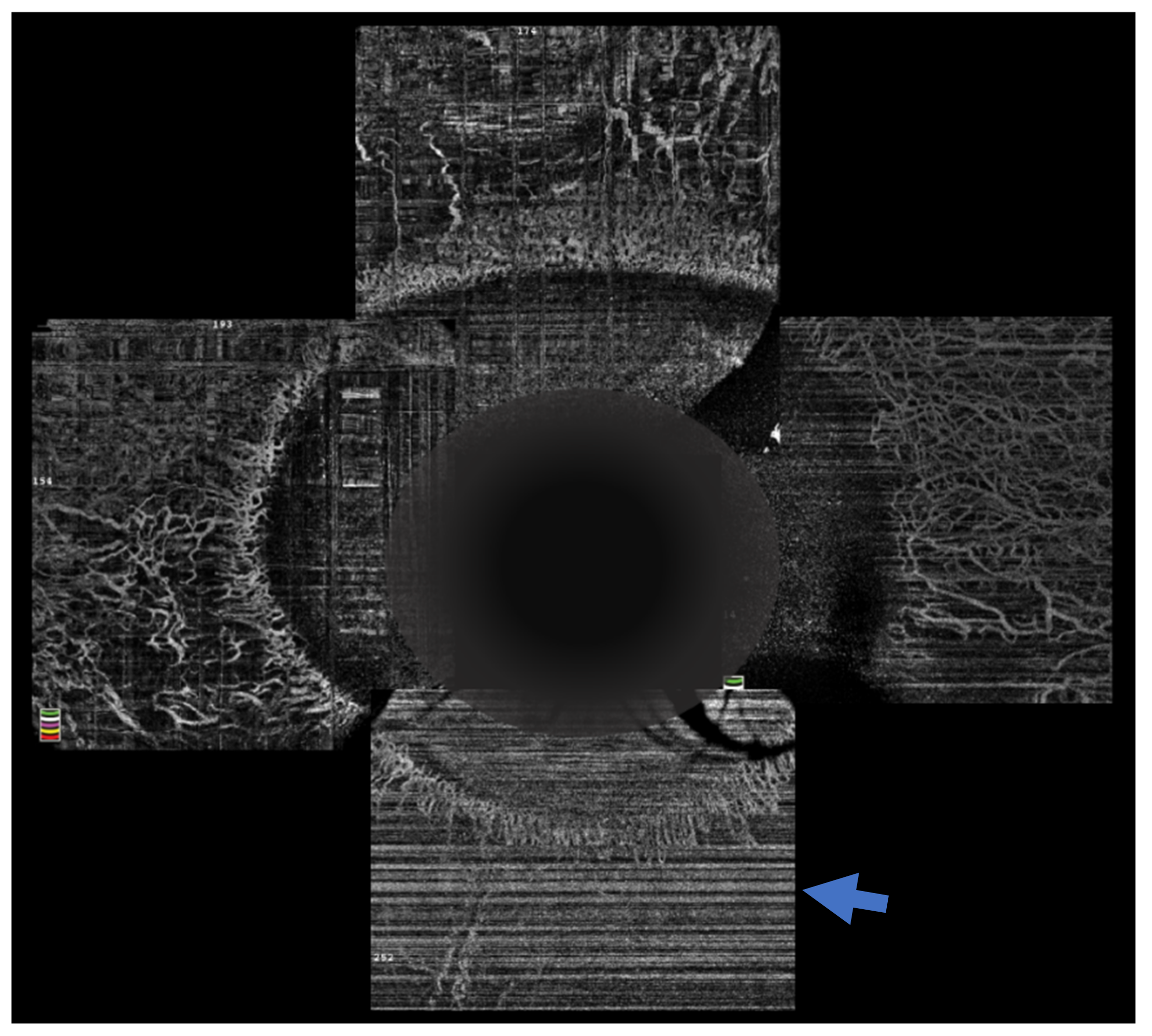
Figure 4. Optical coherence tomography angiography of an eye with chemical injury illustrating lack of vasculature inferiorly (arrow), in the area with ischemia.
4. AS-OCT Angiography
A variety of disease response such as inflammation, infection, degeneration, and trauma could disturb the ocular surface balance and leading to corneal neovascularization (CNV). CNV compromises the transparency of the cornea resulting in poor vision and finally loss of vision [26][27]. The most common way of assessing anterior segment vasculature is through slit-lamp photography (SLP) and fluorescein dye-based angiography. Though widely used, SLP has certain limitations such as poor visualization of blood-vessels in the presence of corneal edema or in fibrotic cornea, resulting in compromised image analysis and poor sensitivity for the smaller vessels [28]. With the recent advancement in image acquisition and analysis technology, OCT uses has recently increased in visualizing and analyzing blood vessels too resulting in OCT angiography (OCTA), an emerging method for imaging ocular vasculature [29][30]. Though OCTA is widely used in posterior segment imaging, its application for the anterior segment is poorly explored. AS-OCTA is a new technology to visualize anterior of the eye blood vessels. It has immense potential for future clinical diagnosis and applications in corneal and iris pathologies, pre-operative surgical planning, evaluating the efficacy of new antiangiogenic drugs and assessing limbal stem cell deficiency [29][30][31][32].
A recent pilot animal model study highlights the importance of AS-OCTA as a useful non-invasive imaging tool for objective assessment of CNV for examining the efficacy of a drug on the angiogenesis., which is essential for further clinical studies [33]. In clinics AS-OCTA has shown to be a promising tool for CNV, LSCD and to detect subclinical neovascularization of iris (NVI) that appeared in its early stages.
Advancement of technology and convergence of health care and computational biology, it is compelling to use artificial intelligence to produce normative databases and carry out deep analysis for appropriate AS-OCTA studies. Further studies are needed for designing the feasibility of combining OCTA with fluorescence angiography using multi-modal approaches. With further development, OCTA for anterior segment imaging in the clinics may become common in the near future.
References
- Gasser, T.; Romano, V.; Seifarth, C.; Bechrakis, N.E.; Kaye, S.B.; Steger, B. Morphometric characterisation of pterygium associated with corneal stromal scarring using high-resolution anterior segment optical coherence tomography. Br. J. Ophthalmol. 2017, 101, 660–664.
- Soliman, W.; Mohamed, T.A. Spectral domain anterior segment optical coherence tomography assessment of pterygium and pinguecula. Acta Ophthalmol. 2012, 90, 461–465.
- Nanji, A.A.; Sayyad, F.E.; Galor, A.; Dubovy, S.; Karp, C.L. High-Resolution Optical Coherence Tomography as an Adjunctive Tool in the Diagnosis of Corneal and Conjunctival Pathology. Ocul. Surf. 2015, 13, 226–235.
- Thomas, B.J.; Galor, A.; Nanji, A.A.; El Sayyad, F.; Wang, J.; Dubovy, S.R.; Joag, M.G.; Karp, C.L. Ultra high-resolution anterior segment optical coherence tomography in the diagnosis and management of ocular surface squamous neoplasia. Ocul. Surf. 2014, 12, 46–58.
- Shousha, M.A.; Karp, C.L.; Canto, A.P.; Hodson, K.; Oellers, P.; Kao, A.A.; Bielory, B.; Matthews, J.; Dubovy, S.R.; Perez, V.L.; et al. Diagnosis of ocular surface lesions using ultra-high-resolution optical coherence tomography. Ophthalmology 2013, 120, 883–891.
- Singh, S.; Mittal, R.; Ghosh, A.; Tripathy, D.; Rath, S. High-Resolution Anterior Segment Optical Coherence Tomography in Intraepithelial Versus Invasive Ocular Surface Squamous Neoplasia. Cornea 2018, 37, 1292–1298.
- Kieval, J.Z.; Karp, C.L.; Abou Shousha, M.; Galor, A.; Hoffman, R.A.; Dubovy, S.R.; Wang, J. Ultra-high resolution optical coherence tomography for differentiation of ocular surface squamous neoplasia and pterygia. Ophthalmology 2012, 119, 481–486.
- Shousha, M.A.; Karp, C.L.; Perez, V.L.; Hoffmann, R.; Ventura, R.; Chang, V.; Dubovy, S.R.; Wang, J. Diagnosis and management of conjunctival and corneal intraepithelial neoplasia using ultra high-resolution optical coherence tomography. Ophthalmology 2011, 118, 1531–1537.
- Tran, A.Q.; Venkateswaran, N.; Galor, A.; Karp, C.L. Utility of high-resolution anterior segment optical coherence tomography in the diagnosis and management of sub-clinical ocular surface squamous neoplasia. Eye Vis. 2019, 6, 27.
- Vempuluru, V.S.; Jakati, S.; Godbole, A.; Mishra, D.K.; Mohamed, A.; Kaliki, S. Spectrum of AS-OCT features of ocular surface tumors and correlation of clinico-tomographic features with histopathology: A study of 70 lesions. Int. Ophthalmol. 2021, 41, 3571–3586.
- Sun, Y.; Hua, R. Ocular surface squamous neoplasia: Angiographic characteristics and response to subconjunctival/perilesional 5-fluorouracil injections. Drug Des. Devel. Ther. 2019, 13, 1323–1334.
- Liu, Z.; Karp, C.L.; Galor, A.; Al Bayyat, G.J.; Jiang, H.; Wang, J. Role of optical coherence tomography angiography in the characterization of vascular network patterns of ocular surface squamous neoplasia. Ocul. Surf. 2020, 18, 926–935.
- Wong, J.R.; Nanji, A.A.; Galor, A.; Karp, C.L. Management of conjunctival malignant melanoma: A review and update. Expert Rev. Ophthalmol. 2014, 9, 185–204.
- Banayan, N.; Georgeon, C.; Grieve, K.; Ghoubay, D.; Baudouin, F.; Borderie, V. In vivo confocal microscopy and optical coherence tomography as innovative tools for the diagnosis of limbal stem cell deficiency. J. Fr. Ophtalmol. 2018, 41, e395–e406.
- Liang, Q.; Le, Q.; Cordova, D.W.; Tseng, C.H.; Deng, S.X. Corneal Epithelial Thickness Measured Using Anterior Segment Optical Coherence Tomography as a Diagnostic Parameter for Limbal Stem Cell Deficiency. Am. J. Ophthalmol. 2020, 216, 132–139.
- Haagdorens, M.; Behaegel, J.; Rozema, J.; Van Gerwen, V.; Michiels, S.; Ni Dhubhghaill, S.; Tassignon, M.J.; Zakaria, N. A method for quantifying limbal stem cell niches using OCT imaging. Br. J. Ophthalmol. 2017, 101, 1250–1255.
- Varma, S.; Shanbhag, S.S.; Donthineni, P.R.; Mishra, D.K.; Singh, V.; Basu, S. High-Resolution Optical Coherence Tomography Angiography Characteristics of Limbal Stem Cell Deficiency. Diagnostics 2021, 11, 1130.
- Shanbhag, S.S.; Patel, C.N.; Goyal, R.; Donthineni, P.R.; Singh, V.; Basu, S. Simple limbal epithelial transplantation (SLET): Review of indications, surgical technique, mechanism, outcomes, limitations, and impact. Indian J. Ophthalmol. 2019, 67, 1265–1277.
- Kate, A.; Basu, S. Mini-conjunctival autograft combined with deep anterior lamellar keratoplasty for chronic sequelae of severe unilateral chemical burn: A case report. Int. J. Surg. Case Rep. 2021, 88, 106508.
- Kate, A.; Mudgil, T.; Basu, S. Longitudinal Changes in Corneal Epithelial Thickness and Reflectivity following Simple Limbal Epithelial Transplantation: An Optical Coherence Tomography-Based Study. Curr. Eye Res. 2021, 1–7.
- Kam, K.W.; Patel, C.N.; Nikpoor, N.; Yu, M.; Basu, S. Limbal ischemia: Reliability of clinical assessment and implications in the management of ocular burns. Indian J. Ophthalmol. 2019, 67, 32–36.
- Luisi, J.; Kraft, E.R.; Giannos, S.A.; Patel, K.; Schmitz-Brown, M.E.; Reffatto, V.; Merkley, K.H.; Gupta, P.K. Longitudinal Assessment of Alkali Injury on Mouse Cornea Using Anterior Segment Optical Coherence Tomography. Transl. Vis. Sci. Technol. 2021, 10, 6.
- Tey, K.Y.; Gan, J.; Foo, V.; Tan, B.; Ke, M.Y.; Schmetterer, L.; Mehta, J.S.; Ang, M. Role of anterior segment optical coherence tomography angiography in the assessment of acute chemical ocular injury: A pilot animal model study. Sci. Rep. 2021, 11, 16625.
- Fung, S.S.M.; Stewart, R.M.K.; Dhallu, S.K.; Sim, D.A.; Keane, P.A.; Wilkins, M.R.; Tuft, S.J. Anterior Segment Optical Coherence Tomographic Angiography Assessment of Acute Chemical Injury. Am. J. Ophthalmol. 2019, 205, 165–174.
- Kate, A.; Basu, S. Role of Anterior Segment-Optical Coherence Tomography Angiography in Acute Ocular Burns. Diagnostics 2022, 12, 607.
- Sharif, Z.; Sharif, W. Corneal neovascularization: Updates on pathophysiology, investigations & management. Rom. J. Ophthalmol. 2019, 63, 15–22.
- Roshandel, D.; Eslani, M.; Baradaran-Rafii, A.; Cheung, A.Y.; Kurji, K.; Jabbehdari, S.; Maiz, A.; Jalali, S.; Djalilian, A.R.; Holland, E.J. Current and emerging therapies for corneal neovascularization. Ocul. Surf. 2018, 16, 398–414.
- Martin, R. Cornea and anterior eye assessment with slit lamp biomicroscopy, specular microscopy, confocal microscopy, and ultrasound biomicroscopy. Indian J. Ophthalmol. 2018, 66, 195–201.
- Ang, M.; Sim, D.A.; Keane, P.A.; Sng, C.C.; Egan, C.A.; Tufail, A.; Wilkins, M.R. Optical Coherence Tomography Angiography for Anterior Segment Vasculature Imaging. Ophthalmology 2015, 122, 1740–1747.
- Lee, W.D.; Devarajan, K.; Chua, J.; Schmetterer, L.; Mehta, J.S.; Ang, M. Optical coherence tomography angiography for the anterior segment. Eye Vis. 2019, 6, 4.
- Roberts, P.K.; Goldstein, D.A.; Fawzi, A.A. Anterior Segment Optical Coherence Tomography Angiography for Identification of Iris Vasculature and Staging of Iris Neovascularization: A Pilot Study. Curr. Eye Res. 2017, 42, 1136–1142.
- Akagi, T.; Uji, A.; Huang, A.S.; Weinreb, R.N.; Yamada, T.; Miyata, M.; Kameda, T.; Ikeda, H.O.; Tsujikawa, A. Conjunctival and Intrascleral Vasculatures Assessed Using Anterior Segment Optical Coherence Tomography Angiography in Normal Eyes. Am. J. Ophthalmol. 2018, 196, 1–9.
- Ong, H.S.; Tey, K.Y.; Ke, M.; Tan, B.; Chua, J.; Schmetterer, L.; Mehta, J.S.; Ang, M. A pilot study investigating anterior segment optical coherence tomography angiography as a non-invasive tool in evaluating corneal vascularisation. Sci. Rep. 2021, 11, 1212.
More
Information
Subjects:
Ophthalmology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
15 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




