Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chen, M.; , .; Hu, Y.; Zhao, L. Non-Coding RNAs in Plants. Encyclopedia. Available online: https://encyclopedia.pub/entry/21755 (accessed on 01 March 2026).
Chen M, , Hu Y, Zhao L. Non-Coding RNAs in Plants. Encyclopedia. Available at: https://encyclopedia.pub/entry/21755. Accessed March 01, 2026.
Chen, Ming, , Yueming Hu, Liang Zhao. "Non-Coding RNAs in Plants" Encyclopedia, https://encyclopedia.pub/entry/21755 (accessed March 01, 2026).
Chen, M., , ., Hu, Y., & Zhao, L. (2022, April 14). Non-Coding RNAs in Plants. In Encyclopedia. https://encyclopedia.pub/entry/21755
Chen, Ming, et al. "Non-Coding RNAs in Plants." Encyclopedia. Web. 14 April, 2022.
Copy Citation
Plant transcriptomes encompass a large number of functional non-coding RNAs (ncRNAs), only some of which have protein-coding capacity. Since their initial discovery, ncRNAs have been classified into two broad categories based on their biogenesis and mechanisms of action, housekeeping ncRNAs and regulatory ncRNAs. With advances in RNA sequencing technology and computational methods, bioinformatics resources continue to emerge and update rapidly, including workflow for in silico ncRNA analysis, up-to-date platforms, databases, and tools dedicated to ncRNA identification and functional annotation.
ncRNA
plant
ncRNA resource
ncRNA function
ncRNA interaction
1. Introduction
In the last few years, a number of non-coding RNAs (ncRNAs) have been described in plants involved in several processes, ranging from RNA maturation, splicing, regulation of transcription, post-transcriptional RNA modifications, and nucleosome remodeling. Therefore, it is unquestionable that ncRNAs play a significant role in gene regulatory network [1][2][3][4]. With extensive transcriptome analysis, up to 90% of the eukaryotic genome is transcribed into RNA, of which only 1–2% corresponds to protein-coding mRNA [5][6]. Although the remaining transcripts lack minimal protein-coding capacity and poorly conserved sequences [2][5][7], the emergence of ncRNAs as novel ribose regulators of gene expression sheds light on the so-called “dark matter” of the genome.
Current studies have revealed that ncRNAs can be transcribed from DNA sequences in protein-coding genes, intergenic or intronic regions [8]. In terms of their regulatory roles, ncRNAs could be divided into two major categories in plants (Figure 1). Among them, housekeeping ncRNAs are necessary for fundamental biological processes of life, so the content is relatively constant. Regulatory ncRNAs vary in size, shape, and accumulation patterns, so their expression is temporal and spatially specific [9]. Notably, any ncRNAs classification system is defined as an intelligent construct that is unlikely to perfectly reflect nature [10]. To date, many ncRNAs have not been described in plants, such as PIWI-interacting RNA (piRNA), an animal-specific small silencing RNA [11][12], enhancer RNAs (eRNAs), which play critical role in transcriptional activation in mammalian cells and are transcribed from enhancers [13][14][15], and Y RNAs, which are necessary for DNA replication in humans [16][17].
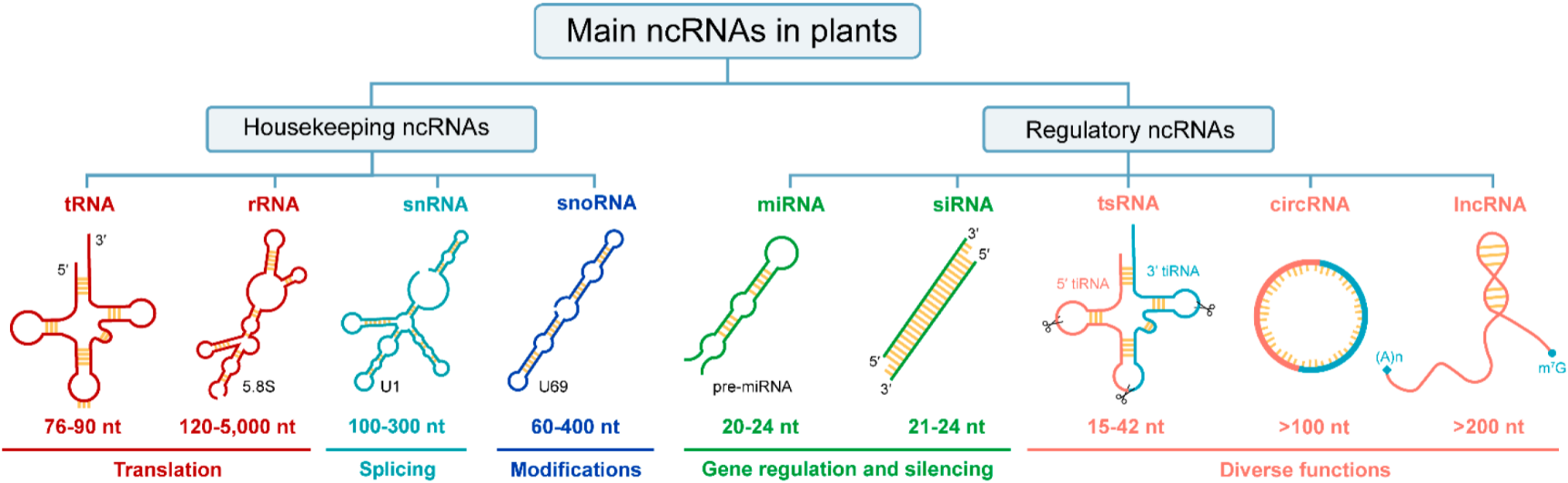
Figure 1. ncRNAs category in plants. From top to bottom, there are primary classification, secondary classification, abbreviations, secondary structures, size, and functions of ncRNAs. Since some ncRNAs contain multiple types, one is selected and annotated with text in the lower right corner of the secondary structure. The sizes of ncRNAs are approximate. Diverse functions include gene expression regulation, translation inhibition, plant immunity, stress response, etc. Abbreviations: tRNA, transfer RNA; rRNA, ribosomal RNA; snRNA, small nuclear RNA; snoRNA, small nucleolar RNA; miRNA, micro RNA; siRNA, small interfering RNA; tsRNA, tRNA-derived small RNA; circRNA, circular RNA; lncRNA, long non-coding RNA; tiRNA, stress-induced tRNA or tRNA halves; nt, nucleotides.
So far, the biogenesis of some regulatory ncRNAs has been clearly described [18][19][20]; however, siRNA and tsRNA are poorly defined in plants. As further studies have shown, ncRNAs participate in the maintenance of homeostasis in plants by ncRNA-associated interaction with other biomolecules and microorganisms, which is of great significance to growth, development, differentiation, and reproduction of plants.
With the advancement of high-throughput RNA-seq technologies, the diversity of ncRNAs world has been unveiled. To date, numerous studies have applied RNA-seq technology to discover known and novel classes of ncRNAs in diverse tissues and developmental stages [21]. These precious data have been mined and stored in public databases.
2. Biogenesis and Functions of ncRNAs in Plants
Currently, massive endogenous ncRNAs with various regulatory potentials have been discovered in various plant species [22][23]. Based on their average size, regulatory ncRNAs could be further categorized into small RNAs (18–30 nt), medium-sized ncRNAs (31–200 nt), and lncRNAs (>200 nt). In addition, it can be classified into linear or circular according to its morphology (Figure 1). In general, 200 nt is regarded as the dividing line in the regulatory ncRNAs world, but this size consideration is arbitrary because circRNAs, eRNAs, and promoter-associated transcripts (PATs) have displayed variable lengths [8]. Recently, regulatory small RNAs (sRNAs), namely, miRNA and siRNA, are considered to have tiny sizes but play important roles in response to stress or environmental changes by regulating the expression of target genes [24][25][26][27]. Likewise, lncRNAs were contemplated as transcriptional noise but later gained importance as one of the wide-ranging and heterogeneous groups of ncRNAs [28]. Notably, unlike other linear regulatory ncRNAs, circRNAs are a novel class of ncRNAs that lack free 5′ and 3′ terminus, which have been extensively explored in the past few years [29]. Besides, many small ncRNAs derived from tRNAs, called tsRNAs, have also been identified in plants with a broad size range of 15–42 nt [30][31][32]. Here, researchers classified tsRNAs as regulatory ncRNA according to their diverse functions (Figure 1). In general, the functions of some regulatory ncRNAs are similar, while a few are distinct, nevertheless overlapping in silencing pathways [33]. Next, researchers will introduce their biogenesis and functions in plants in detail.
2.1. miRNA
miRNA biogenesis is a multistep process involving transcription, processing, modification, and assembly of the RNA-induced silencing complex (RISC) (Figure 2A) [34][35][36]. First, primary miRNAs (pri-miRNAs) are transcribed by RNA POLYMERASE II (Pol II) containing hairpin RNA secondary structures. Then, an RNase III family DICER-LIKE (DCL) enzyme, usually DCL1 [37], assisted by HYPONASTIC LEAVES 1 (HYL1) and SERRATE (SE), cleaves from the base of the pri-miRNA hairpin to yield a precursor-miRNA (pre-miRNA) hairpin and cleaves again to release a miRNA/miRNA* duplex [38]. Next, the 3′-most nucleotides of the initial miRNA/miRNA* duplex are then 2′-O-methylated by the nuclear HUA ENHANCER 1 (HEN1) protein for stabilizing miRNA [39]. Finally, most mature miRNA strands are incorporated into ARGONAUTE 1 (AGO1) in the nucleus (unlike in animals, where it occurs in the cytoplasm [40]), with the removal of the miRNA* strand and the transport of the miRNA-AGO1 complex to the cytoplasm, where miRNAs induce post-transcriptional gene silencing by transcript cleavage and translation repression [24][35][41][42].
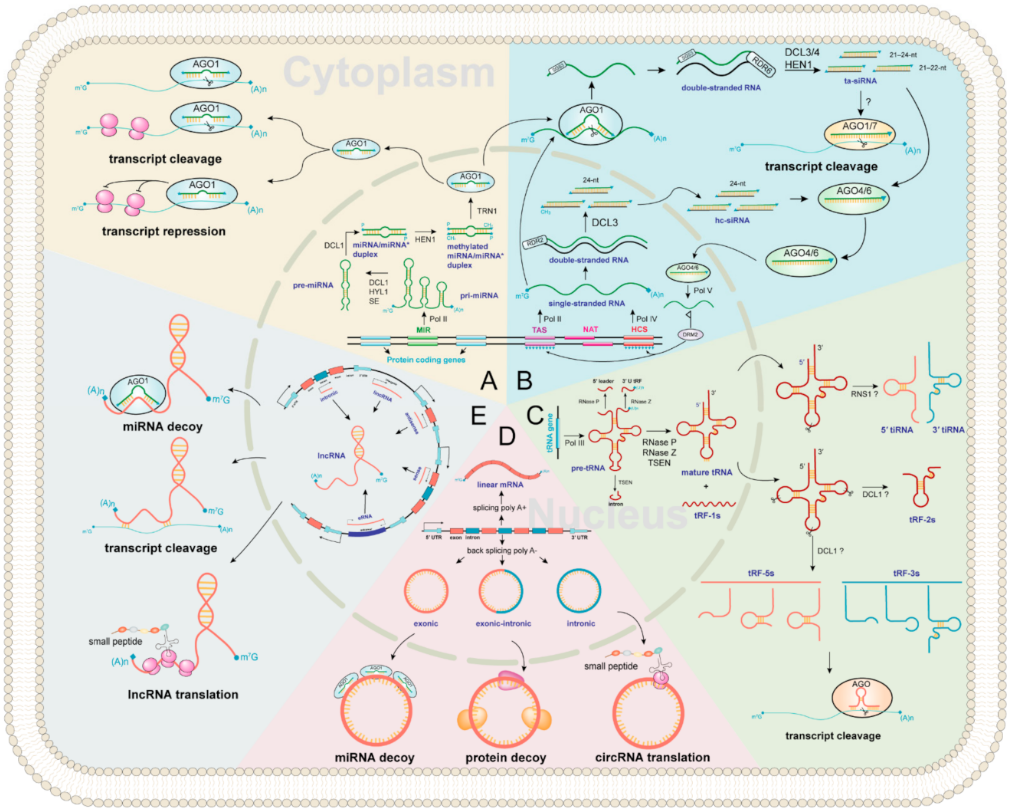
Figure 2. Regulator ncRNAs biogenesis landscape and functions in plants. The inside of the circle represents the nucleus, and the outside represents cytoplasm. All ncRNAs types are marked in purple. The background of each color represents biogenesis and functions of (A) miRNA; (B) siRNA; (C) tsRNA; (D) circRNA; (E) lncRNA, respectively.
As post-transcriptional gene regulators, miRNAs are up- or down-regulated for improving plant productivity and stress tolerance in numerous species [43][44]. Therefore, studying the expression patterns of miRNAs can help researchers better understand the regulatory networks of stress response and environmental adaptation. In general, miRNAs have several features in regulatory pathways. (1) Evolutionarily conserved RNAs tend to have conserved targets in related plant species. For example, miR159 targets MYB genes and is down-regulated in salt-stress responses among Arabidopsis [45], tobacco [46], and kidney bean [47]. miR172 targets AP2 genes for regulating floral development in Arabidopsis, rice, soybean, barley, and maize [27]. A list of conserved miRNAs suggests a common regulatory mechanism across different species. (2) A single miRNA can participate in a variety of stress responses and developmental processes. For instance, cold-inducible miR393 targets TIR1/AFB genes and is up-regulated for enhancing cold tolerance [48]. miR393 is also induced by PAMP flagellin (flg22) to down-regulate the levels of TIR1/AFB genes for antibacterial defense [49]. Besides, miR393 is also involved in regulating arbuscule formation [50], inhibiting root elongation, and promoting lateral root initiation [51]. (3) Multiple miRNAs can participate in one biological process. For example, in rice, miR156, miR396, and miR397 cooperate in the regulation of grain size. miR156, miR393, and miR444 participate in tillering together [27]. (4) The expression patterns of miRNAs rely on the specific condition. As mentioned above, miRNAs are up- or down-regulated according to specific stress or specific tissue. Another example can also be supported. miR1425 will influence the number of fertile pollen grains by regulating a pentatricopeptide repeat (PPR)-containing protein under cold stress [52].
2.2. siRNA
According to the mode of action, siRNA can be simply divided into three secondary categories, namely trans-acting siRNAs (ta-siRNA), heterochromatic siRNAs (hc-siRNA), and natural antisense siRNAs (nat-siRNAs). However, in fact, ta-siRNAs belong to the so-called “secondary siRNAs” category, including ta-siRNA and phased siRNAs. Here, since many of the known ta-siRNAs are also phased [10], researchers use ta-siRNA instead of “secondary siRNAs” for discussion. Generally, they both play a role in transcriptional gene silencing by complementary target mRNAs or directing DNA and histone methylation through RNA-directed DNA methylation (RdDM) process [53][54].
ta-siRNAs are generated from TAS genes (Figure 2B) [24][55]. Firstly, TAS genes are transcribed into single-stranded RNAs by RNA Pol II, and then they loose the cap and mostly also the poly-A end upon miRNA-AGO1 complex guided cleavage. Secondly, the 5′ or 3′ cleavage fragments are protected by SUPPRESSOR OF GENE SILENCING 3 (SGS3) and converted to double-stranded RNA (dsRNA) by RNA-dependent RNA polymerases 6 (RDR6) [56]. Finally, they are methylated and processed into 21–24 nt ta-siRNAs by HEN1 and various DCL activities. The 21–22 nt size class are loaded onto AGO1 or AGO7 to induce post-transcriptional gene silencing of complementary target mRNAs in the cytoplasm, while some ta-siRNAs are incorporated into AGO4/6 to guide RNA Pol V-mediated de novo DNA methylation of TAS genes [54]. In Arabidopsis, miR173 targets TAS1 and TAS2 genes to generate ta-siRNAs [55][57]. The TAS1 ta-siRNAs target the heat stress transcription factor genes, HEAT-INDUCED TAS1 TARGET 1 (HTT1) and HTT2, to regulate plant thermotolerance [58].
nat-siRNAs can be divided into two categories, cis-NAT-siRNAs and trans-NAT-siRNAs. However, only cis-NAT-siRNAs have been described in plants. trans-NAT-siRNAs remain only a hypothetical possibility. Therefore, in this entry, cis-NAT-siRNAs are collectively referred to as nat-siRNAs. Previously, nat-siRNAs were thought to be generated by the hybridization of separately transcribed complementary RNAs. However, to date, many of the nat-siRNAs investigated depend on RDR for their accumulation [10][59][60][61][62]. This RDR dependency suggests that the precursor dsRNA did not derive from the hybridization of two separately transcribed, complementary mRNAs. Thus, the biogenesis of nat-siRNAs is not well defined and appears to be very complex with some important unanswered questions. Based on available data, Zhang et al. speculated that there are at least five possible mechanisms to generate nat-siRNAs [63]. However, it is clear that nat-siRNAs can be induced by salt [59], pathogen [63], and control sperm function during double fertilization in Arabidopsis [61].
The biogenesis of hc-siRNA begins with the transcription of RNA Pol IV from the intergenic or repetitive genomic regions to generate single-stranded siRNA precursors [64][65][66], which are converted into dsRNA and processed into 24 nt siRNA duplexes. Methylated hc-siRNAs are loaded into AGO4 in the cytoplasm and are transported to the nucleus [67], followed by the recruitment of these hc-siRNA-AGO4 complexes to RNA Pol V transcripts. The subsequent recruitment of DOMAINS REARRANGED METHYLASE 2 (DRM2) catalyzes DNA methylation at RdDM target loci [53][67].
2.3. tsRNA
With a broad size range of 15–42 nt, tsRNAs are a new category of regulatory ncRNAs, which are classified into five categories according to the cleavage sites, namely tRF-1s, tRF-2s, tRF-3s, tRF-5s, and tiRNA (Figure 2C). However, the study in plants has just started, and many questions remain to be answered. For example, the biogenesis pathway of tsRNAs in plants is still unclear, and the physiological function of certain tsRNA in plants is currently very limited [68]. In this entry, researchers propose a hypothesis of tsRNAs biogenesis in plants based on previous studies. First, RNA poly III transcribes tRNA gene as precursor tRNA (pre-tRNA) [69], which includes a 5′ leader, a mature tRNA backbone, a 3′ U trailer, and sometimes an intron [70]. Then, the 5′ leader, 3′ U trailer, and intronic sequences are cleaved by RNase P, RNase Z, and tRNA-splicing endonucleases (TSEN) to produce mature tRNA and tRF-1s (tRF-1s could be derived from 3′-end of pre-tRNA) [71][72][73]. The mature tRNA (73–90 nt) forms a secondary cloverleaf structure with a D-loop (left), a T-loop (right), anticodon loop (bottom), a variable loop, and an acceptor stem (Figure 2C). Finally, the mature tRNAs could be cleavaged by Arabidopsis S-like Ribonuclease 1 (RNS1) and/or DCL1 to form tRF-2s, tRF-3s, tRF-5s, and tiRNA (Figure 2C) [30][31]. In mammals, tsRNAs incorporate into silencing AGO and trigger RNA interference [74]. Likewise, AGO-associated tsRNAs have been predicted in Arabidopsis and rice [30][75]. An in vitro assay has shown that certain tsRNAs regulate gene expression by translation inhibition, and tsRNA-AGO1 complex tends to target transposable element transcripts and probably maintains genome stability [31][76][77].
2.4. circRNA
CircRNAs were first discovered in plant viruses by Sanger’s group in 1976. Studies have shown that circRNAs are circular, single-stranded, and covalently closed RNA biomolecules [78]. The composition of circRNAs can be divided into three categories (Figure 2D). (1) Exonic circRNAs are formed by lariat-driven circularization and intron pairing-driven circularization [79]. (2) Intronic circRNAs are the source of introns generated by the partial degradation of introns after the formation of the lasso structure. (3) Exonic-intronic circRNAs, which are composed of exons and introns, are cyclized during splicing. In 2013, Jeck et al., proposed that exon skipping and intron pairing reduced the distance between splicing sites and promoted the reverse splicing of pre-mRNA [80]. This leads to the deletion of the 3′ and 5′ ends of circRNAs [81]. Several distinct functional mechanisms for animal circRNAs have been identified, suggesting that plant circRNAs may exhibit similar conserved functions. These include miRNA decoys [82], transcriptional modulation [83], translation of circRNAs into small peptides [84]. Besides, circRNAs can play an important role in plant development and stress responses. For example, Vv-circATS1 responds to cold stress by regulating the expression of stimulus-responsive genes in grape [85]. Under dehydration-stressed conditions, many differentially expressed circRNAs have been detected in wheat [86], pear [87], maize, and Arabidopsis [88]. These studies suggest that circRNAs have post-transcriptional roles. However, the mechanism of this remains to be elucidated.
2.5. lncRNA
The biogenesis of lncRNAs can be divided into five categories according to the transcribed site by Pol II: (1) sense lncRNAs are transcribed on the same strand as exons; (2) antisense lncRNAs are transcribed on the opposite strand of exons; (3) intronic lncRNAs are transcribed on introns; (4) intergenic lncRNAs are located between two distinct genes; (5) enhancer lncRNAs emerge from an enhancer region of protein-coding genes (Figure 2E) [89]. They can control target regulation by multiple ways, including chromatin remodeling [90][91][92], transcriptional repression, RNA splicing and transcriptional enhancer [93][94]. In addition, lncRNAs may encode small peptides (Figure 2E), which are required for various cellular processes [95]. Notably, numerous plant lncRNAs are regulated by abiotic stresses. For example, many differentially expressed lncRNAs have been identified in Arabidopsis under drought, cold, salinity, heat, and abscisic acid stresses [96]. Besides, biotic stress-responsive lncRNAs have also been identified in wheat [97], Arabidopsis [98], and tomato [99].
References
- Rincon-Riveros, A.; Morales, D.; Rodriguez, J.A.; Villegas, V.E.; Lopez-Kleine, L. Bioinformatic Tools for the Analysis and Prediction of ncRNA Interactions. Int. J. Mol. Sci. 2021, 22, 11397.
- Yu, Y.; Zhang, Y.; Chen, X.; Chen, Y. Plant Noncoding RNAs: Hidden Players in Development and Stress Responses. Annu. Rev. Cell Dev. Biol. 2019, 35, 407–431.
- Jampala, P.; Garhewal, A.; Lodha, M. Functions of long non-coding RNA in Arabidopsis thaliana. Plant Signal. Behav. 2021, 16, 1925440.
- Yao, R.W.; Wang, Y.; Chen, L.L. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019, 21, 542–551.
- Pauli, A.; Rinn, J.L.; Schier, A.F. Non-coding RNAs as regulators of embryogenesis. Nat. Rev. Genet. 2011, 12, 136–149.
- Rai, M.I.; Alam, M.; Lightfoot, D.A.; Gurha, P.; Afzal, A.J. Classification and experimental identification of plant long non-coding RNAs. Genomics 2019, 111, 997–1005.
- Ariel, F.; Romero-Barrios, N.; Jegu, T.; Benhamed, M.; Crespi, M. Battles and hijacks: Noncoding transcription in plants. Trends Plant Sci. 2015, 20, 362–371.
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-Coding RNAs and their Integrated Networks. J. Integr. Bioinform. 2019, 16, 20190027.
- Vivek, A.T.; Kumar, S. Computational methods for annotation of plant regulatory non-coding RNAs using RNA-seq. Brief. Bioinform. 2021, 22, bbaa322.
- Axtell, M.J. Classification and comparison of small RNAs from plants. Annu. Rev. Plant Biol. 2013, 64, 137–159.
- Kim, J.K.; Gabel, H.W.; Kamath, R.S.; Tewari, M.; Pasquinelli, A.; Rual, J.F.; Kennedy, S.; Dybbs, M.; Bertin, N.; Kaplan, J.M.; et al. Functional genomic analysis of RNA interference in C. elegans. Science 2005, 308, 1164–1167.
- Ozata, D.M.; Gainetdinov, I.; Zoch, A.; O’Carroll, D.; Zamore, P.D. PIWI-interacting RNAs: Small RNAs with big functions. Nat. Rev. Genet. 2019, 20, 89–108.
- Ye, R.; Cao, C.; Xue, Y. Enhancer RNA: Biogenesis, function, and regulation. Essays Biochem. 2020, 64, 883–894.
- Kim, T.K.; Hemberg, M.; Gray, J.M.; Costa, A.M.; Bear, D.M.; Wu, J.; Harmin, D.A.; Laptewicz, M.; Barbara-Haley, K.; Kuersten, S.; et al. Widespread transcription at neuronal activity-regulated enhancers. Nature 2010, 465, 182–187.
- Gao, T.; Qian, J. EnhancerAtlas 2.0: An updated resource with enhancer annotation in 586 tissue/cell types across nine species. Nucleic Acids Res. 2020, 48, D58–D64.
- Christov, C.P.; Gardiner, T.J.; Szuts, D.; Krude, T. Functional requirement of noncoding Y RNAs for human chromosomal DNA replication. Mol. Cell. Biol. 2006, 26, 6993–7004.
- Zhang, A.T.; Langley, A.R.; Christov, C.P.; Kheir, E.; Shafee, T.; Gardiner, T.J.; Krude, T. Dynamic interaction of Y RNAs with chromatin and initiation proteins during human DNA replication. J. Cell Sci. 2011, 124, 2058–2069.
- Achkar, N.P.; Cambiagno, D.A.; Manavella, P.A. miRNA Biogenesis: A Dynamic Pathway. Trends Plant Sci. 2016, 21, 1034–1044.
- Wang, H.V.; Chekanova, J.A. Long Noncoding RNAs in Plants. Adv Exp Med Biol 2017, 1008, 133–154.
- Ashwal-Fluss, R.; Meyer, M.; Pamudurti, N.R.; Ivanov, A.; Bartok, O.; Hanan, M.; Evantal, N.; Memczak, S.; Rajewsky, N.; Kadener, S. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell 2014, 56, 55–66.
- Parvathaneni, R.K.; Bertolini, E.; Shamimuzzaman, M.; Vera, D.L.; Lung, P.Y.; Rice, B.R.; Zhang, J.; Brown, P.J.; Lipka, A.E.; Bass, H.W.; et al. The regulatory landscape of early maize inflorescence development. Genome Biol. 2020, 21, 165.
- Bonnet, E.; Van de Peer, Y.; Rouze, P. The small RNA world of plants. New Phytol. 2006, 171, 451–468.
- Liu, X.; Hao, L.; Li, D.; Zhu, L.; Hu, S. Long non-coding RNAs and their biological roles in plants. Genom. Proteom. Bioinform. 2015, 13, 137–147.
- Chen, X. Small RNAs and their roles in plant development. Annu. Rev. Cell Dev. Biol. 2009, 25, 21–44.
- D’Ario, M.; Griffiths-Jones, S.; Kim, M. Small RNAs: Big Impact on Plant Development. Trends Plant Sci. 2017, 22, 1056–1068.
- Martinez, G.; Kohler, C. Role of small RNAs in epigenetic reprogramming during plant sexual reproduction. Curr. Opin. Plant Biol. 2017, 36, 22–28.
- Tang, J.; Chu, C. MicroRNAs in crop improvement: Fine-tuners for complex traits. Nat. Plants 2017, 3, 17077.
- Chekanova, J.A. Long non-coding RNAs and their functions in plants. Curr. Opin. Plant Biol. 2015, 27, 207–216.
- Sablok, G.; Zhao, H.; Sun, X. Plant Circular RNAs (circRNAs): Transcriptional Regulation Beyond miRNAs in Plants. Mol. Plant 2016, 9, 192–194.
- Alves, C.S.; Vicentini, R.; Duarte, G.T.; Pinoti, V.F.; Vincentz, M.; Nogueira, F.T. Genome-wide identification and characterization of tRNA-derived RNA fragments in land plants. Plant Mol. Biol. 2017, 93, 35–48.
- Cognat, V.; Morelle, G.; Megel, C.; Lalande, S.; Molinier, J.; Vincent, T.; Small, I.; Duchene, A.M.; Marechal-Drouard, L. The nuclear and organellar tRNA-derived RNA fragment population in Arabidopsis thaliana is highly dynamic. Nucleic Acids Res. 2017, 45, 3460–3472.
- Megel, C.; Morelle, G.; Lalande, S.; Duchene, A.M.; Small, I.; Marechal-Drouard, L. Surveillance and cleavage of eukaryotic tRNAs. Int. J. Mol. Sci. 2015, 16, 1873–1893.
- Axtell, M.J.; Meyers, B.C. Revisiting Criteria for Plant MicroRNA Annotation in the Era of Big Data. Plant Cell 2018, 30, 272–284.
- Rogers, K.; Chen, X. Biogenesis, turnover, and mode of action of plant microRNAs. Plant Cell 2013, 25, 2383–2399.
- Yu, Y.; Jia, T.; Chen, X. The ‘how’ and ‘where’ of plant microRNAs. New Phytol. 2017, 216, 1002–1017.
- Park, M.Y.; Wu, G.; Gonzalez-Sulser, A.; Vaucheret, H.; Poethig, R.S. Nuclear processing and export of microRNAs in Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 3691–3696.
- Fahlgren, N.; Howell, M.D.; Kasschau, K.D.; Chapman, E.J.; Sullivan, C.M.; Cumbie, J.S.; Givan, S.A.; Law, T.F.; Grant, S.R.; Dangl, J.L.; et al. High-throughput sequencing of Arabidopsis microRNAs: Evidence for frequent birth and death of MIRNA genes. PLoS ONE 2007, 2, e219.
- Kurihara, Y.; Watanabe, Y. Arabidopsis micro-RNA biogenesis through Dicer-like 1 protein functions. Proc. Natl. Acad. Sci. USA 2004, 101, 12753–12758.
- Yu, B.; Yang, Z.; Li, J.; Minakhina, S.; Yang, M.; Padgett, R.W.; Steward, R.; Chen, X. Methylation as a crucial step in plant microRNA biogenesis. Science 2005, 307, 932–935.
- Axtell, M.J.; Westholm, J.O.; Lai, E.C. Vive la difference: Biogenesis and evolution of microRNAs in plants and animals. Genome Biol. 2011, 12, 221.
- Baumberger, N.; Baulcombe, D.C. Arabidopsis ARGONAUTE1 is an RNA Slicer that selectively recruits microRNAs and short interfering RNAs. Proc. Natl. Acad. Sci. USA 2005, 102, 11928–11933.
- Bologna, N.G.; Iselin, R.; Abriata, L.A.; Sarazin, A.; Pumplin, N.; Jay, F.; Grentzinger, T.; Dal Peraro, M.; Voinnet, O. Nucleo-cytosolic Shuttling of ARGONAUTE1 Prompts a Revised Model of the Plant MicroRNA Pathway. Mol. Cell 2018, 69, 709–719.e5.
- Shriram, V.; Kumar, V.; Devarumath, R.M.; Khare, T.S.; Wani, S.H. MicroRNAs as Potential Targets for Abiotic Stress Tolerance in Plants. Front. Plant Sci. 2016, 7, 817.
- Koroban, N.V.; Kudryavtseva, A.V.; Krasnov, G.S.; Sadritdinova, A.F.; Fedorova, M.S.; Snezhkina, A.V.; Bolsheva, N.L.; Muravenko, O.V.; Dmitriev, A.A.; Melnikova, N.V. The role of microRNA in abiotic stress response in plants. Mol. Biol. 2016, 50, 387–394.
- Chen, L.; Wang, T.; Zhao, M.; Tian, Q.; Zhang, W.H. Identification of aluminum-responsive microRNAs in Medicago truncatula by genome-wide high-throughput sequencing. Planta 2012, 235, 375–386.
- Frazier, T.P.; Sun, G.; Burklew, C.E.; Zhang, B. Salt and drought stresses induce the aberrant expression of microRNA genes in tobacco. Mol. Biotechnol. 2011, 49, 159–165.
- Jones-Rhoades, M.W.; Bartel, D.P. Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol. Cell 2004, 14, 787–799.
- Liu, Y.; Wang, K.; Li, D.; Yan, J.; Zhang, W. Enhanced Cold Tolerance and Tillering in Switchgrass (Panicum virgatum L.) by Heterologous Expression of Osa-miR393a. Plant Cell Physiol. 2017, 58, 2226–2240.
- Navarro, L.; Dunoyer, P.; Jay, F.; Arnold, B.; Dharmasiri, N.; Estelle, M.; Voinnet, O.; Jones, J.D. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 2006, 312, 436–439.
- Etemadi, M.; Gutjahr, C.; Couzigou, J.M.; Zouine, M.; Lauressergues, D.; Timmers, A.; Audran, C.; Bouzayen, M.; Becard, G.; Combier, J.P. Auxin perception is required for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Physiol. 2014, 166, 281–292.
- Gifford, M.L.; Dean, A.; Gutierrez, R.A.; Coruzzi, G.M.; Birnbaum, K.D. Cell-specific nitrogen responses mediate developmental plasticity. Proc. Natl. Acad. Sci. USA 2008, 105, 803–808.
- Jeong, D.H.; Park, S.; Zhai, J.; Gurazada, S.G.; De Paoli, E.; Meyers, B.C.; Green, P.J. Massive analysis of rice small RNAs: Mechanistic implications of regulated microRNAs and variants for differential target RNA cleavage. Plant Cell 2011, 23, 4185–4207.
- Du, J.; Johnson, L.M.; Jacobsen, S.E.; Patel, D.J. DNA methylation pathways and their crosstalk with histone methylation. Nat. Rev. Mol. Cell. Biol. 2015, 16, 519–532.
- Matzke, M.A.; Kanno, T.; Matzke, A.J. RNA-Directed DNA Methylation: The Evolution of a Complex Epigenetic Pathway in Flowering Plants. Annu. Rev. Plant Biol. 2015, 66, 243–267.
- Fei, Q.; Xia, R.; Meyers, B.C. Phased, secondary, small interfering RNAs in posttranscriptional regulatory networks. Plant Cell 2013, 25, 2400–2415.
- Peragine, A.; Yoshikawa, M.; Wu, G.; Albrecht, H.L.; Poethig, R.S. SGS3 and SGS2/SDE1/RDR6 are required for juvenile development and the production of trans-acting siRNAs in Arabidopsis. Genes Dev. 2004, 18, 2368–2379.
- Chen, H.M.; Chen, L.T.; Patel, K.; Li, Y.H.; Baulcombe, D.C.; Wu, S.H. 22-Nucleotide RNAs trigger secondary siRNA biogenesis in plants. Proc. Natl. Acad. Sci. USA 2010, 107, 15269–15274.
- Li, S.; Liu, J.; Liu, Z.; Li, X.; Wu, F.; He, Y. HEAT-INDUCED TAS1 TARGET1 Mediates Thermotolerance via HEAT STRESS TRANSCRIPTION FACTOR A1a-Directed Pathways in Arabidopsis. Plant Cell 2014, 26, 1764–1780.
- Borsani, O.; Zhu, J.; Verslues, P.E.; Sunkar, R.; Zhu, J.K. Endogenous siRNAs derived from a pair of natural cis-antisense transcripts regulate salt tolerance in Arabidopsis. Cell 2005, 123, 1279–1291.
- Katiyar-Agarwal, S.; Morgan, R.; Dahlbeck, D.; Borsani, O.; Villegas, A.J.; Zhu, J.K.; Staskawicz, B.J.; Jin, H. A pathogen-inducible endogenous siRNA in plant immunity. Proc. Natl. Acad. Sci. USA 2006, 103, 18002–18007.
- Ron, M.; Alandete, S.M.; Eshed, W.L.; Fletcher, J.C.; McCormick, S. Proper regulation of a sperm-specific cis-nat-siRNA is essential for double fertilization in Arabidopsis. Genes Dev. 2010, 24, 1010–1021.
- Zhang, X.; Xia, J.; Lii, Y.E.; Barrera-Figueroa, B.E.; Zhou, X.; Gao, S.; Lu, L.; Niu, D.; Chen, Z.; Leung, C.; et al. Genome-wide analysis of plant nat-siRNAs reveals insights into their distribution, biogenesis and function. Genome Biol. 2012, 13, R20.
- Zhang, X.; Lii, Y.; Wu, Z.; Polishko, A.; Zhang, H.; Chinnusamy, V.; Lonardi, S.; Zhu, J.K.; Liu, R.; Jin, H. Mechanisms of small RNA generation from cis-NATs in response to environmental and developmental cues. Mol. Plant 2013, 6, 704–715.
- Blevins, T.; Podicheti, R.; Mishra, V.; Marasco, M.; Wang, J.; Rusch, D.; Tang, H.; Pikaard, C.S. Identification of Pol IV and RDR2-dependent precursors of 24 nt siRNAs guiding de novo DNA methylation in Arabidopsis. eLife 2015, 4, e09591.
- Zhai, J.; Bischof, S.; Wang, H.; Feng, S.; Lee, T.F.; Teng, C.; Chen, X.; Park, S.Y.; Liu, L.; Gallego-Bartolome, J.; et al. A One Precursor One siRNA Model for Pol IV-Dependent siRNA Biogenesis. Cell 2015, 163, 445–455.
- Zhou, M.; Palanca, A.; Law, J.A. Locus-specific control of the de novo DNA methylation pathway in Arabidopsis by the CLASSY family. Nat. Genet. 2018, 50, 865–873.
- Law, J.A.; Jacobsen, S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010, 11, 204–220.
- Zhu, L.; Ow, D.W.; Dong, Z. Transfer RNA-derived small RNAs in plants. Sci. China Life Sci. 2018, 61, 155–161.
- Schramm, L.; Hernandez, N. Recruitment of RNA polymerase III to its target promoters. Genes Dev. 2002, 16, 2593–2620.
- Tocchini-Valentini, G.D.; Fruscoloni, P.; Tocchini-Valentini, G.P. Processing of multiple-intron-containing pretRNA. Proc. Natl. Acad. Sci. USA 2009, 106, 20246–20251.
- Ceballos, M.; Vioque, A. tRNase Z. Protein Pept. Lett. 2007, 14, 137–145.
- Frank, D.N.; Pace, N.R. Ribonuclease P: Unity and diversity in a tRNA processing ribozyme. Annu. Rev. Biochem. 1998, 67, 153–180.
- Abelson, J.; Trotta, C.R.; Li, H. tRNA splicing. J. Biol. Chem. 1998, 273, 12685–12688.
- Maute, R.L.; Schneider, C.; Sumazin, P.; Holmes, A.; Califano, A.; Basso, K.; Dalla-Favera, R. tRNA-derived microRNA modulates proliferation and the DNA damage response and is down-regulated in B cell lymphoma. Proc. Natl. Acad. Sci. USA 2013, 110, 1404–1409.
- Loss-Morais, G.; Waterhouse, P.M.; Margis, R. Description of plant tRNA-derived RNA fragments (tRFs) associated with argonaute and identification of their putative targets. Biol. Direct 2013, 8, 6.
- Ivanov, P.; Emara, M.M.; Villen, J.; Gygi, S.P.; Anderson, P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol. Cell 2011, 43, 613–623.
- Nowacka, M.; Strozycki, P.M.; Jackowiak, P.; Hojka-Osinska, A.; Szymanski, M.; Figlerowicz, M. Identification of stable, high copy number, medium-sized RNA degradation intermediates that accumulate in plants under non-stress conditions. Plant Mol. Biol. 2013, 83, 191–204.
- Kolakofsky, D. Isolation and characterization of Sendai virus DI-RNAs. Cell 1976, 8, 547–555.
- Liu, C.X.; Li, X.; Nan, F.; Jiang, S.; Gao, X.; Guo, S.K.; Xue, W.; Cui, Y.; Dong, K.; Ding, H.; et al. Structure and Degradation of Circular RNAs Regulate PKR Activation in Innate Immunity. Cell 2019, 177, 865–880.e21.
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157.
- Li, X.; Liu, S.; Zhang, L.; Issaian, A.; Hill, R.C.; Espinosa, S.; Shi, S.; Cui, Y.; Kappel, K.; Das, R.; et al. A unified mechanism for intron and exon definition and back-splicing. Nature 2019, 573, 375–380.
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388.
- Conn, V.M.; Hugouvieux, V.; Nayak, A.; Conos, S.A.; Capovilla, G.; Cildir, G.; Jourdain, A.; Tergaonkar, V.; Schmid, M.; Zubieta, C.; et al. A circRNA from SEPALLATA3 regulates splicing of its cognate mRNA through R-loop formation. Nat. Plants 2017, 3, 17053.
- Pamudurti, N.R.; Bartok, O.; Jens, M.; Ashwal-Fluss, R.; Stottmeister, C.; Ruhe, L.; Hanan, M.; Wyler, E.; Perez-Hernandez, D.; Ramberger, E.; et al. Translation of CircRNAs. Mol. Cell 2017, 66, 9–21.e7.
- Gao, Z.; Li, J.; Luo, M.; Li, H.; Chen, Q.; Wang, L.; Song, S.; Zhao, L.; Xu, W.; Zhang, C.; et al. Characterization and Cloning of Grape Circular RNAs Identified the Cold Resistance-Related Vv-circATS1. Plant Physiol. 2019, 180, 966–985.
- Wang, Y.; Yang, M.; Wei, S.; Qin, F.; Zhao, H.; Suo, B. Identification of Circular RNAs and Their Targets in Leaves of Triticum aestivum L. under Dehydration Stress. Front. Plant Sci. 2016, 7, 2024.
- Wang, J.; Lin, J.; Wang, H.; Li, X.; Yang, Q.; Li, H.; Chang, Y. Identification and characterization of circRNAs in Pyrus betulifolia Bunge under drought stress. PLoS ONE 2018, 13, e0200692.
- Zhang, P.; Fan, Y.; Sun, X.; Chen, L.; Terzaghi, W.; Bucher, E.; Li, L.; Dai, M. A large-scale circular RNA profiling reveals universal molecular mechanisms responsive to drought stress in maize and Arabidopsis. Plant J. 2019, 98, 697–713.
- Waititu, J.K.; Zhang, C.; Liu, J.; Wang, H. Plant Non-Coding RNAs: Origin, Biogenesis, Mode of Action and Their Roles in Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 8401.
- Chen, M.; Penfield, S. Feedback regulation of COOLAIR expression controls seed dormancy and flowering time. Science 2018, 360, 1014–1017.
- Kim, D.H.; Sung, S. Coordination of the vernalization response through a VIN3 and FLC gene family regulatory network in Arabidopsis. Plant Cell 2013, 25, 454–469.
- Marquardt, S.; Raitskin, O.; Wu, Z.; Liu, F.; Sun, Q.; Dean, C. Functional consequences of splicing of the antisense transcript COOLAIR on FLC transcription. Mol. Cell 2014, 54, 156–165.
- Song, Y.; Xuan, A.; Bu, C.; Ci, D.; Tian, M.; Zhang, D. Osmotic stress-responsive promoter upstream transcripts (PROMPTs) act as carriers of MYB transcription factors to induce the expression of target genes in Populus simonii. Plant Biotechnol. J. 2019, 17, 164–177.
- Bardou, F.; Ariel, F.; Simpson, C.G.; Romero-Barrios, N.; Laporte, P.; Balzergue, S.; Brown, J.W.; Crespi, M. Long noncoding RNA modulates alternative splicing regulators in Arabidopsis. Dev. Cell 2014, 30, 166–176.
- Matsumoto, A.; Nakayama, K.I. Hidden Peptides Encoded by Putative Noncoding RNAs. Cell Struct. Funct. 2018, 43, 75–83.
- Di, C.; Yuan, J.; Wu, Y.; Li, J.; Lin, H.; Hu, L.; Zhang, T.; Qi, Y.; Gerstein, M.B.; Guo, Y.; et al. Characterization of stress-responsive lncRNAs in Arabidopsis thaliana by integrating expression, epigenetic and structural features. Plant J. 2014, 80, 848–861.
- Xin, M.; Wang, Y.; Yao, Y.; Song, N.; Hu, Z.; Qin, D.; Xie, C.; Peng, H.; Ni, Z.; Sun, Q. Identification and characterization of wheat long non-protein coding RNAs responsive to powdery mildew infection and heat stress by using microarray analysis and SBS sequencing. BMC Plant Biol. 2011, 11, 61.
- Zhu, Q.H.; Stephen, S.; Taylor, J.; Helliwell, C.A.; Wang, M.B. Long noncoding RNAs responsive to Fusarium oxysporum infection in Arabidopsis thaliana. New Phytol. 2014, 201, 574–584.
- Wang, J.; Yu, W.; Yang, Y.; Li, X.; Chen, T.; Liu, T.; Ma, N.; Yang, X.; Liu, R.; Zhang, B. Genome-wide analysis of tomato long non-coding RNAs and identification as endogenous target mimic for microRNA in response to TYLCV infection. Sci. Rep. 2015, 5, 16946.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.7K
Revisions:
2 times
(View History)
Update Date:
15 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





