Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Malihe Mehdizadeh Allaf | -- | 3594 | 2022-04-14 16:52:57 | | | |
| 2 | Conner Chen | -11 word(s) | 3583 | 2022-04-15 05:00:04 | | | | |
| 3 | Conner Chen | -10 word(s) | 3573 | 2022-04-15 05:02:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mehdizadeh Allaf, M. Synechocystis sp.: A Model Microorganism. Encyclopedia. Available online: https://encyclopedia.pub/entry/21786 (accessed on 07 February 2026).
Mehdizadeh Allaf M. Synechocystis sp.: A Model Microorganism. Encyclopedia. Available at: https://encyclopedia.pub/entry/21786. Accessed February 07, 2026.
Mehdizadeh Allaf, Malihe. "Synechocystis sp.: A Model Microorganism" Encyclopedia, https://encyclopedia.pub/entry/21786 (accessed February 07, 2026).
Mehdizadeh Allaf, M. (2022, April 14). Synechocystis sp.: A Model Microorganism. In Encyclopedia. https://encyclopedia.pub/entry/21786
Mehdizadeh Allaf, Malihe. "Synechocystis sp.: A Model Microorganism." Encyclopedia. Web. 14 April, 2022.
Copy Citation
Synechocystis sp. is a unicellular, spherical, non-nitrogen-fixing cyanobacterium with 0.7–8 µm in diameter and no or fine and colorless mucilage layer . The cell envelop of Synechocystis sp. contains the outer membrane, a peptidoglycan layer, and cytoplasmic membrane. The thylakoid membranes, derived from the cytoplasmic membrane, cover the peripheral region of the cell . From cyanobacteria to higher plants, thylakoid membranes are the site of photosynthesis converged near the cytoplasmic membrane. Thylakoid centers, fibrous coated cylindrical structures, 40–50 nm in diameter and 50–1000 nm in length, establish and maintain thylakoid membrane organization
cyanobacteria
Synechocystis
1. Synechocystis sp.: A Model Microorganism
Synechocystis sp. is a unicellular, spherical, non-nitrogen-fixing cyanobacterium with 0.7–8 µm in diameter and no or fine and colorless mucilage layer (Figure 1) [1]. The cell envelop of Synechocystis sp. contains the outer membrane, a peptidoglycan layer, and cytoplasmic membrane. The thylakoid membranes, derived from the cytoplasmic membrane, cover the peripheral region of the cell [2][3]. From cyanobacteria to higher plants, thylakoid membranes are the site of photosynthesis [4] converged near the cytoplasmic membrane. Thylakoid centers, fibrous coated cylindrical structures, 40–50 nm in diameter and 50–1000 nm in length, establish and maintain thylakoid membrane organization [5][6]. In the cytoplasm, various components such as carboxysomes, ribosomes, polyphosphate bodies, lipid bodies, and cyanophycin granules are detectable [3][6]. In the central cytoplasmic region, carboxysomes improve the carbon fixation by the cell [3][7], while the stored phosphate in polyphosphate bodies can be used for adenosine triphosphate (ATP), phospholipid, and nucleic acid biosynthesis [3].
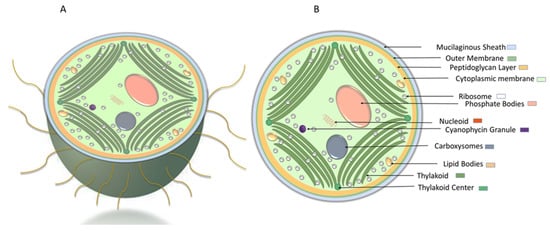
Figure 1. Schematic representation of Synechocystis cell morphology. (A) cross-sectional view of Synechocystis cell with pili. (B) ultrastructure of Synechocystis cell.
Synechocystis sp. cell’s content is more or less homogeneous and proliferates by binary fission (cleavage). The divided cells can be paired for a short time after division [1][8].
Appendages on the surface of Synechocystis sp., called pili, are engaged in motility, adhesion, biofilm formation, and DNA uptake [9]. The pili on the Synechocystis sp. surface are identified as type IV (T4P), after genome sequencing [1]. T4Ps are also responsible for suspending the cells and controlling their position in the water column by increasing the cell viscous drag and also the extension/retraction activity [10]. Pili structures are detectable with a scanning electron microscope and transmission electron microscope after negative staining [9].
The entire genome of Synechocystis sp. strain PCC 6803, as the first photosynthetic autotroph, was sequenced in 1996 by Kaneko et al. [11]. Due to short generation times, the ease of genetic manipulation, and the limited size of genome and proteome, Synechocystis sp. is a suitable model organism to study photosynthesis, lipid metabolism, stress responses, molecular biology, genetic systems, pili system, biofilm formation, etc. [1][12][13][14][15][16]. Genetic manipulation with the aim to improve valuable products has attracted a great deal of attention in the past years; however, it is still in its infancy. In a study on protein–protein interactions (PPIs) it was shown that photosynthesis plays a role in metabolism, cell motility, DNA repair, cell division, and other physiological processes. The additional level of PPIs information greatly enhanced the molecular mechanisms of photosynthesis and therefore improved the understanding of the molecular organization in Synechocystis sp. [17]. The mutant strain of Synechocystis sp. with a knockout gene (slr512) showed a better high light acclimation compared to the wild-type strain [18]. Metabolic engineering design with target genes involved in the process improved the lipid and ethanol productions [19][20] and proliferative growth and certain contents of intracellular pigments including chlorophyll-a and carotenoids [21][22].
2. Motility
2.1. Type IV Pili
Type IV pili are complex multi-protein apparatus with 15–20 kDa pilin subunits, extremely thin (6–9 nm in diameter), and longer than 1 µm, with the ability to form bundles [9][23][24]. After negative staining, three morphologically different type IV pili were identified in Synechocystis sp. PCC 6803; thick, thin, and bundle [13][25][26]. Thick morphotype has a length of 1–3 µm and 6–8 nm in diameter, thin morphotype has 0.5–1 µm in length and 2–3 nm in diameter, and a bundle of pili with a diameter less than 45 nm. The thin morphotype covers the entire outer surface of the Synechocystis cell, while the thick ones are used to link between cells, and are required for motility and transformation competency [25][26]. Type IV pili play important roles in the twitching motility of cells, biofilm formation, aggregation, adhesion, uptake of DNA, and natural competence or secretion [9].
The pilus rod, outer membrane complex, cytoplasmic pilus platform, and secretion ATPases are four distinct subcomplexes of the type IV pili apparatus (Figure 8) [9]. Pilus is made of 500–1000 major structural component protein units called pilin. In addition to pilin, PilQ, PilN, PilO, PilM, PilC, PilB, PilT, and PilD are other proteins in the type IV pilus system [9][26][27][28]. The transport of pilins across the outer membrane and alignment of the pore complex with the pilus platform is facilitated by the protein structure in the membrane complex and powered by secretion ATPases [9][29]. The motility direction of the Synechocystis cell is controlled by the localization of the type IV pili apparatus and strongly correlates with the orientation of the PilB protein adjacent to a specific region of the cytoplasmic membrane (Figure 2) [27][30]. Different studies show two groups of genes: a group of genes that are involved in pilus biogenesis, motility, and assembled proteins [13][25][31][32], and another group that is involved in signal transduction for pilus assembly and phototaxis [32][33][34]. Inactivated genes are involved in pilus biogenesis and abolish cell motility [25].
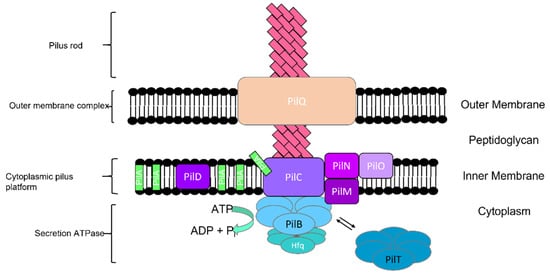
Figure 2. Type IV pilus and four distinct subcomplexes in cyanobacteria.
It was identified that the motility of cyanobacteria is partially controlled by light and several genes that are important for phototaxis [23][35]. The mutant strain of Synechocystis sp. with lacking structural and regulatory components that encode pilin resulted in the loss of motility [15][31][36].
2.2. Cell Movement
Motility is a way to respond to a changing environment [37]. Twitching or gliding motility has been detected in many Gram-negative bacteria, including cyanobacteria, mediated by type IV pili [25]. Twitching is translocation over a moist surface, which requires an extension, tethering, and then retraction activities of pili [38]. Gliding motility is a smooth motion in a direction parallel to the long axis of the cell that occurs at an interface such as solid–liquid, solid–air, and solid–solid [39]. Gliding has been observed in filamentous cyanobacteria such as Oscillatoria and Phormidium species [25] while twitching or gliding motility has been detected especially in Synechocystis sp. [36].
The motile behavior of cyanobacteria, including Synechocystis sp., can be regulated by the direction, intensity, and wavelength of light, which is known as phototaxis [25][28][40]. Cell size, shape, cell refractive index, and the refractive index of the surrounding medium are the parameters that impact phototaxis [28][41]. In phototaxis, Synechocystis cells act as tiny spherical lenses where the image of a light source in the cell’s environment is focused on the cell inner wall. From the focused light, which is detected and analyzed by the light-detecting molecules (called photoreceptors), a signal is sent to the IV pili complexes. Then, the type IV pili complexes, distributed on the cell wall, enable or disable the particular pili for directing the cell towards or away from the light source, leading to a positive or negative phototaxis (Figure 3) [42]. The photosynthetic apparatus also acts as a signaling photoreceptor by absorbing light through chlorophylls, phycobilins, or carotenoids to initiate signal transduction cascades [43]. Red to green wavelengths result in positive phototaxis, while blue wavelength leads to negative phototaxis [44].
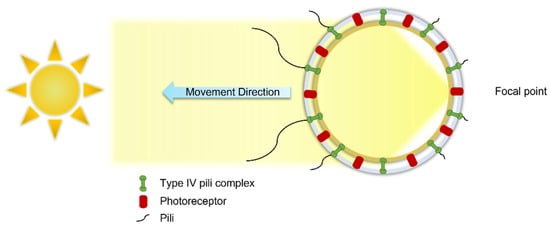
Figure 3. The cyanobacterium Synechocystis sp. cell acts as a lens and focuses the light at the opposite side of a light source (focal point). The photoreceptor proteins deactivate the type IV pili complexes near the focal point and activate the ones at the opposite side of the focal point, which leads the cell towards the light source in positive phototaxis.
The secretion of extracellular substances from the cells improves cell motility, the direction of colony-forming cells, and light focusing by modifying the refractive index near the cell surface [28][40][46]. Single-cell shows limited motility behavior compared to the small colonies [46], by increasing the number of cells in a colony, as a result of cell division or cellular aggregation, the number of motile cells increases [46].
Single Synechocystis cells display an intermittent motion in cell suspensions with two phases; a high-motility “run” and a low-motility “tumble” (Figure 4) [35]. The two phases can be modified under various external stressors. Increasing the light intensity, uniformly over the space, increases the probability of Synechocystis being in the run state randomly in all directions. This feature, however, vanishes after a typical characteristic time of about 1 h, when the initial probability is recovered. These results were well described by a mathematical model based on the linear response theory proposed by Vourc’h et al. [35].
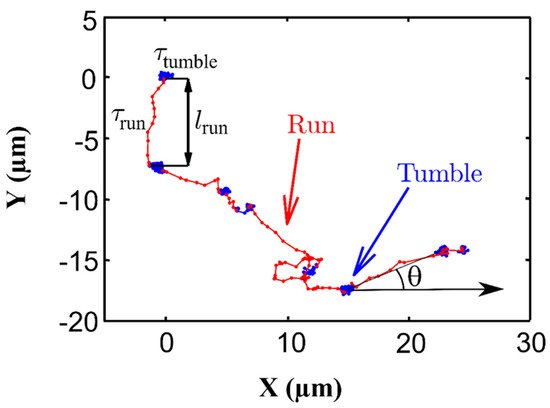
Figure 4. Illustration of run-and-tumble motion of a Synechocystis cell extracted from the experimental trajectory of a single cell. During run the cell moves quickly from one point to another, while during tumble it remains constrained in a given area and tends to change directions.
Synechocystis cells can also undergo biased motility under directional illumination. Under directional light flux, Synehcocystis cells perform phototactic motility and head toward the light source (in positive phototaxis). Vourc’h et al. [35] showed that this biased motility stems from the averaged displacements during run periods, which is no longer random (as it was in the uniform illumination). They showed the bias is the result of the number of runs, which is greater toward the light source, and not of longer runs in this direction. Brought together, these results suggest distinct pathways for the recognition of light intensity and light direction in this prokaryotic microorganism. This effect can be used in the active control of bacterial flows.
It has also been observed that very strong local illumination inactivates the motility apparatus [42]. Increasing the light intensity of more than ~475 µmol m−2 s−1 reverses the direction of Synechocystis cells to move away from the high levels of radiation source [47][48]. Moreover, Synechocystis cells show a negative phototaxis behavior under ultraviolet radiation as an effective escape mechanism to avoid damage to DNA and other cellular components of Synechocystis [44][48][49].
Contrary to the run phase that can extend from a fraction of a second to several minutes, the tumble lasts only a fraction of a second [50]. The tumbling phase is a clockwise rotation that allows the cell to change the motility direction of the next run [51][52].
Chemotaxis is another scheme that allows an organism to move toward or away from gradients of nutrients or other chemical stimuli. Detecting by transmembrane chemoreceptors [38][50][53] the microorganism performs a three-dimensional random walk is observed in a homogenous environment, and the direction of each run is identified after a tumble [51].
3. Biofilm Formation
Bacteria exist predominantly within biofilm in natural, industrial, and clinical settings. Recent research has established that more than 99% of microorganisms in natural settings are fixed on surfaces, due to nutritional and protective benefits linked with life in the adherent populations. In contact with a solid surface, cyanobacteria can aggregate and form a biofilm to grow and survive particularly under environmental stresses [15]. The formation of biofilm by pathogenetic organisms in industrial settings and clinical instruments can infect the living host and can cause chronic infections [54][55]. Alternatively, phototrophic biofilms can be employed in various useful applications such as wastewater purification, bioremediation, aquaculture, and agriculture [56][57][58][59].
Nutrient gradient, cell differentiation, quorum sensing, bacterial motion, and their interaction with the environment can affect the biofilm structure [60]. Microcolonies can form once the bacteria contact the solid surface [61]. Then, once the biofilm developed and matured, some cells secrete chemical molecules that allow the cells to disperse in the environment [62].
Recent research suggests that in cyanobacteria, signaling by secretion of extracellular polysaccharides reduces the diffusion coefficient with time (Figure 5) [15] and, therefore, enhances the formation of biofilm. The hardness of the substrate surface also can affect the motility and the proportion of the motile cells that in turn impact the shape of microcolonies [63].
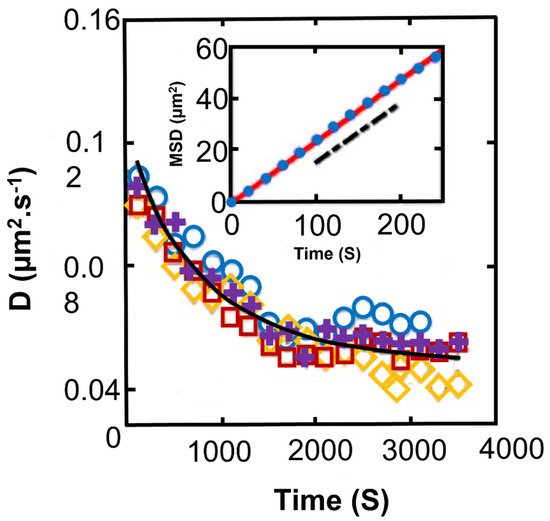
Figure 5. Temporal evolution of the diffusion coefficient of Synechocystis for different experiments; black line: a phenomenological model. Inset: experimental MSD at long times (circles) and as computed from numerical simulations (line). The dashed black line indicates the slope given by the phenomenological model [15]. Among different species of bacteria and cyanobacteria, Synechocystis sp. is a suitable photosynthetic model organism to study biofilm formation and physical characteristics. At present, Synechocystis is probably the only cyanobacterium, which has been investigated in such details, making it an interesting lab model bacterium for biotechnological applications [64].
On a surface exposed to light, phototrophic microorganisms such as unicellular and filamentous cyanobacteria, green algae, and diatoms, are able to form surface-attached communities, called phototrophic biofilms [65][66]. Biofilms are the most successful form of life on the planet earth and the oldest fossilized phototrophic biofilms date back approximately 3.5 billion years ago [56][67][68].
The morphology of biofilms shows various degrees of porosity in the form of mushroom-like macrocolonies surrounded by water-filled voids. They can be smooth and flat, rough, fluffy, or filamentous. However, all morphologies immobilize biofilm cells and permit very diverse habitats of microorganisms on a small scale [69]. The material properties of the surfaces such as surface charge, hydrophobicity, roughness, topography, and stiffness affect adhesion and biofilm formation [70][71][72].
Biofilm initiation depends on the motility of Synechocystis in the first place, followed by physicochemical and electrostatic interactions between the surface and the microorganism’s envelope, and microorganism cells together [60][73][74]. Focusing on the dynamics at the cell scale, the balance between nucleation-division and diffusion-aggregation processes controls the emergence of microcolonies. For example, motility can either favor bacterial aggregation by enabling cell-cell encounters, but also can prevent localized aggregates by enhancing dispersion. The growth of microcolonies of Synechocystis on soft and different hard surfaces was studied by Vourc’h et al. [63]. The results showed that soft surfaces promote higher amounts of motile bacteria pm than the hard ones, and the number of cell clusters at long times was a decreasing function of pm. Therefore, it was proposed that motility allows the bacteria to escape from clusters while non-motile ones are trapped [63]. This study highlighted that for an adequate description of the biofilm formation of Synechocystis it is necessary to account for subpopulations of variable dynamics among a given cell strain. A kinetic model that emphasizes specific interactions between cells, complemented by extensive numerical simulations considering various amounts of cell motility, described adequately the experimental results of this study [63], the high proportion of motile cells enhances dispersion rather than aggregation.
Photosynthetic metabolisms, in general, convert chemical energy to mechanical energy for overcoming the frictional forces between cells and their environment in biofilms [75]. In Synechocystis, pili are essential to initiate biofilm formation and adhesion to both biotic and abiotic surfaces. Pili provides intercellular interactions through aggregation and twitching motility in the secondary structure of the biofilm [74].
To hold the biofilm together, microorganisms, such as Synechocystis produce extracellular polymeric substances (EPS). EPS forms around 90% of the dry mass of biofilms and is composed of mostly polysaccharides, which are complex polymeric carbohydrates, proteins, nucleic acids, and lipids [60][69]. The extracellular polysaccharides generated by cyanobacteria are unique compared to other bacteria and contain sulfate groups. The sulfated extracellular polysaccharides in Synechocystis known as “synechan” and the whole set of genes that regulated synechan biosynthesis and its transcriptional regulation have been recently identified. Synechan is responsible for the buoyancy and floating of cyanobacteria cells. In addition, many sulfated polysaccharides have antiviral, antitumor, or anti-inflammatory characteristics that can be used for human health [76]. EPS provides also the cohesion of biofilms together, immobilizes biofilm cells, mediates the adhesion of the biofilms to the surfaces, creates a three-dimensional polymer network, and provides an external digestive system [69][77]. On water surface or sewage treatment effluents, the produced EPS by Synechocystis species protects the cell death induced by TiO2 nanoparticles [78]. In the bloom-forming cyanobacteria and the presence of proper nutrients and light intensity, the high photosynthetic activity of cells leads to O2 supersaturation, which nucleates into bubbles. These bubbles trapped within the EPS, migrate the biomass upward and aggregate on the surface [79]. In cyanobacteria, the produced EPS improves the soil water-holding capacity, which prevents erosion [80].
In Synechocystis species, in addition to EPS, the S-layer (surface layers of bacterial cell walls), as well as pili, assist to attach the biofilms to surfaces [66][81]. However, the presence of an S-layer may be more important for the initial attachment of cell-glass binding than in cell-cell binding, which can be influenced by the pH and ionic strength of the growth medium [66]. Synechocystis biofilm formation could be induced or altered by stress responses such as pH, salinity, nutrient level, and osmolarity of the culture. It has been observed that the Synechocystis biofilm was initiated for strains subjected to poor nutrient conditions due to the precipitation with calcium in hard water [66]. Previous research showed that the rate of produced EPS in different strains of Synechocystis species (PCC 6803 and PCC 6714) is similar and is related to the stage of growth. The EPS extracted from the above two strains constituted of a minimum of 11–12 mono-oses (number of carbons in open-chain monosaccharides with the suffixes “-ose”) with the ability to form various types of polymers, 15–20% (w/w) uronic derivatives, 10–15% (w/w) osamies, and 7–8% molar ratio sulfate residues. Around 20–40% of the total weight of EPS is made of proteins [82].
Occasionally, some biofilms have shown vertically laminated multilayered regions ranging from millimeters to several centimeters, known as microbial mats or phototrophic mats [56][83]. These types of niches are dominated by the various groups of cyanobacteria, colorless sulfur bacteria, purple bacteria, and sulfur-reducing bacteria [84][85]. Mats are boosted by the photosynthetic behavior of cyanobacteria as they provide nutrients for other microorganisms as well as physical strength [84].
The undesired development of biofilms in the wrong place and at the wrong time is known as biofouling, which has resulted in a considerable economic loss due to material decay, blockage of flow-through membranes, and cleaning procedures on medical devices, water purification systems, ship hulls, pipelines and reservoirs, and desalination plants [68][86][87]. The biofouling organisms present a higher tolerance against biocides, and disinfectants [68]. However, it has been observed that the antibacterial, antialgal, antifungal, cytotoxic, immunosuppressive, and enzyme inhibiting activities of cyanobacteria metabolites have the ability to prevent biofouling [88].
4. Synechocystis in Suspensions
In many natural or industrial (photobioreactor) situations, Synechocystis cells are suspended in a fluid medium; the suspension is often called “active” or “living” fluid, in which cells act as microstructural elements of the fluid and convert the chemical energy of nutrients into mechanical energy for driving the flow. Therefore, active fluids can develop complex spontaneous motions in the absence of external pressure or velocity gradients. In active fluids flow, there may exist a reciprocal interaction between the cell and the carrying fluid. Fluid mechanics, in conjunction with the microorganism motility, governs the dynamics of active fluids. On the one hand, cell motility can modify the physical and rheological properties of the active fluid; and on the other hand, flow shear can affect cell growth and cell motility. In addition, concentrated populations of cells can modify the influence of the environment that acts on the cell, e.g., by shading from the light or depleting of nutrients. Recent experimental results [89] reveal that shear flow affects the growth rate, doubling per day, biomass yield, pigments and lipid production, and rheological properties of Synechocystis sp. CPCC 534 suspensions. The results showed a significant increase in biomass, doubling per day, yield production, and the total amount of chlorophyll-a and carotenoid due to the induced shear flow in comparison with the non-sheared suspension [89]. Meanwhile, shear showed a negative impact on lipid production and cell size [89]. In addition, it is shown the mixing method by which shear has been imposed on the flow can directly impact the growth and pigment production of Synechocystis sp. [90][91]. The growth and pigment production were higher in cultures that were grown under turbulent mixing compared to orbitally shaking [90]. In addition, the growth and pigment production presented an improvement in an agitated photobioreactor (turbulent mixing) and airlift bubble column photobioreactor compared to the stationary culture [91]. The highest level of cellular pigments were detected in the early stages of cultural growth when the cells were preparing for the rapid growth phase [91].
The rheological behavior of active fluids, in terms of the viscosity of Synechocystis cell suspensions, which experienced various shear rates during growth, was also studied [89]. It was observed that the suspension viscosity, normalized at a constant bio-volume fraction of Synechocystis, showed Newtonian behavior (viscosity independent from shear stress) at all cell concentrations. This behavior was observed for both pre-sheared and non-sheared samples, implying that the shear history does not have an influence on the rheological behavior of Synechocystis suspensions [89]. Concerning the effect of cell motility on viscosity, the experiments showed no significant difference between the viscosity of live-cell and dead-cell suspensions [89]. This behavior was attributed to the low and twitching nature of the Synechocystis motility, in contrast to the high motility of swimmers such as Chlamydomonas reinhardtii. However, Synechocystis concentration showed a noticeable increase in the viscosity of cell suspensions. It was observed that the viscosity is a linearly increasing function of the cell volume fraction, as is for passive rigid particles; and a correlation was proposed for this variation. However, the viscosity increase in Synechocystis suspensions was smaller than the one observed in suspensions of rigid spherical particles of similar size [89]. The smaller observed increase in viscosity with cell volume fraction was attributed to the fact that the elastic soft suspended particles (Synechocystis cells) can deform; thus, some of the energy of the flow is dissipated in deforming the cells. This decreases the amount of energy dissipated by hydrodynamic interactions compared to rigid particles, so the viscosity is less than it would be for rigid particles. Another (or concomitant) cause can be the effect of the shape dynamics of soft elastic particles as is discussed in Gao et al. [92].
References
- Ikeuchi, M.; Tabata, S. Synechocystis sp. PCC 6803—A useful tool in the study of the genetics of cyanobacteria. Photosynth. Res. 2001, 70, 73–83.
- Liberton, M.; Berg, R.H.; Heuser, J.; Roth, R.; Pakrasi, H.B. Ultrastructure of the membrane systems in the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. Protoplasma 2006, 227, 129–138.
- Liberton, M.; Austin, J.R.; Berg, R.H.; Pakrasi, H.B. Unique thylakoid membrane architecture of a unicellular N2 -fixing cyanobacterium revealed by electron tomography. Plant Physiol. 2011, 155, 1656–1666.
- Demé, B.; Cataye, C.; Block, M.A.; Maréchal, E.; Jouhet, J. Contribution of galactoglycerolipids to the 3-dimensional architecture of thylakoids. FASEB 2014, 28, 3373–3383.
- Kunkel, D.D. Thylakoid centers: Structures associated with the cyanobacterial photosynthetic membrane system. Arch. Microbiol. 1982, 133, 97–99.
- van de Meene, A.M.L.; Hohmann-Marriott, M.H.; Vermaas, W.F.J.; Roberson, R.W. The three-dimensional structure of the cyanobacterium Synechocystis sp. PCC 6803. Arch. Microbiol. 2006, 184, 259–270.
- Klein, M.G.; Zwart, P.; Bagby, S.C.; Cai, F.; Chisholm, S.W.; Heinhorst, S.; Cannon, G.C.; Kerfeld, C.A. Identification and structural analysis of a novel carboxysome shell protein with implications for metabolite transport. J. Mol. Biol. 2009, 392, 319–333.
- Komárek, J. Coccoid and colonial cyanobacteria. In Freshwater Algae of North America, Ecology and Classification; Wehr, J.D., Sheath, R.G., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 59–116.
- Schuergers, N.; Wilde, A. Appendages of the cyanobacterial cell. Life 2015, 5, 700–715.
- Aguilo-Ferretjans, M.D.M.; Bosch, R.; Puxty, R.J.; Latva, M.; Zadjelovic, V.; Chhun, A.; Sousoni, D.; Polin, M.; Scanlan, D.J.; Christie-Oleza, J.A. Pili allow dominant marine cyanobacteria to avoid sinking and evade predation. Nat. Commun. 2021, 12, 1857.
- Kaneko, T.; Sato, S.; Kotani, H.; Tanaka, A.; Asamizu, E.; Nakamura, Y.; Miyajima, N.; Hirosawa, M.; Sugiura, M.; Sasamoto, S.; et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996, 3, 109–136.
- Dvořák, P.; Casamatta, D.A.; Hašler, P.; Jahodářová, E.; Norwich, A.R.; Poulíčková, A. Diversity of the cyanobacteria. In Modern Topics in the Phototrophic Prokaryotes; Hallenbeck, P., Ed.; Springer: Cham, Switzerland, 2017; pp. 3–46.
- Yoshihara, S.; Geng, X.X.; Okamoto, S.; Yura, K.; Murata, T.; Go, M.; Ohmori, M.; Ikeuchi, M. Mutational analysis of genes involved in pilus structure, motility and transformation competency in the unicellular motile cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2001, 42, 63–73.
- Sarsekeyeva, F.; Zayadan, B.K.; Usserbaeva, A.; Bedbenov, V.S.; Sinetova, M.A.; Los, D.A. Cyanofuels: Biofuels from cyanobacteria. Reality and perspectives. Photosynth. Res. 2015, 125, 329–340.
- Vourc’h, T.; Peerhossaini, H.; Léopoldès, J.; Mejean, A.; Chauvat, F.; Cassier-Chauvat, C. Slowdown of the surface diffusion during early stages of bacterial colonization. Phys. Rev. E 2018, 97, 032407.
- Plohnke, N.; Seidel, T.; Kahmann, U.; Rögner, M.; Schneider, D.; Rexroth, S. The proteome and lipidome of Synechocystis sp. PCC 6803 cells grown under light-activated heterotrophic conditions. Mol. Cell Proteom. 2015, 14, 572–584.
- Xu, C.; Wang, B.; Yang, L.; Hu, L.Z.; Yi, L.; Wang, Y.; Chen, S.; Emili, A.; Wan, C. Global landscape of native protein complexes in Synechocystis sp. PCC 6803. Mol. Cell Proteom. 2021; in press.
- Xie, Y.; Chen, L.; Sun, T.; Jiang, J.; Tian, L.; Cui, J.; Zhang, W. A transporter Slr1512 involved in bicarbonate and pH-dependent acclimation mechanism to high light stress in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148336.
- Eungrasamee, K.; Miao, R.; Incharoensakdi, A.; Lindblad, P.; Jantaro, S. Improved lipid production via fatty acid biosynthesis and free fatty acid recycling in engineered Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2019, 12, 1–13.
- Pembroke, J.T.; Armshaw, P.; Ryan, M.P. Metabolic engineering of the model photoautotrophic cyanobacterium synechocystis for ethanol production: Optimization strategies and challenges. In Fuel Ethanol Production from Sugarcane; Basso, T.P., Basso, L.C., Eds.; IntechOpen: London, UK, 2019; pp. 199–219.
- Utharn, S.; Yodsang, P.; Incharoensakdi, A.; Jantaro, S. Cyanobacterium Synechocystis sp. PCC 6803 lacking adc1 gene produces higher polyhydroxybutyrate accumulation under modified nutrients of acetate supplementation and nitrogen-phosphorus starvation. Biotechnol. Rep. 2021, 31, e00661.
- Mittermair, S.; Lakatos, G.; Nicoletti, C.; Ranglová, K.; Manoel, J.C.; Grivalský, T.; Kozhan, D.M.; Masojídek, J.; Richter, J. Impact of glgA1, glgA2 or glgC overexpression on growth and glycogen production in Synechocystis sp. PCC 6803. J. Biotechnol 2021, 340, 47–56.
- Craig, L.; Volkmann, N.; Arvai, A.S.; Pique, M.E.; Yeager, M.; Egelman, E.H.; Tainer, J.A. Type IV pilus structure by cryo-electron microscopy and crystallography: Implications for pilus assembly and functions. Mol Cell 2006, 23, 651–662.
- Craig, L.; Li, J. Type IV pili: Paradoxes in form and function. Curr. Opin. Struct. Biol. 2009, 18, 267–277.
- Bhaya, D.; Bianco, N.R.; Bryant, D.; Grossman, A.R. Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC6803. Mol. Microbiol. 2000, 37, 941–951.
- Bhaya, D.; Watanabe, N.; Ogawa, T.; Grossman, A.R. The role of an alternative sigma factor in motility and pilus formation in the cyanobacterium Synechocystis sp. strain PCC6803. Proc. Natl. Acad. Sci. USA 1999, 96, 3188–3193.
- Schuergers, N.; Nürnberg, D.J.; Wallner, T.; Mullineaux, C.W.; Wilde, A. PilB localization correlates with the direction of twitching motility in the cyanobacterium. Microbiology 2015, 161, 960–966.
- Schuergers, N.; Mullineaux, C.W.; Wilde, A. Cyanobacteria in motion. Curr. Opin. Plant Biol. 2017, 37, 109–115.
- Maier, B.; Wong, G.C.L. How bacteria use type IV pili machinery on surfaces. Trends Microbiol. 2015, 23, 775–788.
- Nakane, D.; Nishizaka, T. Asymmetric distribution of type IV pili triggered by directional light in unicellular cyanobacteria. Proc. Natl. Acad. Sci. USA 2017, 114, 6593–6598.
- Song, W.; Zang, S.; Li, Z.; Dai, G.; Liu, K.; Chen, M.; Qiu, B. Sycrp2 is essential for twitching motility in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 2018, 200, e00436-18.
- Yoshihara, S.; Ikeuchi, M. Phototactic motility in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Photochem. Photobiol. Sci. 2004, 3, 512–518.
- Bhaya, D.; Takahashi, A.; Grossman, A.R. Light regulation of type IV pilus-dependent motility by chemosensor-like elements in Synechocystis PCC6803. Proc. Natl. Acad. Sci. USA 2001, 98, 7540–7545.
- Bhaya, D.; Takahashi, A.; Shahi, P.; Grossman, A.R. Novel motility mutants of Synechocystis strain PCC 6803 generated by in vitro transposon mutagenesis. J. Bacteriol. 2001, 183, 6140–6143.
- Vourc’h, T.; Léopoldès, J.; Peerhossaini, H. Light control of the diffusion coefficient of active fluids. J. Fluid Eng. 2020, 142, 031109-1–031109-7.
- Chandra, A.; Joubert, L.; Bhaya, D. Modulation of type IV pili phenotypic plasticity through a novel Chaperone-Usher system in Synechocystis sp. BioRxiv 2017, 130278.
- Wadhams, G.H.; Armitage, J.P. Making sense of it all: Bacterial chemotaxis. Nature 2004, 5, 1024–1037.
- Mattick, J.S. Type IV pili and twitching motility. Annu. Rev. Microbiol. 2002, 56, 289–314.
- Wall, D.; Kaiser, D. Type IV pili and cell motility. Mol. Microbiol. 1999, 32, 1–10.
- Ursell, T.; Chau, R.M.W.; Wisen, S.; Bhaya, D.; Huang, K.C. Motility enhancement through surface modification is sufficient for cyanobacterial community organization during phototaxis. PLoS Comput. Biol. 2013, 9, e1003205.
- Samadi, Z.; Johlin, E.; DeGroot, C.; Peerhossaini, H. Modelling optical properties of algae using the finite-difference time domain method. In Proceedings of the Fluids Engineering Division Summer Meeting, Online, 10–12 August 2021; p. 85307, V003T05A016.
- Schuergers, N.; Lenn, T.; Kampmann, R.; Meissner, M.V.; Esteves, T.; Temerinac-ott, M.; Korvink, J.G.; Lowe, A.R.; Mullineaux, C.W.; Wilde, A. Cyanobacteria use micro-optics to sense light direction. eLife 2016, 5, e12620.
- Mullineaux, C.W. How do cyanobacteria sense and respond to light? Mol. Microbiol. 2001, 41, 965–971.
- Chau, R.M.W.; Bhaya, D.; Huang, K.C. Emergent phototactic responses of cyanobacteria under complex light regimes. ASM 2017, 8, 1–15.
- Nilsson, D.E.; Colley, N.J. Comparative vision: Can bacteria really see? Curr. Biol. 2016, 26, R369–R371.
- Burriesci, M.; Bhaya, D. Tracking phototactic responses and modeling motility of Synechocystis sp. strain PCC6803. J. Photochem. Photobiol. B 2008, 91, 77–86.
- Ng, W.; Grossman, A.R.; Bhaya, D. Multiple light inputs control phototaxis in Synechocystis sp. strain PCC6803. J. Bacteriol. 2003, 185, 1599–1607.
- Moon, Y.; Kim, S.; Chung, Y. Sensing and responding to UV-A in cyanobacteria. Int. J. Mol. Sci. 2012, 13, 16303–16332.
- Choi, J.; Chungl, Y.; Moon, Y.; Kim, C.; Watanabe, M.; Song, P.; Joe, C.; Bogorad, L.; Park, Y.M. Photomovement of the Gliding Cyanobacterium Synechocystis sp. PCC 6803. Photochem. Photobiol. 1999, 70, 95–102.
- Webre, D.J.; Wolanin, P.M.; Stock, J.B. Bacterial chemotaxis. Curr. Biol. 2003, 13, 47–49.
- Parkinson, J.S. Signal transduction schemes of bacteria. Cell 1993, 73, 857–871.
- Copeland, M.F.; Weibel, D.B. Bacterial swarming: A model system for studying dynamic self-assembly. Soft Matter 2013, 5, 1174–1187.
- Sourjik, V.; Wingreen, N.S. Responding to chemical gradients: Bacterial chemotaxis. Curr. Opin. Cell Biol. 2012, 24, 262–268.
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 28, 1318–1323.
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nature 2003, 2, 114–122.
- Roeselers, G.; van Loosdrecht, M.C.M.; Muyzer, G. Phototrophic biofilms and their potential applications. J. Appl. Phycol. 2008, 20, 227–235.
- Valiente, E.F.; Ucha, A.; Quesada, A.; Leganés, F.; Carreres, R. Contribution of N2 fixing cyanobacteria to rice production: Availability of nitrogen from 15N-labelled cyanobacteria and ammonium sulphate to rice. Plant Soil 2000, 1, 107–112.
- Bender, J.; Phillips, P. Microbial mats for multiple applications in aquaculture and bioremediation. Bioresour. Technol. 2004, 94, 229–238.
- Ivnitsky, H.; Katz, I.; Minz, D.; Volvovic, G.; Shimoni, E.; Kesselman, E.; Semiat, R.; Dosoretz, C.G. Bacterial community composition and structure of biofilms developing on nanofiltration membranes applied to wastewater treatment. Water Res. 2007, 41, 3924–3935.
- Mazza, M.G. The physics of biofilms-an introduction. J. Phys. D Appl. Phys. 2016, 49, 203001.
- Taktikos, J.; Lin, Y.T.; Stark, H.; Biais, N.; Zaburdaev, V. Pili-induced clustering of N. gonorrhoeae Bacteria. PLoS ONE 2015, 10, e0137661.
- Vlamakis, H.; Chai, Y.; Beauregard, P.; Losick, R.; Kolter, R. Sticking together: Building a biofilm the Bacillus subtilis way. Nat. Rev. Microbiol. 2013, 11, 157–168.
- Vourc’h, T.; Léopoldès, J.; Peerhossaini, H. Clustering of bacteria with heterogeneous motility. Phys. Rev. E 2020, 101, 022612.
- Vermaas, W. Molecular genetics of the cyanobacterium Synechocystis sp. PCC 6803: Principles and possible biotechnology applications. J. Appl. Phycol. 1996, 8, 263–273.
- Mazard, S.; Penesyan, A.; Ostrowski, M.; Paulsen, I.T.; Egan, S. Tiny microbes with a big impact: The role of cyanobacteria and their metabolites in shaping our future. Mar. Drugs 2016, 14, 97.
- Allen, R.; Rittmann, B.E.; Curtiss, R. Axenic biofilm formation and aggregation by Synechocystis concentration and require cell surface structures. Appl. Environ. Microb. 2019, 85, e02192-18.
- Des Marais, D.J. Microbial mats and the early evolution of life. Trends Ecol. Evol 1990, 5, 140–144.
- Flemming, H.C. Biofouling in water systems—Cases, causes and countermeasures. Appl. Microbiol. Biotechnol. 2002, 59, 629–640.
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633.
- Lichter, J.A.; Thompson, M.T.; Delgadillo, M.; Nishikawa, T.; Rubner, M.F.; Van Vliet, K.J. Substrata mechanical stiffness can regulate adhesion of viable bacteria. Biomacromolecules 2008, 9, 1571–1578.
- Guégan, C.; Garderes, J.; Le Pennec, G.; Gaillard, F.; Fay, F.; Linossier, I.; Herry, J.M.; Bellon Fontaine, V.R.K. Alteration of bacterial adhesion induced by the substrate stiffness. Colloid Surface B 2014, 114, 193–200.
- Song, F.; Koo, H.; Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 2015, 94, 1027–1034.
- Bell, G.I. Models for the specific adhesion of cells to cells. Science 1978, 200, 618–627.
- Berne, C.; Ducret, A.; Hardy, G.G.; Brun, Y.V. Adhesins involved in attachment to abiotic surfaces by gram-negative bacteria. Microbiol. Spectr. 2015, 3, 1–27.
- Moore, K.A.; Altus, S.; Tay, J.W.; Meehl, J.B.; Johnson, E.B.; Bortz, D.M.; Cameron, J.C. Mechanical regulation of photosynthesis in cyanobacteria. Nat. Microbiol. 2020, 5, 757–767.
- Maeda, K.; Okuda, Y.; Enomoto, G.; Watanabe, S.; Ikeuchi, M. Biosynthesis of a sulfated exopolysaccharide, synechan, and bloom formation in the model cyanobacterium Synechocystis sp. strain PCC 6803. eLife 2021, 10, e66538.
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectrum. 2015, 3, 1–30.
- Planchon, M.; Jittawuttipoka, T.; Cassier-Chauvat, C.; Guyot, F.; Gelabert, A.; Benedetti, M.F.; Chauvat, F.; Spalla, O. Exopolysaccharides protect Synechocystis against the deleterious effects of titanium dioxide nanoparticles in natural and artificial waters. J. Colloid Interface Sci. 2013, 405, 35–43.
- Dervaux, J.; Mejean, A.; Brunet, P. Irreversible collective migration of cyanobacteria in eutrophic conditions. PLoS ONE 2015, 10, e0120906.
- Rao, D.L.N.; Burns, R.G. The effect of surface growth of blue-green algae and bryophytes on some microbiological, biochemical, and physical soil properties. Biol. Fertil. Soils 1990, 9, 239–244.
- Šmarda, J.; Šmajs, D.; Komrska, J.; Krzyžánek, V. S-layers on cell walls of cyanobacteria. Micron 2002, 33, 257–277.
- Panoff, J.; Priem, B.; Morvan, H.; Joset, F. Sulphated exopolysaccharides produced by two unicellular strains of cyanobacteria, Synechocystis PCC 6803 and 6714. Arch. Microbiol. 1988, 150, 558–563.
- Prieto-Barajas, C.M.; Valencia-Cantero, E.; Santoyo, G. Microbial mat ecosystems: Structure types, functional diversity, and biotechnological application. Electron. J. Biotechnol. 2018, 31, 48–56.
- van Gemerden, H. Microbial mats: A joint venture. Mar. Geol. 1993, 113, 3–25.
- Klatt, J.M.; Meyer, S.; Häusler, S.; Macalady, J.L.; de Beer, D.; Polerecky, L. Structure and function of natural sulphide-oxidizing microbial mats under dynamic input of light and chemical energy. ISME J. 2016, 10, 921–933.
- Baker, J.S.; Dudley, L.Y. Biofouling in membrane systems—A review. Desalination 1998, 118, 81–90.
- Parnasa, R.; Nagar, E.; Sendersky, E.; Reich, Z.; Simkovsky, R.; Golden, S.; Schwarz, R. Small secreted proteins enable biofilm development in the cyanobacterium Synechococcus elongatus. Sci. Rep. 2016, 6, 32209.
- Dahms, H.; Ying, X.; Pfeiffer, C. Antifouling potential of cyanobacteria: A mini-review. Biofouling 2006, 22, 317–327.
- Mehdizadeh Allaf, M.; Habib, Z.; de Bruyn, J.R.; DeGroot, C.T.; Peerhossaini, H. Rheological and Biophysical Properties of Living Fluids Under Shear: Active Suspensions of Synechocystis sp. CPCC 534. J. Fluid Eng. 2022, 144, 021208.
- Samadi, Z.; Mehdizadeh Allaf, M.; Saifi, R.; De Groot, C.T.; Peerhossaini, H. Effects of turbulent mixing and orbitally shaking on cell growth and biomass production in active fluids. AJBSR 2022, 15, 396–404.
- Mehdizadeh Allaf, M.; Fadlallah, H.; Jarrahi, M.; Peerhossaini, H. Growth and pigment production of Synechocystis sp. PCC 6803 under shear stress. Can. J. Chem. Eng. 2022; in press.
- Gao, T.; Hu, H.H.; Castaneda, P.P. Shape dynamics and rheology of soft elastic particles in a shear flow. Phys. Rev. Lett. 2012, 108, 058302.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.1K
Revisions:
3 times
(View History)
Update Date:
15 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




