
Video Upload Options
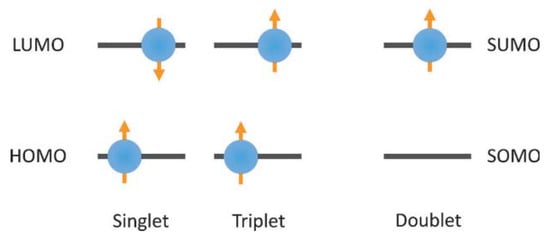
Perchlorotrityl radical (PTM), tris (2,4,6-trichlorophenyl) methyl radical (TTM), (3,5-dichloro-4-pyridyl) bis (2,4,6 trichlorophenyl) methyl radical (PyBTM), (N-carbazolyl) bis (2,4,6-trichlorophenyl) methyl radical (CzBTM), and their derivatives are stable organic radicals that exhibit light emissions at room temperature. Since these triarylmethyl radicals have an unpaired electron, their electron spins at the lowest excited state and ground state are both doublets, and the transition from the lowest excited state to the ground state does not pose the problem of a spin-forbidden reaction. When used as OLED layers, these triarylmethyl radicals exhibit unique light-emitting properties, which can increase the theoretical upper limit of the OLED’s internal quantum efficiency (IQE) to 100%.
1. Introduction
2. Introduction to OLED
2.1. OLED’s Working Principle and Material Structure Composition
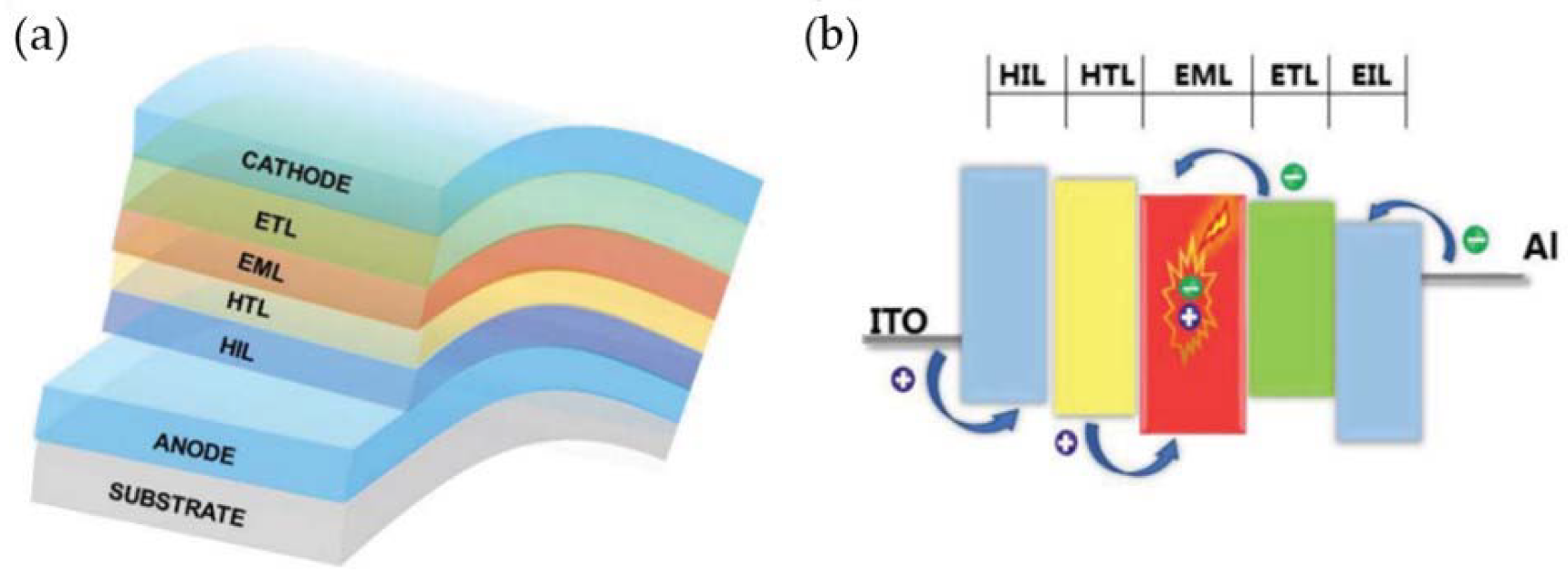
2.2. Classification of OLED Lighting Methods
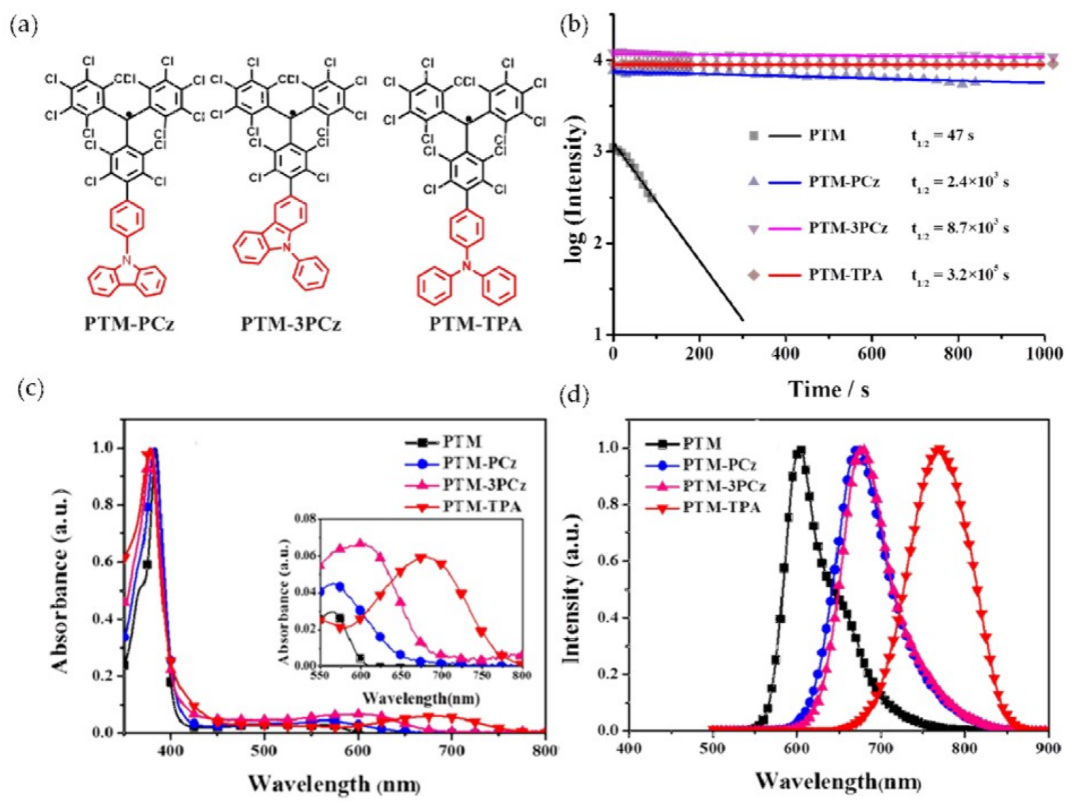
| Molecule | Solvent | Λf a (nm) | Φf | Ref. |
|---|---|---|---|---|
| PTM-PCz | cyclohexane | 673 | 0.44 | [88] |
| PTM-3PCz | cyclohexane | 679 | 0.57 | [88] |
| PTM-TPA | cyclohexane | 767 | 0.26 | [88] |
| PTM-3PCz | cyclohexane | 663 | 0.043 | [88] |
| PTM-TPA | cyclohexane | 664 | 0.29 | [88] |
| 3I-PTMR b | cyclohexane | 605 | 0.016 | [89] |
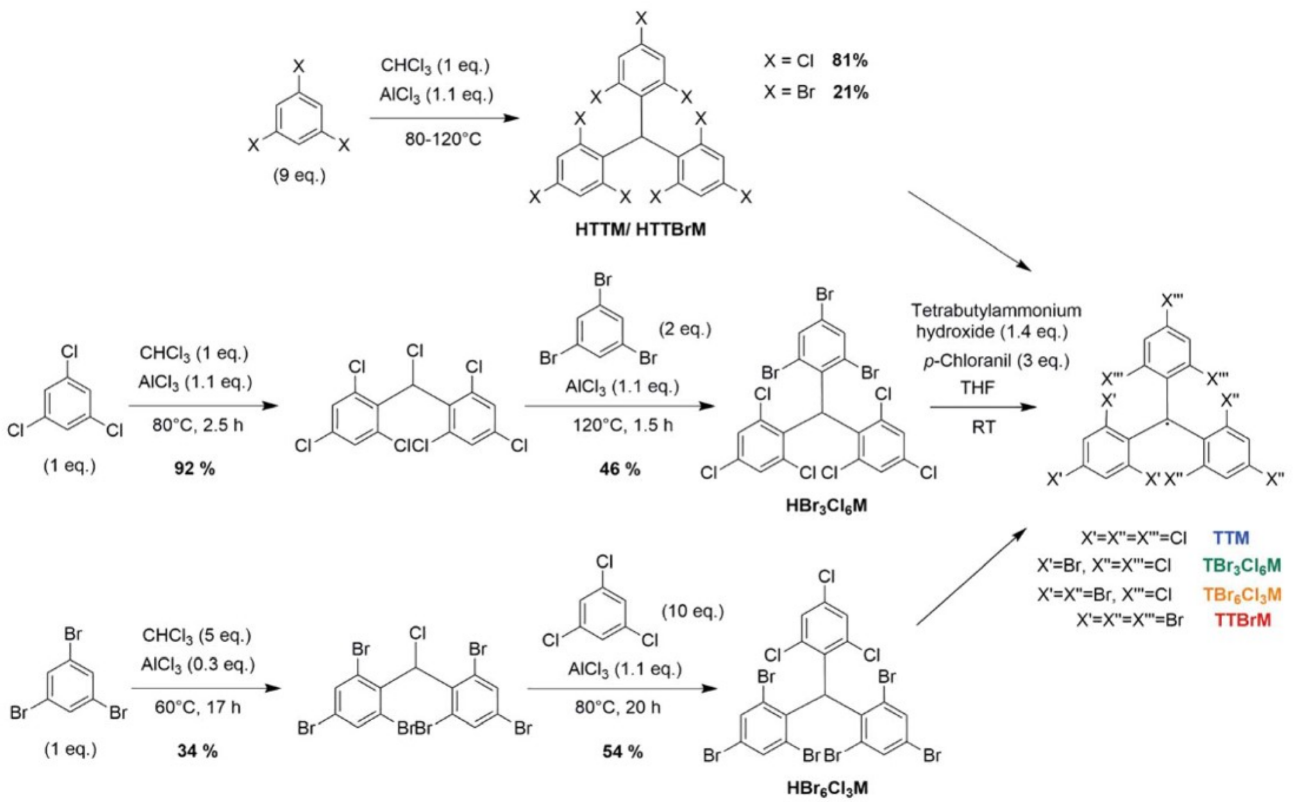
| TTM | cyclohexane | 680 | 0.54 | [89] |
| TTM-1Cz | / | 611 | 0.91 | [90] |
| TTM-2Bi | cyclohexane | 562 | 0.008 | [91] |
| TTM-3Bi | cyclohexane | 692 | 0.58 | [92] |
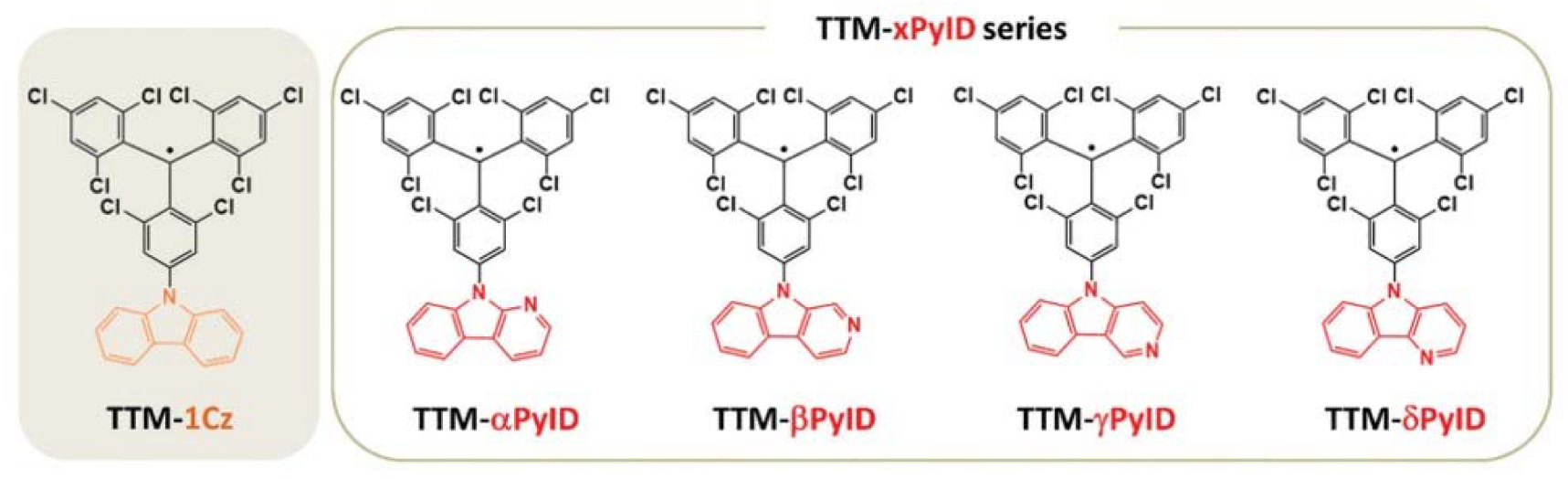
| TTM-αPyID | chloroform | 588 | 0.16 | [93] |
| TTM-βPyID | chloroform | 593 | 0.30 | [93] |
| TTM-γPyID | cyclohexane | 599 | 0.63 | [94] |
| TTM-δPyID | cyclohexane | 610 | 0.98 | [94] |
| 2αPyID-TTM | cyclohexane | 598 | 0.37 | [94] |
| 2δPyID-TTM | cyclohexane | 614 | 0.89 | [94] |
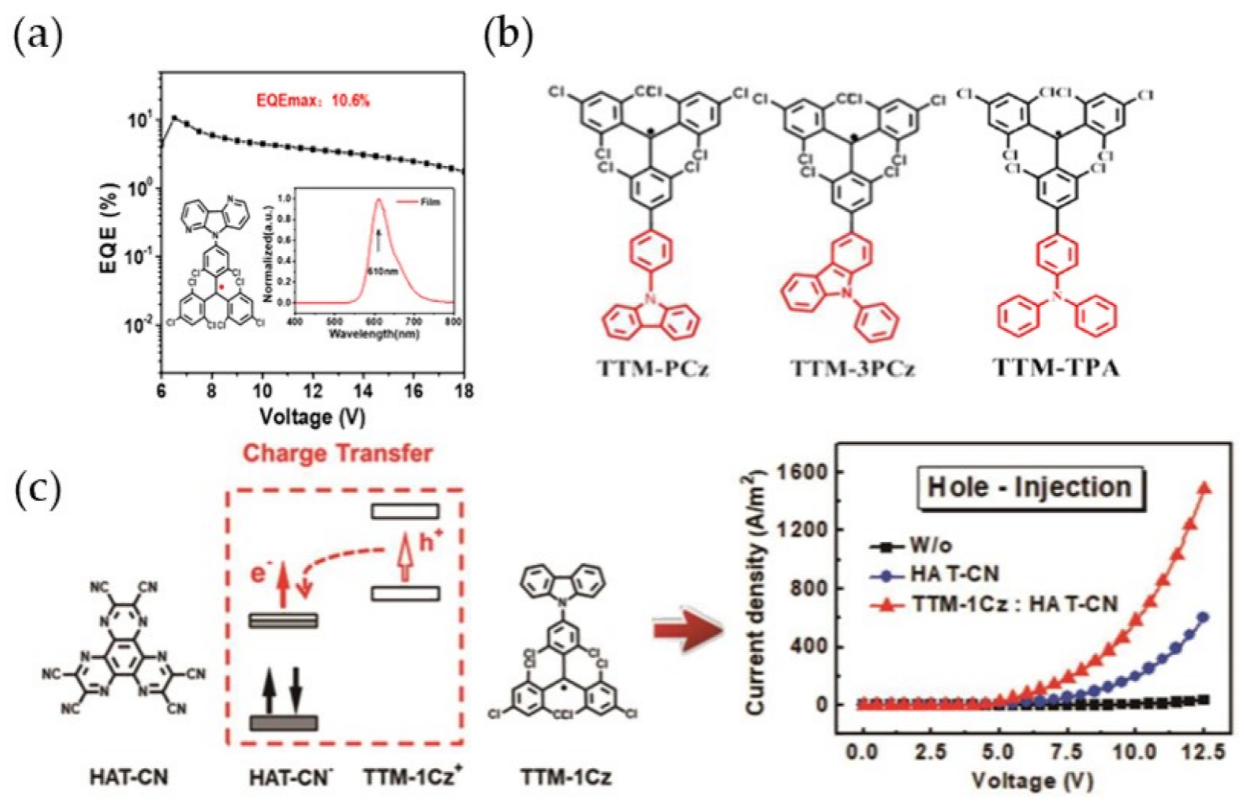
| TTM-PCz | cyclohexane | 608 | 0.65 | [95] |
| TTM-3PCz | cyclohexane | 627 | 0.92 | [95] |
| TBr3Cl6M | DCM | / | 0.018 | [96] |
| TBr6Cl3M | DCM | / | 0.012 | [96] |
| TTBrM | DCM | / | 0.008 | [96] |
| TTM-DPA | cyclohexane | 705 | 0.65 | [97] |
| TTM-DBPA | cyclohexane | 748 | 0.28 | [97] |
| TTM-DFA | cyclohexane | 809 | 0.05 | [97] |
| PyBTM | EPA c (77 K) | / | 0.81 | [98] |
| Br2PyBTM | chloroform | 593 | 0.02 | [99] |
| F2PyBTM | chloroform | 566 | 0.06 | [99] |
| PyBTM d | / | 680 | 0.89 | [100] |
| bisPyTM | dichloromethane | 650 | 0.009 | [101] |
| mBr2-F2PyBTM | EPA c (77 K) | / | 0.11 | [102] |
| mPh2-F2PyBTM | EPA c (77 K) | / | 0.12 | [102] |
| mClPh2-F2PyBTM | EPA c (77 K) | / | 0.11 | [102] |
| mPy2-F2PyBTM | EPA c (77 K) | / | 0.07 | [102] |
| trisPyM | CH2Cl2 | 700 | 0.0085 | [103] |
| metaPyBTM | dichloromethane | 571 | 0.017 | [104] |
| CzBTM | cyclohexane | 697 | 0.020 | [105] |
| PyID-BTM | cyclohexane | 664 | 0.195 | [106] |
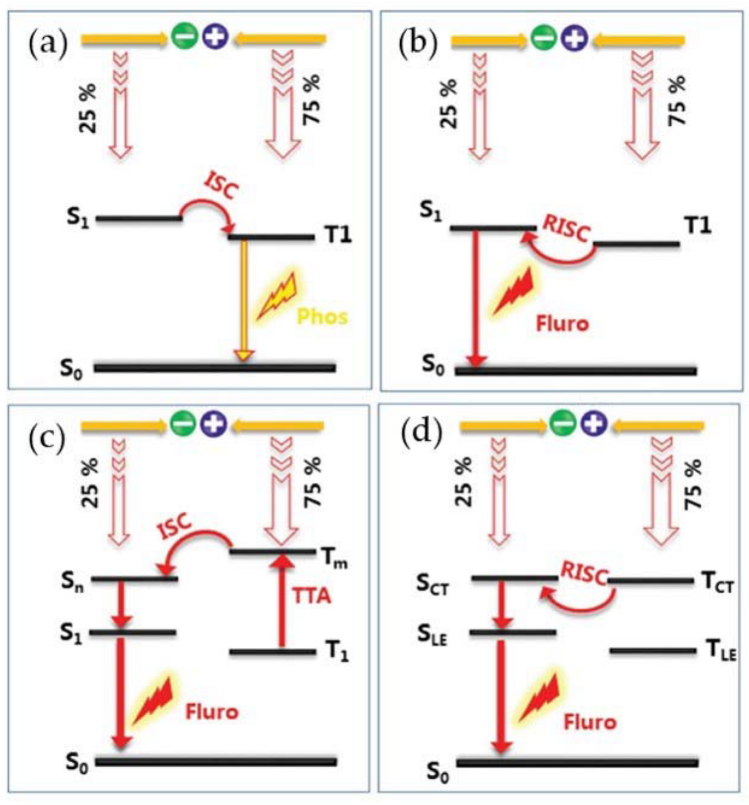
3. PTM-Based Luminescent Organic Radicals
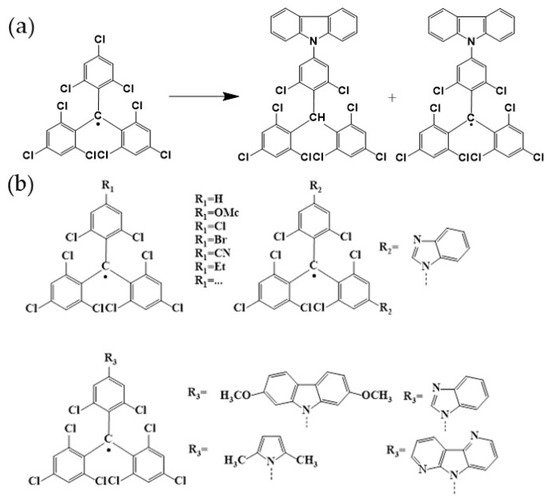
4. TTM-Based Luminescent Organic Radicals
References
- Hayeck, N.; Mussa, I.; Perrier, S.; George, C. Production of peroxy radicals from the photochemical reaction of fatty acids at the air–water interface. ACS Earth Space Chem. 2020, 4, 1247–1253.
- Ao, C.; Ruan, S.; He, W.; He, C.; Xu, K.; Zhang, L. Theoretical investigation of chemical reaction kinetics of CO2 and vinyl radical under catalytic combustion. Fuel 2021, 305, 121566.
- Tao, Y.; Jasper, A.W.; Georgievskii, Y.; Klippenstein, S.J.; Sivaramakrishnan, R. Termolecular chemistry facilitated by radical-radical recombinations and its impact on flame speed predictions. Proc. Combust. Inst. 2021, 38, 515–522.
- Bergwik, J.; Akerstrom, B. α1-Microglobulin binds Illuminated flavins and has a protective effect against sublethal riboflavin-induced damage in retinal epithelial cells. Front. Physiol. 2020, 11, 295.
- Di Meo, S.; Venditti, P. Evolution of the knowledge of free radicals and other oxidants. Oxid. Med. Cell. Longevity 2020, 2020, 9829176.
- Hui, Y.; Wen, S.; Lihong, W.; Chuang, W.; Chaoyun, W. Molecular structures of nonvolatile components in the haihong fruit wine and their free radical scavenging effect. Food Chem. 2021, 353, 129298.
- Gridnev, A.A.; Gudkov, M.V.; Bekhli, L.S.; Mel’nikov, V.P. Possible Mechanism of thermal reduction of graphite oxide. Russ. J. Phys. Chem. B 2019, 12, 1008–1016.
- F Marcos, C.; Neo, A.G.; Díaz, J.; Martínez-Caballero, S. A safe and green benzylic radical bromination experiment. J. Chem. Educ. 2020, 97, 582–585.
- Li, G.; Luo, Z.; Wang, W.; Cen, J. A study of the mechanisms of guaiacol pyrolysis based on free radicals detection technology. Catalysts 2020, 10, 295.
- Haikal, R.R.; Soliman, A.B.; Pellechia, P.J.; Heißler, S.; Tsotsalas, M.; Ali, S.S.; Alkordi, M.H. Functional microporous polymers through Cu-mediated, free-radical polymerization of buckminster fullerene. Carbon 2017, 118, 215–224.
- Sun, H.; Choi, W.; Zang, N.; Battistella, C.; Thompson, M.P.; Cao, W.; Zhou, X.; Forman, C.; Gianneschi, N.C. Bioactive peptide brush polymers via photoinduced reversible-deactivation radical polymerization. Angew. Chem. Int. Ed. 2019, 58, 17359–17364.
- Zhu, Z.; Shi, S.; Wang, H. Radical chain polymerization catalyzed by graphene oxide and cooperative hydrogen bonding. Macromol. Rapid Comm. 2016, 37, 187–194.
- Wang, Y.; Li, H.; Dong, J.; Hu, L.; Wei, D.; Bai, L.; Yang, H.; Chen, H. Recyclable bio-based photoredox catalyst in metal-free atom transfer radical polymerization. Macromol. Chem. Phys. 2021, 222, 2000406.
- Kamath, D.; Mezyk, S.P.; Minakata, D. Elucidating the elementary reaction pathways and kinetics of hydroxyl radical-induced acetone degradation in aqueous phase advanced oxidation processes. Environ. Sci. Technol. 2018, 52, 7763–7774.
- Yang, Q.; Ma, Y.; Chen, F.; Yao, F.; Sun, J.; Wang, S.; Yi, K.; Hou, L.; Li, X.; Wang, D. Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water. Chem. Eng. J. 2019, 378, 122149.
- Hadley, J.H.; Gordy, W. Nuclear coupling of 33S and the nature of free radicals in irradiated crystals of N-acetyl-l-cysteine. Proc. Natl. Acad. Sci. USA 1977, 74, 216–220.
- Alagappan, A.; Costen, M.L.; McKendrick, K.G. Frequency modulated spectroscopy as a probe of molecular collision dynamics. Spectrochim. Acta Part A 2006, 63, 910–922.
- Blumenschein, F.; Tamski, M.; Roussel, C.; Smolinsky, E.Z.B.; Tassinari, F.; Naaman, R.; Ansermet, J.P. Spin-dependent charge transfer at chiral electrodes probed by magnetic resonance. Phys. Chem. Chem. Phys. 2020, 22, 997–1002.
- Varshney, D.; Kumar, A.; Prakash, J.; Meena, R.; Asokan, K. Gamma irradiation induced dielectric modulation and dynamic memory in nematic liquid crystal materials. J. Mol. Liq. 2020, 320, 114374.
- Zhou, Z.; Qiao, C.; Yao, J.; Yan, Y.; Zhao, Y.S. Exciton funneling amplified photoluminescence anisotropy in organic radical-doped microcrystals. J. Mater. Chem. C 2022, 10, 2551–2555.
- Iwasaki, A.; Hu, L.; Suizu, R.; Nomura, K.; Yoshikawa, H.; Awaga, K.; Noda, Y.; Kanai, K.; Ouchi, Y.; Seki, K.; et al. Interactive radical dimers in photoconductive organic thin films. Angew. Chem. Int. Ed. 2009, 48, 4022–4024.
- Huang, X.; Zhang, Y.; Bai, J.; Li, J.; Li, L.; Zhou, T.; Chen, S.; Wang, J.; Rahim, M.; Guan, X.; et al. Efficient degradation of N-containing organic wastewater via chlorine oxide radical generated by a photoelectrochemical system. Chem. Eng. J. 2020, 392, 123695.
- Hudson, J.M.; Hele, T.J.H.; Evans, E.W. Efficient light-emitting diodes from organic radicals with doublet emission. J. Appl. Phys. 2021, 129, 180901.
- Mu, Y.; Liu, Y.; Tian, H.; Ou, D.; Gong, L.; Zhao, J.; Zhang, Y.; Huo, Y.; Yang, Z.; Chi, Z. Sensitive and repeatable photoinduced luminescent radicals from a simple organic crystal. Angew. Chem. Int. Ed. 2021, 60, 6367–6371.
- Cho, J.M.; Song, C.E.; Moon, S.-J.; Shin, W.S.; Hong, S.; Kim, S.H.; Cho, S.; Lee, J.-K. Scavenging of galvinoxyl spin 1/2 radicals in the processing of organic spintronics. Org. Electron. 2018, 55, 21–25.
- Poggini, L.; Cucinotta, G.; Sorace, L.; Caneschi, A.; Gatteschi, D.; Sessoli, R.; Mannini, M. Nitronyl nitroxide radicals at the interface: A hybrid architecture for spintronics. Rend. Fisi. Acc. Lincei. 2018, 29, 623–630.
- Ramos-Berdullas, N.; Gil-Guerrero, S.; Pena-Gallego, A.; Mandado, M. The effect of spin polarization on the electron transport of molecular wires with diradical character. Phys. Chem. Chem. Phys. 2021, 23, 4777–4783.
- Rane, V. Achieving maximal enhancement of electron spin polarization in stable nitroxyl radicals at room temperature. J. Phys. Chem. B 2021, 125, 5620–5629.
- Kong, X.J.; Zheng, C.; Lan, Y.H.; Chi, S.S.; Dong, Q.; Liu, H.L.; Peng, C.; Dong, L.Y.; Xu, L.; Wang, X.H. Synthesis of multirecognition magnetic molecularly imprinted polymer by atom transfer radical polymerization and its application in magnetic solid-phase extraction. Anal. Bioanal. Chem. 2018, 410, 247–257.
- Xiong, Y.C.; Zhou, W.H.; Li, W.; Huang, H.M.; Laref, A.; Nan, N.; Zhang, J.; Yang, J.T. Emergent electronically-controllable local-field-inducer based on a molecular break-junction with magnetic radical. Phys. Chem. Chem. Phys. 2019, 21, 21693–21697.
- Xie, H.; Vignesh, K.R.; Zhang, X.; Dunbar, K.R. From spin-crossover to single molecule magnetism: Tuning magnetic properties of Co(ii) bis-ferrocenylterpy cations via supramolecular interactions with organocyanide radical anions. J. Mater. Chem. C 2020, 8, 8135–8144.
- Yan, H.; Wu, B.; Meng, Y.S.; Zhang, W.X.; Xi, Z. Synthesis, structure, and magnetic properties of rare-earth bis(diazabutadiene) diradical complexes. Inorg. Chem. 2021, 60, 1315–1319.
- Gomberg, M. An instance of trivalent carbon: Triphenylmethyl. J. Am. Chem. Soc. 1900, 22, 757–771.
- Wilhelm, S.; Tobias, W. III. ueber analoga des triphenylmethyls in der biphenylreihe. Justus Liebigs Ann. Chem. 1909, 368, 295–304.
- Metzmacher, K.D.; Brignell, J.E. Apparent charge mobility from breakdown times in a dielectric liquid. J. Phys. Appl. Phys. 1970, 3, 2215–2225.
- Chen, B.; Liu, B.; Zeng, J.; Nie, H.; Xiong, Y.; Zou, J.; Ning, H.; Wang, Z.; Zhao, Z.; Tang, B.Z. Efficient bipolar blue AIEgens for high-performance nondoped blue OLEDs and hybrid white OLEDs. Adv. Funct. Mater. 2018, 28, 1803369.
- Salehi, A.; Fu, X.; Shin, D.H.; So, F. Recent advances in OLED optical design. Adv. Funct. Mater. 2019, 29, 1808803.
- Chen, D.; Li, W.; Gan, L.; Wang, Z.; Li, M.; Su, S.-J. Non-noble-metal-based organic emitters for OLED applications. Mater. Sci. Eng. R 2020, 142, 100581.
- Helfrich, W.; Schneider, W.G. Recombination radiation in anthracene crystals. Phys. Rev. Lett. 1965, 14, 229–231.
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915.
- Chiu, T.-L.; Xianyu, H.; Ge, Z.; Lee, J.-H.; Liu, K.-C.; Wu, S.-T. Transflective device with a transparent organic light-emitting diode and a reflective liquid-crystal device. J. Soc. Inf. Disp. 2009, 17, 1009–1013.
- Ding, B.-F.; Alameh, K. High-contrast tandem organic light-emitting devices employing semitransparent intermediate layers of LiF/Al/C60. J. Phys. Chem. C 2012, 116, 24690–24694.
- Sim, B.; Moon, C.K.; Kim, K.H.; Kim, J.J. Quantitative analysis of the efficiency of OLEDs. ACS Appl. Mater. Interfaces 2016, 8, 33010–33018.
- Zeng, L.; Chime, A.C.; Chakaroun, M.; Bensmida, S.; Nkwawo, H.; Boudrioua, A.; Fischer, A.P.A. Electrical and optical impulse response of high-speed micro-OLEDs under ultrashort pulse excitation. IEEE Trans. Electron. Devices 2017, 64, 2942–2948.
- Elsamnah, F.; Bilgaiyan, A.; Affiq, M.; Shim, C.H.; Ishidai, H.; Hattori, R. Reflectance-based organic pulse meter sensor for wireless monitoring of photoplethysmogram signal. Biosensors 2019, 9, 87.
- Peng, Q.; Obolda, A.; Zhang, M.; Li, F. Organic light-emitting diodes using a neutral pi radical as emitter: The emission from a doublet. Angew. Chem. Int. Ed. 2015, 54, 7091–7195.
- Zhang, C.; Feng, Y.; Yong, G.; Shen, C.; Zhao, Y. Proton-shared hydrogen bond: Promoting generation of novel triradicals, and serving as phosphorescent and magnetic switch. Synth. Met. 2016, 220, 477–483.
- Wu, X.; Lv, X.; Wang, J.; Sun, L.; Yan, Y. Surface molecular imprinted polymers based on Mn-doped ZnS quantum dots by atom transfer radical polymerization for a room-temperature phosphorescence probe of bifenthrin. Anal. Methods 2017, 9, 4609–4615.
- Ni, L.; Yong, G. Crystal structures, phosphorescent and magnetic properties of novel 1,2-dihydroisoquinoline radicals. J. Mol. Struct. 2018, 1171, 614–618.
- Kosai, J.; Masuda, Y.; Chikayasu, Y.; Takahashi, Y.; Sasabe, H.; Chiba, T.; Kido, J.; Mori, H. S-vinyl sulfide-derived pendant-type sulfone/phenoxazine-based polymers exhibiting thermally activated delayed fluorescence: Synthesis and photophysical property characterization. ACS Appl. Polym. Mater. 2020, 2, 3310–3318.
- Poisson, J.; Tonge, C.M.; Paisley, N.R.; Sauvé, E.R.; McMillan, H.; Halldorson, S.V.; Hudson, Z.M. Exploring the scope of through-space charge-transfer thermally activated delayed fluorescence in acrylic donor–acceptor copolymers. Macromolecules 2021, 54, 2466–2476.
- Zhang, Z.; Chen, W.; Zhang, Y.; Wang, Y.; Tian, Y.; Fang, L.; Ba, X. Photoredox organocatalysts with thermally activated delayed fluorescence for visible-light-driven atom transfer radical polymerization. Macromolecules 2021, 54, 4633–4640.
- Wang, Z.; Gao, Y.; Hussain, M.; Kundu, S.; Rane, V.; Hayvali, M.; Yildiz, E.A.; Zhao, J.; Yaglioglu, H.G.; Das, R.; et al. Efficient radical-enhanced intersystem crossing in an NDI-TEMPO dyad: Photophysics, electron spin polarization, and application in photodynamic therapy. Chem. Eur. J. 2018, 24, 18663–18675.
- Awwad, N.; Bui, A.T.; Danilov, E.O.; Castellano, F.N. Visible-light-initiated free-radical polymerization by homomolecular triplet-triplet annihilation. Chem 2020, 6, 3071–3085.
- Dartar, S.; Ucuncu, M.; Karakus, E.; Hou, Y.; Zhao, J.; Emrullahoglu, M. BODIPY-vinyl dibromides as triplet sensitisers for photodynamic therapy and triplet-triplet annihilation upconversion. Chem. Commun. 2021, 57, 6039–6042.
- Tan, G.; Li, J.; Zhang, L.; Chen, C.; Zhao, Y.; Wang, X.; Song, Y.; Zhang, Y.Q.; Driess, M. The charge transfer approach to heavier main-group element radicals in transition-metal complexes. Angew. Chem. Int. Ed. 2017, 56, 12741–12745.
- Kawanishi, Y.; Mutoh, K.; Abe, J.; Kobayashi, Y. Extending the lifetimes of charge transfer states generated by photoinduced heterolysis of photochromic radical complexes. Asian J. Org. Chem. 2021, 10, 891–900.
- Xiao, S.; Zhang, S.-T.; Gao, Y.; Yang, X.; Liu, H.; Li, W.; Yang, B. Efficient and stable deep-blue narrow-spectrum electroluminescence based on hybridized local and charge-transfer (HLCT) state. Dye. Pigm. 2021, 193, 109482.
- Brandt, J.R.; Wang, X.; Yang, Y.; Campbell, A.J.; Fuchter, M.J. Circularly polarized phosphorescent electroluminescence with a high dissymmetry factor from PHOLEDs based on a platinahelicene. J. Am. Chem. Soc. 2016, 138, 9743–9746.
- Dhbaibi, K.; Abella, L.; Meunier-Della-Gatta, S.; Roisnel, T.; Vanthuyne, N.; Jamoussi, B.; Pieters, G.; Racine, B.; Quesnel, E.; Autschbach, J.; et al. Achieving high circularly polarized luminescence with push-pull helicenic systems: From rationalized design to top-emission CP-OLED applications. Chem. Sci. 2021, 12, 5522–5533.
- Jin, Q.; Chen, S.; Sang, Y.; Guo, H.; Dong, S.; Han, J.; Chen, W.; Yang, X.; Li, F.; Duan, P. Circularly polarized luminescence of achiral open-shell pi-radicals. Chem. Commun. 2019, 55, 6583–6586.
- Wei, Q.; Fei, N.; Islam, A.; Lei, T.; Hong, L.; Peng, R.; Fan, X.; Chen, L.; Gao, P.; Ge, Z. Small-molecule emitters with high quantum efficiency: Mechanisms, structures, and applications in OLED devices. Adv. Opt. Mater. 2018, 6, 1800512.
- Xu, Y.; Xu, P.; Hu, D.; Ma, Y. Recent progress in hot exciton materials for organic light-emitting diodes. Chem. Soc. Rev. 2021, 50, 1030–1069.
- Kimura, K.; Miwa, K.; Imada, H.; Imai-Imada, M.; Kawahara, S.; Takeya, J.; Kawai, M.; Galperin, M.; Kim, Y. Selective triplet exciton formation in a single molecule. Nature 2019, 570, 210–213.
- Kafle, T.R.; Kattel, B.; Wanigasekara, S.; Wang, T.; Chan, W.L. Spontaneous exciton dissociation at organic semiconductor interfaces facilitated by the orientation of the delocalized electron–hole wavefunction. Adv. Energy Mater. 2020, 10, 1904013.
- Hu, T.; Xia, L.; Liu, W.; Xie, J.; Jiang, Z.; Xiong, F.; Hu, W. Dy3+-doped LaInO3: A host-sensitized white luminescence phosphor with exciton-mediated energy transfer. J. Mater. Chem. C 2021, 9, 13410–13419.
- Burdov, V.A.; Vasilevskiy, M.I. Exciton-photon interactions in semiconductor nanocrystals: Radiative transitions, non-radiative processes and environment effects. Appl. Sci. 2021, 11, 497.
- Li, D.; Hu, Y.; Zhang, N.; Lv, Y.; Lin, J.; Guo, X.; Fan, Y.; Luo, J.; Liu, X. Near-infrared to visible organic upconversion devices based on organic light-emitting field effect transistors. ACS Appl. Mater. Interfaces 2017, 9, 36103–36110.
- Yang, D.; Zhou, X.; Ma, D.; Vadim, A.; Ahamad, T.; Alshehri, S.M. Near infrared to visible light organic up-conversion devices with photon-to-photon conversion efficiency approaching 30%. Mater. Horiz. 2018, 5, 874–882.
- Yu, B.H.; Cheng, Y.; Li, M.; Tsang, S.W.; So, F. Sub-band gap turn-on near-infrared-to-visible up-conversion device enabled by an organic-inorganic hybrid perovskite photovoltaic absorber. ACS Appl. Mater. Interfaces 2018, 10, 15920–15925.
- Matsushima, T.; Qin, C.; Goushi, K.; Bencheikh, F.; Komino, T.; Leyden, M.; Sandanayaka, A.S.D.; Adachi, C. Enhanced electroluminescence from organic light-emitting diodes with an organic-inorganic perovskite host layer. Adv. Mater. 2018, 30, 1802662.
- Fadavieslam, M.R. The effect of thickness of light emitting layer on physical properties of OLED devices. Optik 2019, 182, 452–457.
- Segal, M.; Baldo, M.A.; Holmes, R.J.; Forrest, S.R.; Soos, Z.G. Excitonic singlet-triplet ratios in molecular and polymeric organic materials. Phys. Rev. B 2003, 68, 075211.
- Wang, S.; Yan, X.; Cheng, Z.; Zhang, H.; Liu, Y.; Wang, Y. Highly efficient near-infrared delayed fluorescence organic light emitting diodes using a phenanthrene-based charge-transfer compound. Angew. Chem. Int. Ed. 2015, 54, 13068–13072.
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238.
- Thilagam, A. Effect of the Pauli exclusion principle on the singlet exciton yield in conjugated polymers. Appl. Phys. A 2016, 122, 254.
- Law, C.K. Quantum entanglement as an interpretation of bosonic character in composite two-particle systems. Phys. Rev. A 2005, 71, 034306.
- Kim, J.W.; Yoo, S.I.; Kang, J.S.; Yoon, G.J.; Lee, S.E.; Kim, Y.K.; Kim, W.Y. Quenching in single emissive white phosphorescent organic light-emitting devices. Org. Electron. 2016, 38, 230–237.
- Strassner, T. Phosphorescent platinum(II) complexes with C(wedge)C* cyclometalated NHC ligands. Acc. Chem. Res. 2016, 49, 2680–2689.
- Lee, J.; Aizawa, N.; Numata, M.; Adachi, C.; Yasuda, T. Versatile molecular functionalization for inhibiting concentration quenching of thermally activated delayed fluorescence. Adv. Mater. 2017, 29, 1604856.
- Zhou, J.; Chen, P.; Wang, X.; Wang, Y.; Wang, Y.; Li, F.; Yang, M.; Huang, Y.; Yu, J.; Lu, Z. Charge-transfer-featured materials-promising hosts for fabrication of efficient OLEDs through triplet harvesting via triplet fusion. Chem. Commun. 2014, 50, 7586–7589.
- Li, W.; Pan, Y.; Xiao, R.; Peng, Q.; Zhang, S.; Ma, D.; Li, F.; Shen, F.; Wang, Y.; Yang, B.; et al. Employing ∼100% excitons in OLEDs by utilizing a fluorescent molecule with hybridized local and charge-transfer excited state. Adv. Funct. Mater. 2014, 24, 1609–1614.
- Constantinides, C.P.; Koutentis, P.A. Stable N- and N/S-rich heterocyclic radicals. Adv. Heterocycl. Chem. 2016, 119, 173–207.
- Ji, L.; Shi, J.; Wei, J.; Yu, T.; Huang, W. Air-stable organic radicals: New-generation materials for flexible electronics? Adv. Mater. 2020, 32, 1908015.
- Wang, P.; Lin, S.; Lin, Z.; Peeks, M.D.; Van Voorhis, T.; Swager, T.M. A semiconducting conjugated radical polymer: Ambipolar redox activity and faraday effect. J. Am. Chem. Soc. 2018, 140, 10881–10889.
- Ota, K.; Nagao, K.; Ohmiya, H. Synthesis of sterically hindered alpha-hydroxycarbonyls through radical-radical coupling. Org. Lett. 2021, 23, 4420–4425.
- Frisenda, R.; Gaudenzi, R.; Franco, C.; Mas-Torrent, M.; Rovira, C.; Veciana, J.; Alcon, I.; Bromley, S.T.; Burzuri, E.; van der Zant, H.S. Kondo effect in a neutral and stable all organic radical single molecule break junction. Nano. Lett. 2015, 15, 3109–3114.
- Dong, S.; Xu, W.; Guo, H.; Yan, W.; Zhang, M.; Li, F. Effects of substituents on luminescent efficiency of stable triaryl methyl radicals. Phys. Chem. Chem. Phys. 2018, 20, 18657–18662.
- Guo, H.; Peng, Q.; Chen, X.K.; Gu, Q.; Dong, S.; Evans, E.W.; Gillett, A.J.; Ai, X.; Zhang, M.; Credgington, D.; et al. High stability and luminescence efficiency in donor-acceptor neutral radicals not following the Aufbau principle. Nat. Mater. 2019, 18, 977–984.
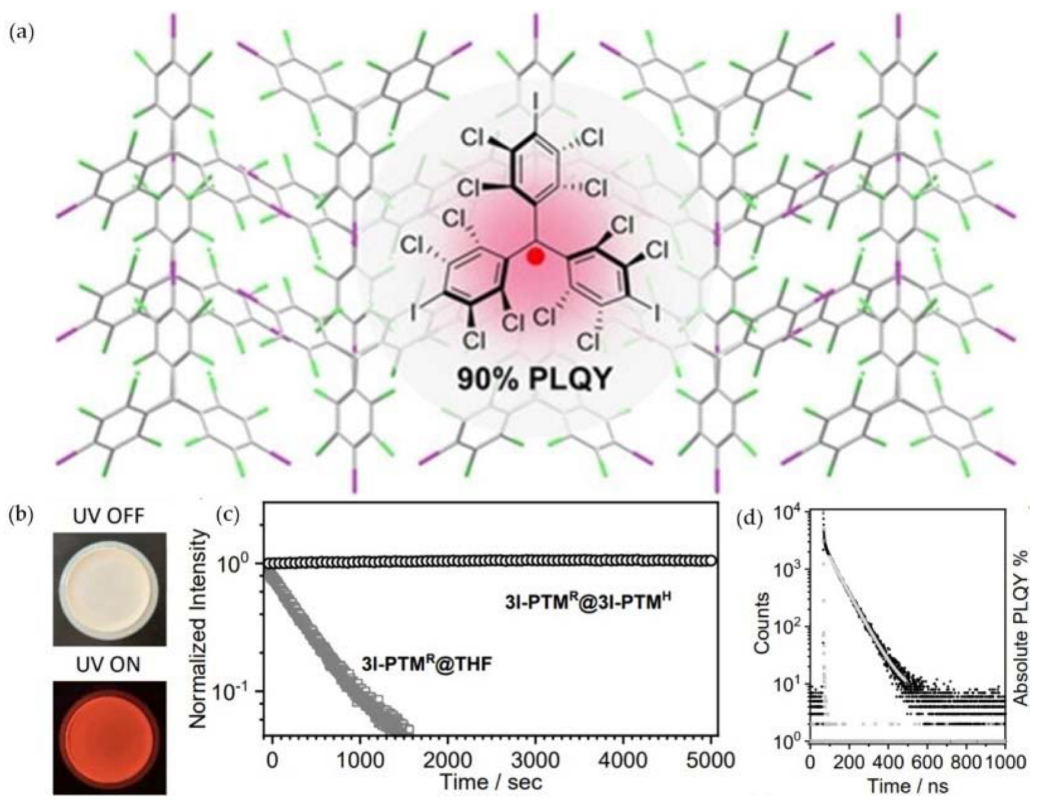
- Liu, C.H.; Hamzehpoor, E.; Sakai-Otsuka, Y.; Jadhav, T.; Perepichka, D.F. A pure-red doublet emission with 90% quantum yield: Stable, colorless, iodinated triphenylmethane solid. Angew. Chem. Int. Ed. 2020, 59, 23030–23034.
- Fajari, L.; Papoular, R.; Reig, M.; Brillas, E.; Jorda, J.L.; Vallcorba, O.; Rius, J.; Velasco, D.; Julia, L. Charge transfer states in stable neutral and oxidized radical adducts from carbazole derivatives. J. Org. Chem. 2014, 79, 1771–1777.
- Obolda, A.; Ai, X.; Zhang, M.; Li, F. Up to 100% formation ratio of doublet exciton in deep-red organic light-emitting diodes based on neutral pi-radical. ACS Appl. Mater. Inter. 2016, 8, 35472–35478.
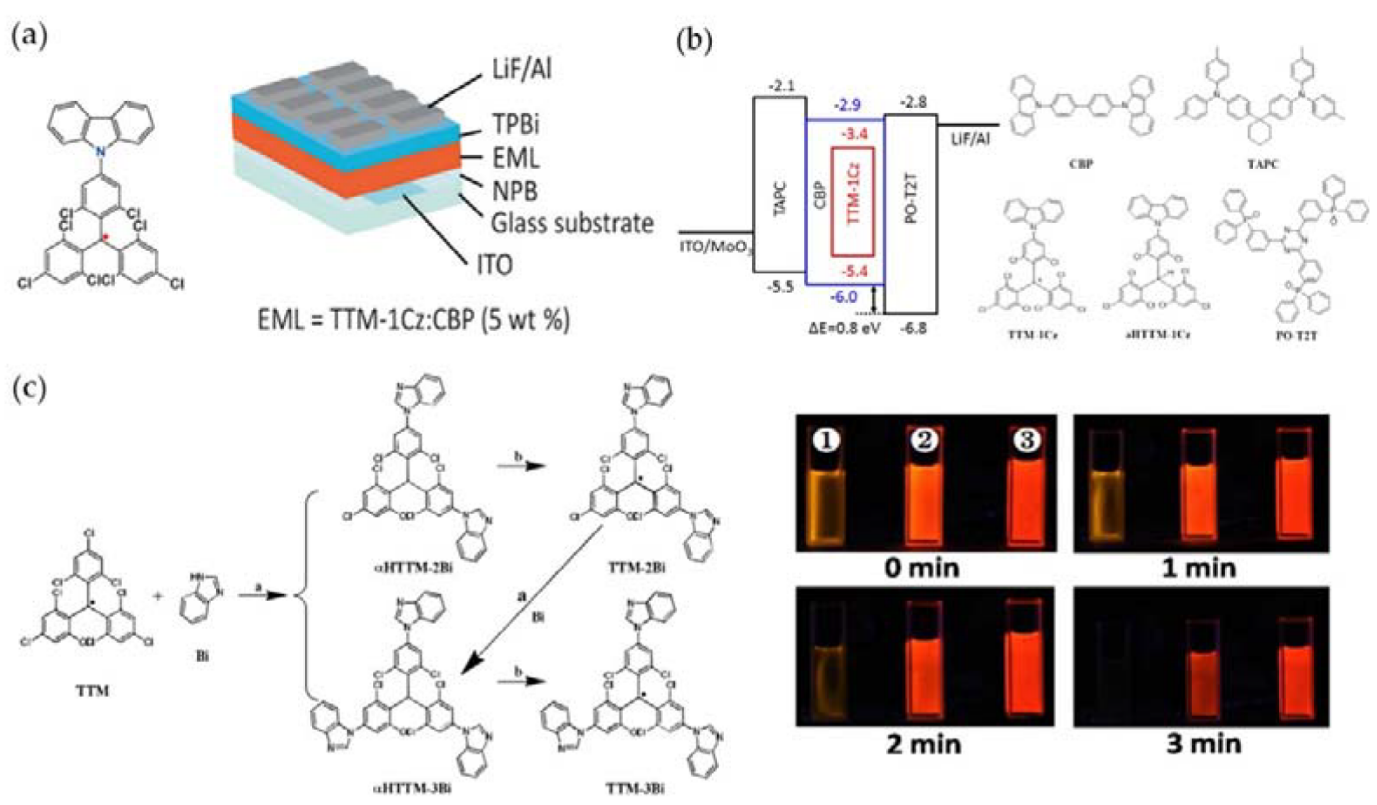
- Gao, Y.; Xu, W.; Ma, H.; Obolda, A.; Yan, W.; Dong, S.; Zhang, M.; Li, F. Novel luminescent benzimidazole-substituent tris(2,4,6-trichlorophenyl)methyl radicals: Photophysics, stability, and highly efficient red-orange electroluminescence. Chem. Mater. 2017, 29, 6733–6739.
- Abdurahman, A.; Hele, T.J.H.; Gu, Q.; Zhang, J.; Peng, Q.; Zhang, M.; Friend, R.H.; Li, F.; Evans, E.W. Understanding the luminescent nature of organic radicals for efficient doublet emitters and pure-red light-emitting diodes. Nat. Mater. 2020, 19, 1224–1229.
- Zhao, Y.; Abdurahman, A.; Zhang, Y.; Zheng, P.; Zhang, M.; Li, F. Highly efficient multifunctional luminescent radicals. CCS Chem. 2021, 938–947.
- Chen, L.; Arnold, M.; Blinder, R.; Jelezko, F.; Kuehne, A.J.C. Mixed-halide triphenyl methyl radicals for site-selective functionalization and polymerization. RSC Adv. 2021, 11, 27653–27658.
- Yan, C.; An, D.; Chen, W.; Zhang, N.; Qiao, Y.; Fang, J.; Lu, X.; Zhou, G.; Liu, Y. Stable diarylamine-substituted tris (2,4,6-trichlorophenyl) methyl radicals: One-step synthesis, near-infrared emission, and redox chemistry. CCS Chem. 2021, 3405–3418.
- Hattori, Y.; Kusamoto, T.; Nishihara, H. Luminescence, stability, and proton response of an open-shell (3,5-dichloro-4-pyridyl) bis (2,4,6-trichlorophenyl) methyl radical. Angew. Chem. Int. Ed. 2014, 53, 11845–11848.
- Hattori, Y.; Kusamoto, T.; Nishihara, H. Highly photostable luminescent open-shell (3,5-dihalo-4-pyridyl) bis (2,4,6-trichlorophenyl) methyl radicals: Significant effects of halogen atoms on their photophysical and photochemical properties. RSC Adv. 2015, 5, 64802–64805.
- Kimura, S.; Kusamoto, T.; Kimura, S.; Kato, K.; Teki, Y.; Nishihara, H. Magnetoluminescence in a photostable, brightly luminescent organic radical in a rigid environment. Angew. Chem. Int. Ed. 2018, 57, 12711–12715.
- Kimura, S.; Tanushi, A.; Kusamoto, T.; Kochi, S.; Sato, T.; Nishihara, H. A luminescent organic radical with two pyridyl groups: High photostability and dual stimuli-responsive properties, with theoretical analyses of photophysical processes. Chem. Sci. 2018, 9, 1996–2007.
- Hattori, Y.; Tsubaki, S.; Matsuoka, R.; Kusamoto, T.; Nishihara, H.; Uchida, K. Expansion of photostable luminescent radicals by meta-substitution. Chem. Asian. J. 2021, 16, 2538–2544.
- Kimura, S.; Uejima, M.; Ota, W.; Sato, T.; Kusaka, S.; Matsuda, R.; Nishihara, H.; Kusamoto, T. An open-shell, luminescent, two-dimensional coordination polymer with a honeycomb lattice and triangular organic radical. J. Am. Chem. Soc. 2021, 143, 4329–4338.
- Matsuoka, R.; Kimura, S.; Kusamoto, T. Solid-state room-temperature near-infrared photoluminescence of a stable organic radical. ChemPhotoChem 2021, 5, 669–673.
- Ai, X.; Chen, Y.; Feng, Y.; Li, F. A stable room-temperature luminescent biphenylmethyl radical. Angew. Chem. Int. Ed. 2018, 57, 2869–2873.
- Abdurahman, A.; Chen, Y.; Ai, X.; Ablikim, O.; Gao, Y.; Dong, S.; Li, B.; Yang, B.; Zhang, M.; Li, F. A pure red luminescent β-carboline-substituted biphenylmethyl radical: Photophysics, stability and OLEDs. J. Mater. Chem. C 2018, 6, 11248–11254.
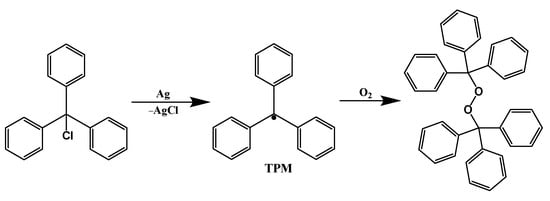
- Ballester, M.; Riera-Figueras, J.; Rodríguez-Siurana, A. Synthesis and isolation of a perchlorotriphenylcarbonium salt. Tetrahedron Lett. 1970, 11, 3615–3618.
- Sholle, V.D.; Rozantsev, E.G. Advances in the chemistry of stable hydrocarbon radicals. Russ. Chem. Rev. 1973, 42, 1011–1019.
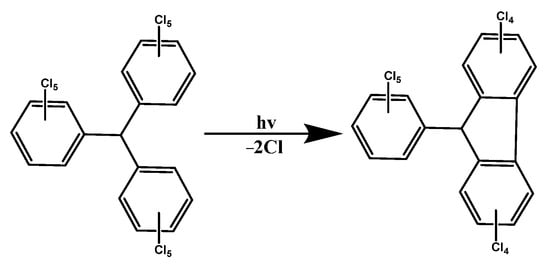
- Fox, M.A.; Gaillard, E.; Chen, C.C. Photochemistry of stable free radicals: The photolysis of perchlorotriphenylmethyl radicals. J. Am. Chem. Soc. 1987, 109, 7088–7094.
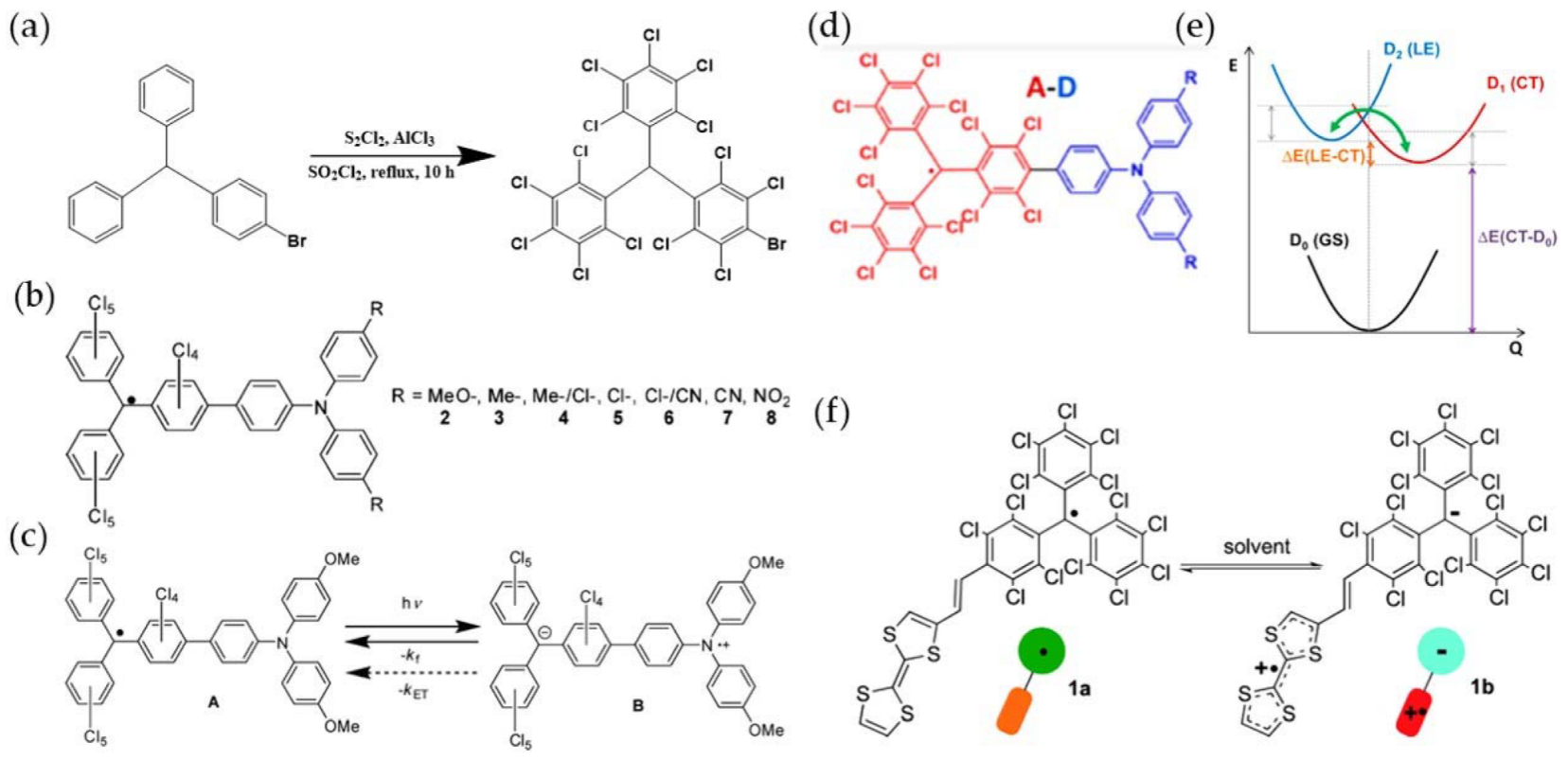
- Heckmann, A.; Lambert, C. Neutral organic mixed-valence compounds: synthesis and all-optical evaluation of electron-transfer parameters. J. Am. Chem. Soc. 2007, 129, 5515–5527.
- Heckmann, A.; Dümmler, S.; Pauli, J.; Margraf, M.; Köhler, J.; Stich, D.; Lambert, C.; Fischer, I.; Resch-Genger, U. Highly fluorescent open-shell NIR dyes: The time-dependence of back electron transfer in triarylamine-perchlorotriphenylmethyl radicals. J. Phys. Chem. C 2009, 113, 20958–20966.
- Heckmann, A.; Lambert, C.; Goebel, M.; Wortmann, R. Synthesis and photophysics of a neutral organic mixed-valence compound. Angew. Chem. Int. Ed. 2004, 43, 5851–5856.
- Cho, E.; Coropceanu, V.; Bredas, J.L. Organic neutral radical emitters: Impact of chemical substitution and electronic-state hybridization on the luminescence properties. J. Am. Chem. Soc. 2020, 142, 17782–17786.
- Guasch, J.; Grisanti, L.; Souto, M.; Lloveras, V.; Vidal-Gancedo, J.; Ratera, I.; Painelli, A.; Rovira, C.; Veciana, J. Intra- and intermolecular charge transfer in aggregates of tetrathiafulvalene-triphenylmethyl radical derivatives in solution. J. Am. Chem. Soc. 2013, 135, 6958–6967.
- Armet, O.; Veciana, J.; Rovira, C.; Riera, J.; Castaner, J.; Molins, E.; Rius, J.; Miravitlles, C.; Olivella, S.; Brichfeus, J. Inert carbon free radicals. 8. Polychlorotriphenylmethyl radicals: Synthesis, structure, and spin-density distribution. J. Phys. Chem. 1987, 91, 5608–5616.
- Carriedo, G.A.; Alonso, F.J.G.; Elipe, P.G.; Brillas, E.; Julia, L. Incorporation of stable organic radicals into cyclotriphosphazene: Preparation and characterization of mono- and diradical adducts. Org. Lett. 2001, 3, 1625–1628.
- Torres, J.L.; Varela, B.; Brillas, E.; Julia, L. Tris(2,4,6-trichloro-3,5-dinitrophenyl)methyl radical: A new stable coloured magnetic species as a chemosensor for natural polyphenols. Chem. Commun. 2003, 1, 74–75.
- Viadel, L.; Carilla, J.; Brillas, E.; Labarta, A.; Julia’, L. Two spin-containing fragments connected by a two-electron one-center heteroatom p spacer. A new open-shell organic molecule with a singlet ground state. J. Mater. Chem. A 1998, 8, 1165–1172.
- Gamero, V.; Velasco, D.; Latorre, S.; López-Calahorra, F.; Brillas, E.; Juliá, L. bis(2,4,6-trichlorophenyl)methyl radical an efficient red light-emitting paramagnetic molecule. Tetrahedron Lett. 2006, 47, 2305–2309.
- Cho, E.; Coropceanu, V.; Brédas, J.-L. Impact of chemical modifications on the luminescence properties of organic neutral radical emitters. J. Mater. Chem. C 2021, 9, 10794–10801.
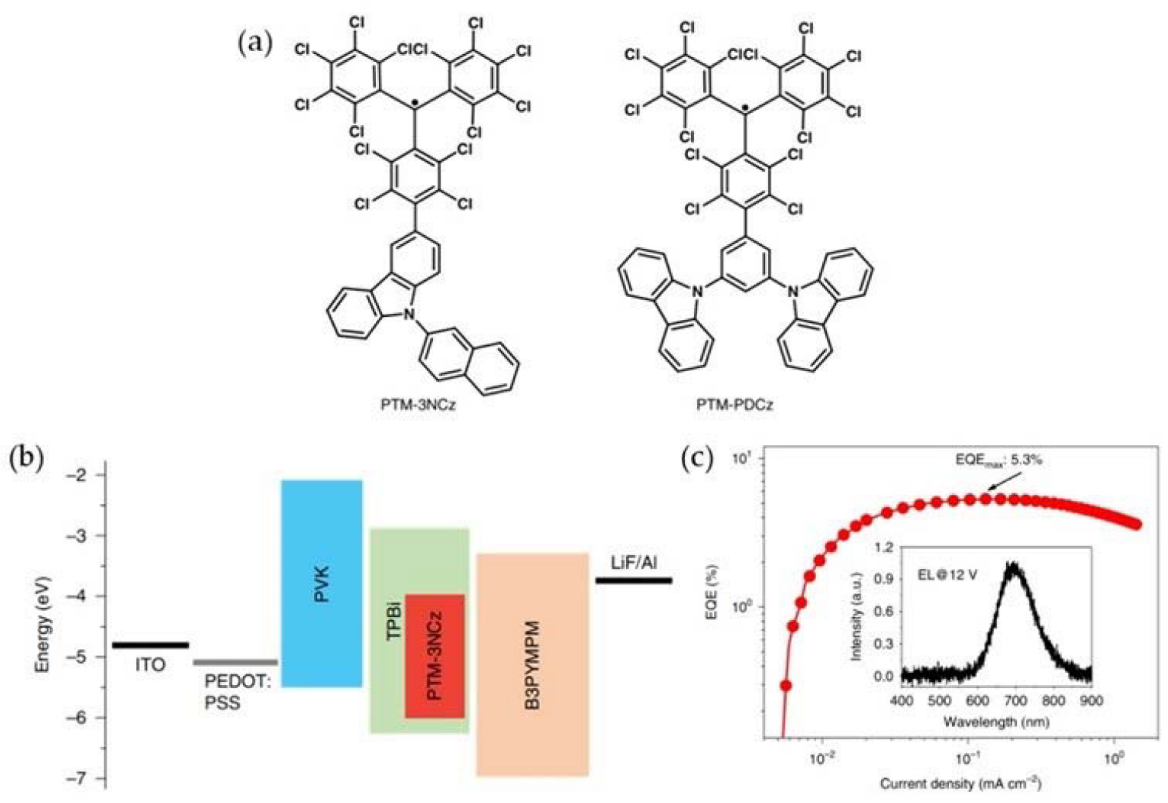
- Cui, Z.; Ye, S.; Wang, L.; Guo, H.; Obolda, A.; Dong, S.; Chen, Y.; Ai, X.; Abdurahman, A.; Zhang, M.; et al. Radical-based organic light-emitting diodes with maximum external quantum efficiency of 10.6. J. Phys. Chem. Lett. 2018, 9, 6644–6648.
- Bin, Z.; Guo, H.; Liu, Z.; Li, F.; Duan, L. Stable organic radicals as hole injection dopants for efficient optoelectronics. ACS Appl. Mater. Inter. 2018, 10, 4882–4886.
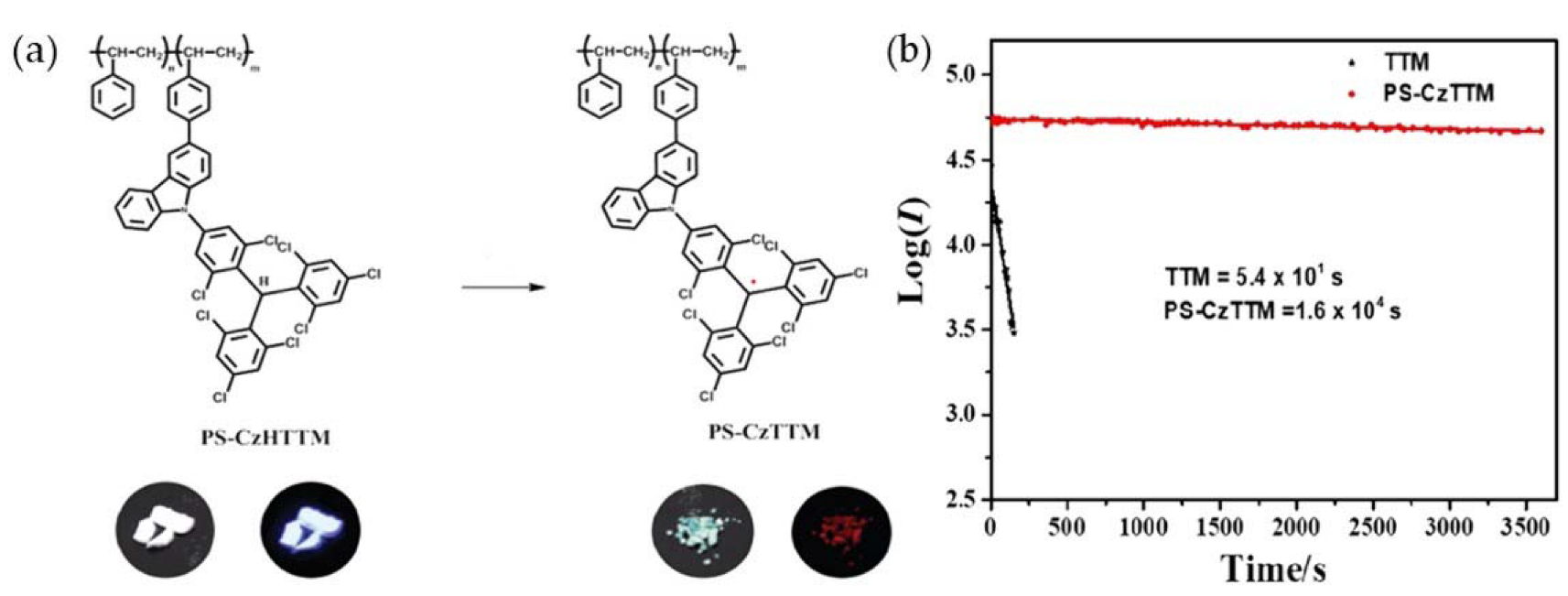
- Abdurahman, A.; Peng, Q.; Ablikim, O.; Ai, X.; Li, F. A radical polymer with efficient deep-red luminescence in the condensed state. Mater. Horiz. 2019, 6, 1265–1270.




